Diabetes mellitus is a common complication of pregnancy associated with increased risks of hypertension and preeclampsia. We identified impaired activity of intermediate-conductance Ca2+-activated K+ channels as an important mechanism of diabetes-induced uterine endothelial dysfunction. Targeting intermediate-conductance Ca2+-activated K+ channels may be a novel therapeutic strategy for improving maternal uterine blood flow in diabetic pregnancies.
Keywords: Ca2+-activated K+ channels, fura-2, membrane potential, pressurized arteries, patch clamp
Abstract
Diabetes in rat pregnancy is associated with impaired vasodilation of the maternal uteroplacental vasculature. In the present study, we explored the role of endothelial cell (EC) Ca2+-activated K+ channels of small conductance (SKCa channels) and intermediate conductance (IKCa channels) in diabetes-induced uterine vascular dysfunction. Diabetes was induced by injection of streptozotocin to second-day pregnant rats and confirmed by the development of maternal hyperglycemia. Control rats were injected with citrate buffer. Changes in smooth muscle cell intracellular Ca2+ concentration, membrane potential, and vasodilation induced by SKCa/IKCa channel activators were studied in uteroplacental arteries of control and diabetic rats. The impact of diabetes on SKCa- and IKCa-mediated currents was explored in freshly dissociated ECs. NS309 evoked a potent vasodilation that was effectively inhibited by TRAM-34 but not by apamin. NS309-induced smooth muscle cell intracellular Ca2+ concentration, membrane potential, and dilator responses were significantly diminished by diabetes; N-cyclohexyl-N-2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine (CyPPA)-evoked responses were not affected. Ca2+-activated ion currents in ECs were insensitive to paxilline, markedly inhibited by charybdotoxin (ChTX), and diminished by apamin. NS309-induced EC currents were generated mostly due to activation of ChTX-sensitive channels. Maternal diabetes resulted in a significant reduction in ChTX-sensitive currents with no effect on apamin-sensitive or CyPPA-induced currents. We concluded that IKCa channels play a prevalent role over SKCa channels in the generation of endothelial K+ currents and vasodilation of uteroplacental arteries. Impaired function of IKCa channels importantly contributes to diabetes-induced uterine endothelial dysfunction. Therapeutic restoration of IKCa channel function may be a novel strategy for improvement of maternal uteroplacental blood flow in pregnancies complicated by diabetes.
NEW & NOTEWORTHY
Diabetes mellitus is a common complication of pregnancy associated with increased risks of hypertension and preeclampsia. We identified impaired activity of intermediate-conductance Ca2+-activated K+ channels as an important mechanism of diabetes-induced uterine endothelial dysfunction. Targeting intermediate-conductance Ca2+-activated K+ channels may be a novel therapeutic strategy for improving maternal uterine blood flow in diabetic pregnancies.
diabetes mellitus is one of the most common medical complications of pregnancy and significantly contributes to maternal and fetal morbidity and mortality (3, 4, 6, 9, 32). A current epidemiological study (9) has indicated an increase in the rate of diabetes during pregnancy in part due to the worldwide obesity epidemic. Diabetic pregnant women have a significantly greater risk of developing hypertension, preeclampsia, and other pregnancy complications than nondiabetic women (3, 5, 44, 45, 53).
In humans, diabetes during pregnancy is associated with an abnormal regulation of peripheral vascular tone in part due to vascular endothelial dysfunction (2, 33, 42). Impaired endothelial function was also detected in the peripheral vasculature of women with a history of gestational diabetes (1, 30). Reduced endothelium-dependent vasodilation has been identified in the main uterine artery of diabetic rats and mice and in myometrial arteries of diabetic pregnant women (11, 49, 50). We recently demonstrated that diabetes during rat pregnancy impairs endothelium-mediated dilation of small uteroplacental radial arteries. Specifically, nitric oxide (NO)- and prostacyclin-independent vasodilation was severely reduced, implicating the EDHF system as a key target of diabetic damage in the uterine resistance vasculature (24). Available data provide evidence that endothelial dysfunction might be an important underlying mechanism of decreased maternal uterine blood flow in pregnancies complicated by diabetes (10, 16, 43, 50).
An increase in the intracellular Ca2+ concentration ([Ca2+]i) translates the effects of mechanical or agonist stimulation of endothelial cells (ECs) into cellular responses (41). A [Ca2+]i rise in response to ACh or ATP stimulation of ECs of the maternal uterine vasculature is a critical step in generating both NO and EDHF (25, 52). Experimental diabetes during rat pregnancy significantly attenuates ACh-induced [Ca2+]i responses in ECs of rat uteroplacental arteries (24). Likewise, diabetes impairs UTP-induced Ca2+ signaling in cardiac ECs (47). These findings suggest that impaired endothelial Ca2+ signaling is an important mechanism contributing to diabetes-induced endothelial dysfunction.
We have previously shown that EDHF-mediated uterine vasodilation was severely reduced by diabetic pregnancy despite partial preservation of EC [Ca2+]i responses to ACh (24). These data suggest that, in addition to impaired endothelial Ca2+ signaling, there are other mechanisms responsible for inhibiting EDHF-induced vasodilation in diabetic pregnancy. The nature of EDHF in rat maternal uteroplacental arteries is not fully understood. Recently, we have shown that Ca2+-activated K+ channels of small conductance (SKCa channels) and intermediate conductance (IKCa channels) play a key role in EDHF-mediated vasodilation of these vessels (26). SKCa and IKCa channels also mediate NO- and prostacyclin-independent EDHF-mediated vasodilation to bradykinin in myometrial arteries from term pregnant women (23).
Ca2+-activated K+ channels comprise a group of SKCa channels, IKCa channels, and large-conductance channels (BKCa channels) that importantly regulate the function of vascular ECs and smooth muscle cells (SMCs). It is generally accepted that SKCa and IKCa channels are critically involved in EDHF-mediated vasodilation in the microcirculatory vasculature (19, 36, 37, 51). Activation of these channels may also contribute to Ca2+-dependent generation of NO due to hyperpolarization of ECs and a subsequent increase in the driving force for Ca2+ influx (41). The role of BKCa channels in regulating endothelial function is still debatable and has been demonstrated mostly in cultured ECs (46). Activity of endothelial Ca2+-activated K+ channels can be modulated by different pathological conditions contributing to altered vascular tone in disease states (18, 51). The role of BKCa channels in regulating endothelial function of the uteroplacental vasculature remains unknown. We hypothesized that the function of SKCa/IKCa channels is impaired in diabetic pregnancy, resulting in reduced generation of endothelial K+ currents, diminished EC and SMC hyperpolarization, and decreased vasodilation. In the present study, we characterized the role of each type of Ca2+-activated K+ channel in endothelium-mediated vasodilation of uterine arteries based on the effects of their specific activators and inhibitors. The effects of experimental diabetes were defined by studying changes in SMC [Ca2+]i or SMC membrane potential concurrently with vasodilation in response to activators of SKCa/IKCa channels in pressurized arteries. To assess the impact of maternal diabetes on channel function, ion currents mediated by Ca2+-activated K+ channels were characterized in ECs that were freshly dissociated from uteroplacental radial arteries of control and diabetic rats.
METHODS
Animals and preparation of arteries.
All experiments were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Pub No. 85-23, Revised 1996). Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Vermont.
Virgin Sprague-Dawley rats (10–11 wk of age) were purchased from Charles River Laboratories (St. Constant, QC, Canada) and housed at the University of Vermont animal care facility. The estrus cycle for female rats was determined by examining vaginal smears. Rats in proestrus were used for breeding. Female rats were bred with Sprague-Dawley male rats overnight in isolated pairs. If copulative plugs were observed the following morning, that day was designated as day 1 of pregnancy. On day 2 of pregnancy, female rats were anesthetized with 4% isoflurane, and 50–55 mg/kg streptozotocin (STZ) in 1 ml citrate buffer was injected intraperitoneally. Control pregnant rats were injected with 1 ml citrate buffer. Both STZ- and citrate buffer-injected rats were weighed, and maternal blood glucose levels were determined from a tail nick using a Freestyle glucometer every other day.
On day 20 of pregnancy, rats were euthanized with 4% isoflurane followed by decapitation. The abdominal wall was transected, and the entire uterus and uterine vasculature were rapidly removed and pinned in a dissecting dish filled with aerated cold physiological salt solution (PSS; see Solutions and drugs for the composition of PSS). Second-order uterine radial arteries were identified within the mesometrial arcade and dissected free of connective tissue. Only radial arteries feeding the placenta (uteroplacental arteries) were used for this study. Both ends of arterial segments were attached to the glass cannulas in an arteriograph and continuously superfused at 3 ml/min with aerated (10% O2, 5% CO2, and 85% N2) PSS at 37°C. Cannulated arteries were initially pressurized to 10 mmHg using the servo pressure system (Living System Instrumentation, Burlington, VT). After a 1-h equilibration period, intraluminal pressure was elevated to 50 mmHg. All experiments were performed at 50 mmHg and under no intraluminal flow conditions. Uteroplacental radial arteries from late pregnant rats can develop vasoconstriction (myogenic tone) in response to elevations of pressure exceeding 50 mmHg. To avoid the development of myogenic tone and its interference with phenylephrine (PE)-induced constriction, arteries were pressurized at 50 mmHg.
Fetuses and their placentas were carefully dissected from each uterine horn and individually weighed without membranes and umbilical cords. Litter size and number of fetal resorptions were recorded for each control or diabetic rat.
Selective loading of SMCs with fura-2 and measurement of [Ca2+]i.
SMC loading with fura-2 was performed by extraluminal incubation of arteries in fura-2 AM (5 μM)-containing solution at room temperature in the dark for 60 min. Fura-2-loaded arteries were washed two to three times and then continuously superfused with aerated PSS at 37°C. Ratiometric measurements of fura-2 fluorescence from SMCs were performed with a photomultiplier system (IonOptix, Milton, MA). Experimental ratios were corrected for background fluorescence taken from each artery before being loaded with fura-2. Background-corrected ratios of 510-nm emission were obtained at a sampling rate of 5 Hz from arteries alternately excited at 340 and 380 nm. All experimental protocols were started after an additional 15-min equilibration period at 10 mmHg to allow intracellular deesterification of fura-2 AM.
Measurements of SMC membrane potential from pressurized arteries.
For intracellular measurement of SMC membrane potential, we used short arterial segments (400–500 μm) that were carefully cleaned of any residual connective tissue. Glass microelectrodes were filled with 0.5M KCl and had tip resistances of 110–150 MΩ; an Ag-AgCl pellet was used as an indifferent electrode. A microelectrode was connected to a motorized micromanipulator (World Precision Instruments), and membrane potential was recorded using a high-input impedance amplifier (Electro 705, World Precision Instruments). Changes in membrane potential and arterial diameter were simultaneously recorded using a data-acquisition program (IonOptix). The following criteria were used for the acceptance of membrane potential recordings: 1) an abrupt negative change in voltage upon impalement of the cells, 2) a sharp return to zero voltage after withdrawal of the microelectrode tip, 3) a tip potential of <7 mV, and 4) an unchanged resistance of microelectrodes after impalement. A stable membrane potential recording for at least 1 min was accepted for data collection.
Enzymatic isolation of ECs and SMCs.
Our protocol for isolating ECs from radial uteroplacental arteries was adopted from Sonkusare et al. (48). After the dissection and careful cleaning from perivascular connective tissue, arterial segments were placed in dissociation media (see composition in Solutions and drugs) containing neutral protease (dispase; 0.5 mg/ml) and elastase (0.5 mg/ml) for 60 min at 37°C; 0.5 mg/ml collagenase type 1 was included for the final 2 min. Gentle trituration of arteries with a glass pipette yielded small sheets of endothelial cells (EC sheets) as well as single ECs.
For the isolation of SMCs, uteroplacental radial arteries were placed in dissociation media (see composition in Solutions and drugs) containing papain (0.3 mg/ml) and dithioerythritol (1 mg/ml) for 20 min at 37°C. Arteries were then transferred to the solution containing a 1.0 mg/ml mixture of collagenase type F (70%) and type H (30%) for 5 min at 37°C. Arteries were gently triturated with a glass pipette that yielded spindle-shaped elongated SMCs. The length of single SMCs was ∼10 times greater than single ECs.
Quantitative real-time PCR.
Radial uteroplacental arteries were collected from control and diabetic 20-day pregnant rats for real-time PCR analysis of SKCa (SK3) and IKCa (SK4) expression. RNA was extracted from arteries using a TRIzol (Invitrogen) extraction protocol and further purified using a RNeasy Micro Kit (Qiagen). All samples were quantified using a Nanodrop Spectrophotometer, and RNA integrity was assessed on the Agilent 2100 Bioanalyzer (Agilent Technologies). Fifty nanograms of total RNA were reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad). Quantitative PCRs were done using 150 nM of forward and reverse primers in Power Sybrgreen Master Mix (Applied Biosystems) with 1 μl of cDNA template. Target mRNA transcripts for SK3, SK4, and two housekeeping genes [hypoxanthine guanine phosphoribosyl transferase (HPRT) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein (Ywaz)] were amplified using an ABI Prism 7000 Sequence Detection System. Standard curves were generated for each primer set and used to calculate the relative quantities of all samples. Relative target mRNA values were normalized using the mean of the control gene quantities. Negative water controls were run for each primer set to ensure no contamination in the reagents and that no secondary primer structures were amplified. In each primer set, the PCR amplicon was designed to span at least one intron region to ensure only cDNA was amplified. We used the following primers: SKCa (SK3), forward 5′-TGTCTTCTCACTGGCATCATGG-3′ and reverse 5′-ACATTGGCGGCAGCGTTCTT-3′; IKCa (SK4) forward 5′-TCAATGCCACGGGACACCT-3′ and reverse 5′-GCCACCACAGCCAATAGTAGAGC-3′; HPRT, forward 5′-CAGTCCCAGCGTCGTGAT-3′ and reverse 5′-CAAGTCTTTCAGTCCTGTCCATAA-3′; and Ywhaz, forward 5′-GCAACGACGTACTGTCTCTTTTGG-3′ and reverse 5′-GTCCACAATTCCTTTCTTGTCATC-3′.
Western blot analysis.
Protein expression of SK3 (SKCa) and SK4 (IKCa) was quantified by Western blot analysis. Radial uterine arteries feeding the placenta were dissected from four control and four diabetic late pregnant rats. Arteries were cleaned of connective tissue in the presence of protease and phosphatase inhibitor cocktail (Thermo Scientific) and kept at −80°C. On the day of the experiment, samples were homogenized in 300 μl lysis buffer. Cell lysates were centrifuged at 800 g to remove cellular debris. Total protein content within cell lysates was measured using the BCA protein assay kit (Pierce), and samples containing equivalent amounts of protein (15–25 μg) were analyzed by SDS-PAGE and Western blot analysis using polyclonal antibodies (Santa Cruz Biotechnology) against SK3 (1:200), SK4 (1:200), and β-actin (1:5,000). Primary antibody binding was visualized using horseradish-conjugated secondary antibody (Cell Signaling Technology) and enhanced chemiluminescence (Pierce). Protein bands were quantified using ImageJ software (NIH).
Protocols for studying the effects of diabetes on uteroplacental artery responses to activators of SKCa/IKCa channels.
Our previous study (26) demonstrated that activation of SKCa and IKCa channels is critical for EDHF-mediated uterine vasodilation. To assess the role of SKCa and IKCa channels in diabetes-impaired uterine vasodilation, we tested the effects of NS309 and N-cyclohexyl-N-2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine (CyPPA) on arteries of control and diabetic rats. NS309 has been shown to be a specific activator of both IKCa and SKCa channels in ECs; CyPPA has been recently introduced as a potent activator of SKCa channels (27). To avoid concomitant production of NO and prostacyclin, arteries were preincubated with 200 μM N-nitro-l-argine (l-NNA; NO synthase inhibitor) and 10 μM indomethacin (cyclooxygenase inhibitor) for 20 min. Vessels were then preconstricted with PE to 60–70% of their initial diameter. After stabilization of vasoconstriction, NS309 was applied in increasing concentrations. A combination of papaverine (100 μM, a phosphodiesterase inhibitor) and diltiazem (10 μM, a Ca2+ channel blocker) was added at the end of the experiment to obtain the diameter under maximally dilated conditions (Dmax). NS309-induced changes in lumen diameters were calculated at the end of the 10-min application period.
PE-induced constrictions were expressed as the percentage of Dmax. Vasodilation to NS309 was expressed as the percentage of maximal vasodilation in response to papaverine and diltiazem. In an additional set of the experiments, vasodilator responses were studied in combination with recordings of changes in SMC [Ca2+]i. Maximal levels of [Ca2+]i in response to each tested concentration of NS309 were compared between experimental groups.
Similar experimental protocols were used to characterize the effect of experimental diabetes on CyPPA-induced diameter and SMC [Ca2+]i responses of arteries preconstricted with PE. In separate subsets of experiments, vasodilator responses to NS309 or CyPPA were studied in arteries treated with apamin or TRAM-34. Apamin was applied intraluminally and extraluminally. Effects of NS309 and CyPPA were also tested in arteries denuded of the endothelium. The denudation procedure was performed by passing air bubbles through the lumen of vessels for 7–10 min. Successful denudation was confirmed by the absence of a vasodilator response to 1 μM ACh.
SMC membrane potential responses to NS309 and CyPPA.
In our electrophysiological experiments, each artery was initially pressurized to 10 mmHg and equilibrated for 1 h. Intraluminal pressure was elevated to 50 mmHg, and PE was added to preconstrict arteries to 60–70%. After stabilization of the PE-induced constriction, microelectrode impalement of SMCs was performed, and membrane potential was recorded for 2–3 min. NS309 or CyPPA was applied in increasing concentrations during the next 15–20 min. SMC membrane potential was recorded after stabilization of the response to each concentration of tested compounds (typically, on 5–8 min of application). Arterial diameters were continuously monitored before and during administration of NS309 or CyPPA. A combination of papaverine and diltiazem was added at the end of each experiment to maximally dilate arteries.
Protocols to study ion currents in ECs and SMCs.
Membrane currents were recorded from ECs or SMCs freshly dissociated from radial uteroplacental arteries of control or diabetic rats. We used the conventional whole cell configuration of the patch-clamp technique and voltage-ramp protocols (200 ms); holding potentials for ECs and SMCs were −45 and −60 mV, respectively. The concentration of Ca2+ in the pipette solutions was 1 μM for experimentation on SMCs and 300 nM or 3 μM in experiments using ECs. The patch-clamp pipette serial resistance was 5–7 MΩ. Ion currents were sampled at 20 kHz, acquired in a voltage-clamp mode using Axopatch 200B, and analyzed with pCLAMP software (Axon Instruments). Patch-clamp experiments were performed at room temperature (20–22°C); all tested compounds were added directly to the experimental chamber.
Solutions and drugs.
PSS contained (in mM) 119 NaCl, 4.7 KCl, 24.0 NaHCO3, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, and 11.0 glucose (pH 7.4). For the fura-2 calibration procedure, we used a solution of the following composition: 140 mM KCl, 20 mM NaCl, 5 mM HEPES, 5 mM EGTA, 1 mM MgCl2, 5 μM nigericin, and 10 μM ionomycin (pH adjusted to 7.1). Dissociation media for enzymatic isolation of ECs contained (in mM) 55 NaCl, 80 Na glutamate, 5.6 KCl, 2 MgCl2, 0.1 CaCl2, 10 glucose, and 10 HEPES (pH adjusted to 7.3). The solution for enzymatic dissociation of SMCs contained (in mM) 110 NaCl, 10 NaHCO3, 5 KCl, 0.5 NaH2PO4, 0.5 KH2PO4, 2 MgCl2, 0.16 CaCl2, 10 HEPES, and 10 glucose (pH adjusted to 7.0). The bath solution for patch-clamp experiments was of the following composition (in mM): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose (pH adjusted to 7.4). For experiments using a conventional patch-clamp configuration, the pipette solution for SMCs (1 μM Ca2+) contained (in mM) 110 KCl, 30 KOH, 10 HEPES, 10 EGTA, 1.07 MgCl2, 8.56 CaCl2, and 10 NaCl (pH adjusted to 7.2). The pipette solution (free concentration of Ca2+: 3 μM) used for ECs was (in mM) 134 KCl, 5.47 MgCl2, 0.181 CaCl2, 5 hydroxy-EDTA, and 10 HEPES (adjusted to pH 7.2 with KOH). The pipette solution (free concentration of Ca2+: 300 nM) used for ECs was (in mM) 132 KCl, 10 NaOH, 10 HEPES, 5 EGTA, 1.088 MgCl2, and 3.19 CaCl2 (adjusted to pH 7.2 with KOH). The concentrations of ions for pipette solutions of desirable concentration of Ca2+ were calculated using the WEBMAXC STANDARD program. The composition of lysis buffer for Western blot analysis was 50 mM HEPES, 250 mM NaCl, 1.5 mM MgCl2, 1 mM PMSF, 1 mM EGTA, 2 mM Na3VO4, 10% glycerol, 1% Triton, 10 μg/ml aprotinin, and 10 μg/ml leupeptin (pH 7.4). The majority of chemicals was purchased from Sigma Chemical (St. Louis, MO). Ionomycin and nigericin were obtained from Calbiochem (La Jolla, CA). Fura-2 AM and pluronic acid were purchased from Invitrogen (Carlsbad, CA). Protease, elastase, and collagenase were purchased from Worthington Biochemical (Lakewood, NJ). Apamin and charybdotoxin (ChTX) were from Peptide International (Louisville, KY). TRAM-34 was from R&D Systems (Minneapolis, MN). Fura-2 AM was dissolved in dehydrated DMSO as a 1 mM stock solution, refrigerated in small aliquots, and used within 1 wk of preparation. NS309, CyPPA, and TRAM-34 were prepared as stock solutions in DMSO and stored at −20°C until use. Apamin, ChTX, and paxilline were dissolved in deionized water and stored at −20°C. PE and papaverine were prepared as water stock solutions on the day of the experiment. l-NNA was dissolved in PSS before the use. Diltiazem and indomethacin were prepared as 10 mM stock solutions in deionized water and alcohol, respectively, and kept refrigerated until use. Ionomycin and nigericin were dissolved in ethanol (10 mM) and kept at −20°C.
Calculations and statistical analysis.
SMC [Ca2+]i was calculated using the following equation (28): [Ca2+]i = Kdβ(R − Rmin)/(Rmax − R), where β is the ratio of the fluorescence intensities at 380-nm excitation wavelength at Rmin and Rmax, R is the experimentally measured ratio (340/380 nm) of fluorescence intensities, Rmin is the ratio in the absence of [Ca2+]i, and Rmax is the ratio at Ca2+-saturated fura-2 conditions. Rmin, Rmax, and β were determined by an in situ calibration procedure from fura-2-loaded arteries treated with ionomycin (10 μM) and nigericin (5 μM). These values were then pooled and used to convert the ratio values into [Ca2+]i. Kd was 282 nM, as determined by in situ titration of Ca2+ in fura-2-loaded small arteries (34). Arterial diameter and intraluminal pressure in combination with membrane potential or 340-to-380-nm ratios were simultaneously recorded using an IonOptix data-acquisition program and imported into the SigmaPlot program for graphical representation, calculations, and statistical analysis. Considering significant oscillatory activity in uterine arteries, all measurements were made by averaging records of arterial diameters, membrane potential, or SMC [Ca2+]i. Each calculated mean value represents averaged data points over a time period of 15–20 s obtained with IonOptix software and includes two to three oscillations when present. At least two lowest and two highest oscillatory points were included in the average. In case of prolonged (>30 s) waves in membrane potential, SMC [Ca2+]i, or arterial diameter, the average value included one lowest and highest point. Data are expressed as means ± SE; n is the number of arterial segments studied. One or two arteries from the same animal were used on each experimental day with 1 vessel/animal used for each protocol. A paired or unpaired Student's t-test or two-way repeated-measures ANOVA were used to determine the significance of differences between sets of data, with P < 0.05 considered significant.
RESULTS
Maternal and fetal pregnancy outcome in a rat model of diabetic pregnancy.
STZ injection produced sustained hyperglycemia on days 4–5 of pregnancy with average nonfasting glucose levels on day 20 of pregnancy of 381.8 ± 8.5 mg/dl (n = 65). Control rats injected with citrate buffer remain normoglycemic throughout pregnancy (77.0 ± 1.2 mg/dl on day 20 of pregnancy, n = 69). Diabetic pregnancy resulted in significantly reduced fetal (1.87 ± 0.05 vs. 2.30 ± 0.02 g) and increased placental (0.541 ± 0.014 vs. 0.444 ± 0.008 g) weights. There was a small but significant reduction in the litter size of diabetic (13.7 ± 0.4) versus control rats (15.4 ± 0.3).
Diabetes in pregnancy impairs NS309- but not CyPPA-induced uterine vasodilation and SMC [Ca2+]i responses.
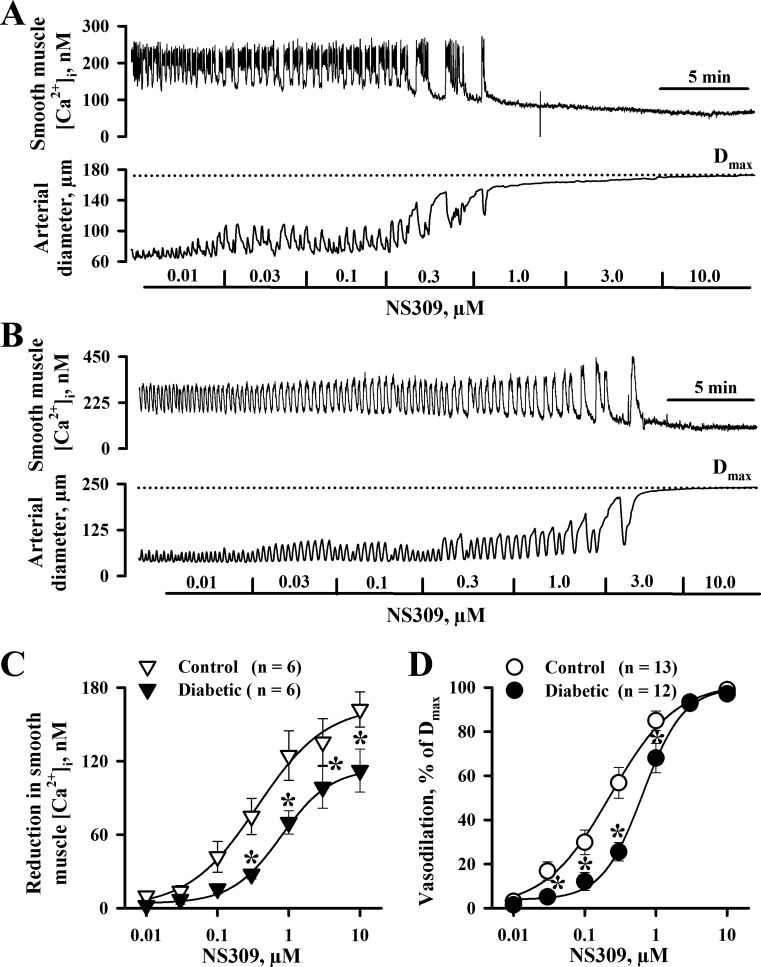
In rat diabetic pregnancy, NO- and prostacyclin-independent EDHF-mediated uterine vasodilation was markedly reduced (24). Here, we defined the relative role of SKCa and IKCa channels in diabetes-impaired vasodilation by testing the vascular effects of NS309 and CyPPA. Arteries of control and diabetic rats were pretreated with l-NNA and indomethacin and preconstricted with PE before NS309 or CyPPA was tested. PE-induced constriction of both control and diabetic vessels was associated with significant SMC [Ca2+]i elevation and the appearance of frequent SMC [Ca2+]i transients (Fig. 1, A and B). NS309 application resulted in a reduction in SMC [Ca2+]i levels and reduced frequency of Ca2+ oscillations or abolishing them in a concentration-dependent manner. Oscillatory changes in [Ca2+]i were followed by oscillations in the arterial diameter, and maximal reduction in SMC [Ca2+]i was associated with maximal vasodilation. NS309-induced SMC [Ca2+]i and dilator responses were significantly diminished in arteries of diabetic rats (Fig. 1, C and D).
Fig. 1.
Diabetes during pregnancy impairs smooth muscle cell (SMC) intracellular Ca2+ concentration ([Ca2+]i) and dilator responses of uterine arteries induced by the activation of small-conductance (SKCa)/intermediate-conductance Ca2+-activated K+ (IKCa) channels with NS309. A and B: representative tracings showing the concentration-dependent reduction in SMC [Ca2+]i and dilatation of an uteroplacental artery from a control (A) and diabetic (B) rat in response to NS309. Arteries were pretreated with 200 μM N-nitro-l-arginine (l-NNA) and 10 μM indomethacin and preconstricted with phenylephrine (0.3 μM) before NS309 was tested. The dotted line indicates the level of maximal dilatation (Dmax) of arteries in response to papaverine and diltiazem. C and D: summary graphs demonstrating reduced SMC [Ca2+]i (C) and dilator (D) responses to NS309 in arteries from control compared with diabetic rats. Vasodilation is expressed as a percentage of Dmax. Numbers in parentheses are numbers of tested arteries. *Significantly different from controls at P < 0.05.
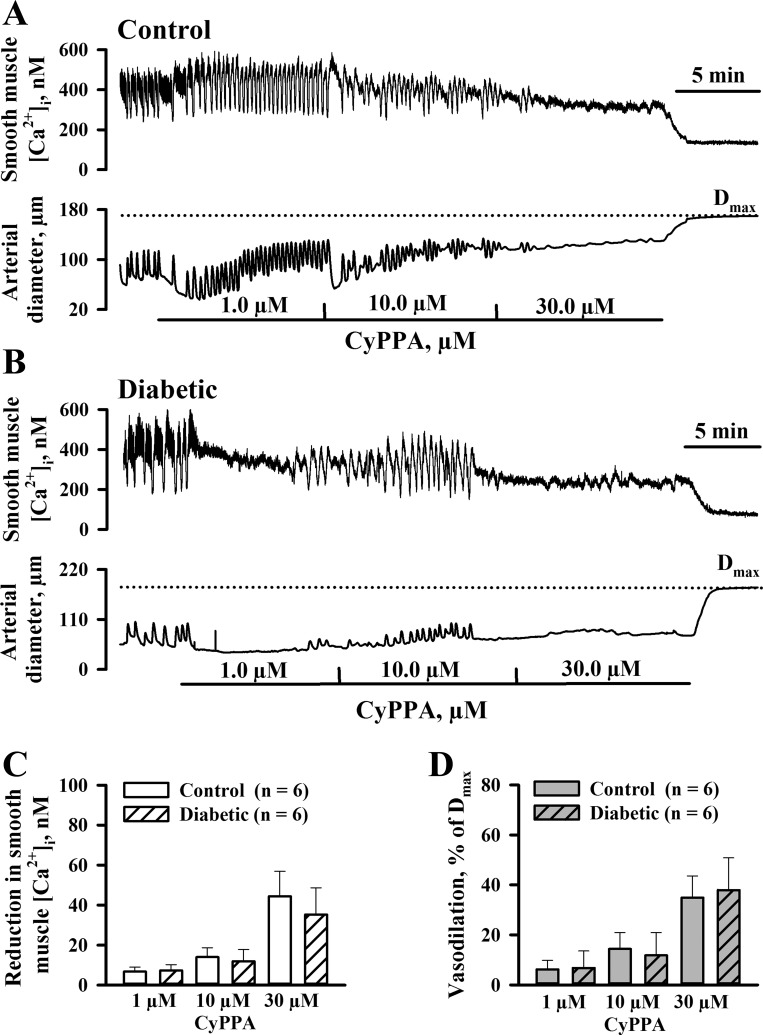
Effects of diabetes on CyPPA-induced vasodilation and SMC [Ca2+]i were tested next. As shown in Fig. 2, A and B, CyPPA application to PE-preconstricted vessels resulted in a modest SMC [Ca2+]i reduction and vasodilation. No significant differences were found in CyPPA-induced responses of arteries from control and diabetic rats (Fig. 2, C and D). Maximal CyPPA-induced responses in vessels of control and diabetic rats were significantly smaller compared with maximal NS309-induced changes in SMC [Ca2+]i (35.3 ± 13.4 vs. 112.3 ± 17.6 nM in diabetics) and diameters (36.7 ± 13.3% vs. 97.1 ± 0.9% in diabetics).
Fig. 2.
Experimental diabetes does not affect uterine vascular responses to activation of SKCa channels with N-cyclohexyl-N-2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine (CyPPA). A and B: representative records demonstrating the reduction in SMC [Ca2+]i and vasodilation of uteroplacental arteries from control (A) and diabetic (B) rats in response to application of CyPPA in increasing concentrations. Vessels were preconstricted with phenylephrine (0.1 and 0.2 μM) in the presence of l-NNA and indomethacin before CyPPA was tested. The dotted line indicates Dmax induced by papaverine and diltiazem. C and D: summary graphs showing the lack of differences between CyPPA-induced changes in SMC [Ca2+]i (C) and diameters of arteries (D) from control and diabetic pregnant rats. Vasodilation is expressed as a percentage of Dmax. Numbers in parentheses are numbers of tested arteries.
Role of IKCa and SKCa channels in NS309- and CyPPA-induced vasodilation.
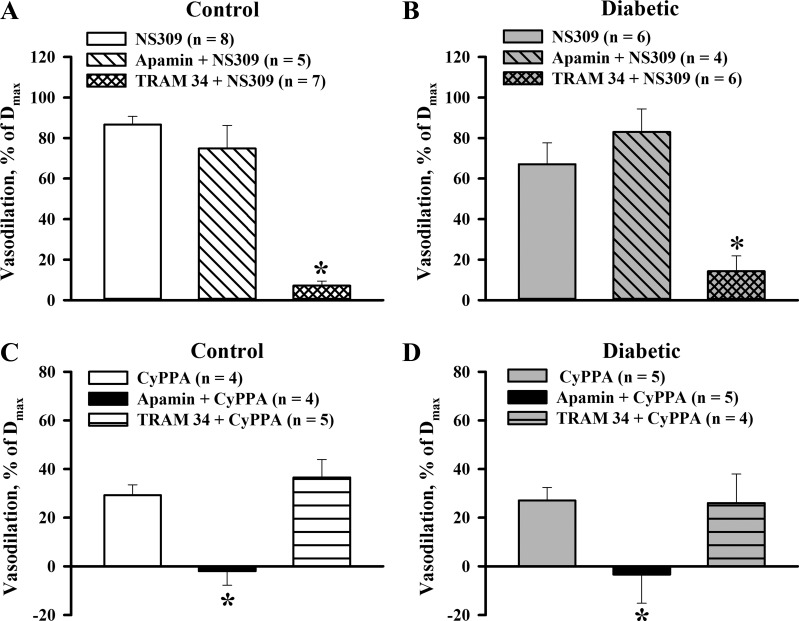
The relative contribution of IKCa and SKCa channels in NS309-induced vasodilation was explored by studying the vasodilation after blockade of SKCa channels with apamin or IKCa channels with TRAM-34. As shown in Fig. 3, A and B, 1 μM NS309 effectively dilated arteries from both control and diabetic rats in the presence of l-NNA and indomethacin. These dilator responses were not affected by apamin but were abolished by TRAM-34. The TRAM-34-sensitive component of NS309-induced vasodilation of diabetic arteries (80 ± 10%) was not significantly different from controls (93 ± 3%). CyPPA-induced vasodilation was blocked by apamin but was not affected by TRAM-34 (Fig. 3, C and D). These data demonstrate that NS309-induced uterine vasodilation is preferentially mediated by IKCa channel activation and that CyPPA effects are induced by activation of SKCa channels.
Fig. 3.
A and B: bar graphs summarizing the effects of SKCa and IKCa channel inhibition with 300 nM apamin and 5 μM TRAM-34 on vasodilation induced by 1 μM NS309 in uteroplacental arteries of control (A) and diabetic (B) rats. C and D: summary graphs demonstrating blockade of CyPPA-induced (30 μM) vasodilation with apamin in arteries of control (C) and diabetic (D) rats. TRAM-34 did not change CyPPA-induced vasodilation. Vasodilator responses are expressed as a percentage of Dmax of each artery induced by application of papaverine and diltiazem. Numbers in parentheses are numbers of tested arteries. *Significantly different from control responses at P < 0.05.
Dilator effects of 1 μM NS309 and 30 μM CyPPA were markedly reduced in endothelium-denuded arteries of control (6.3 ± 3.9% and 4.8 ± 3.5%, n = 4) and diabetic (1.2 ± 0.7% and 0.9 ± 0.9%, n = 4) rats. In these experiments, successful denudation was confirmed by abolition of ACh-induced dilation (5.3 ± 1.9%, n = 8). These data demonstrate that both NS309- and CyPPA-induced dilator responses of uteroplacental arteries are mediated by the activation of IKCa and/or SKCa channels located on ECs.
Experimental diabetes impairs SMC hyperpolarization induced by activators of SKCa/IKCa channels.
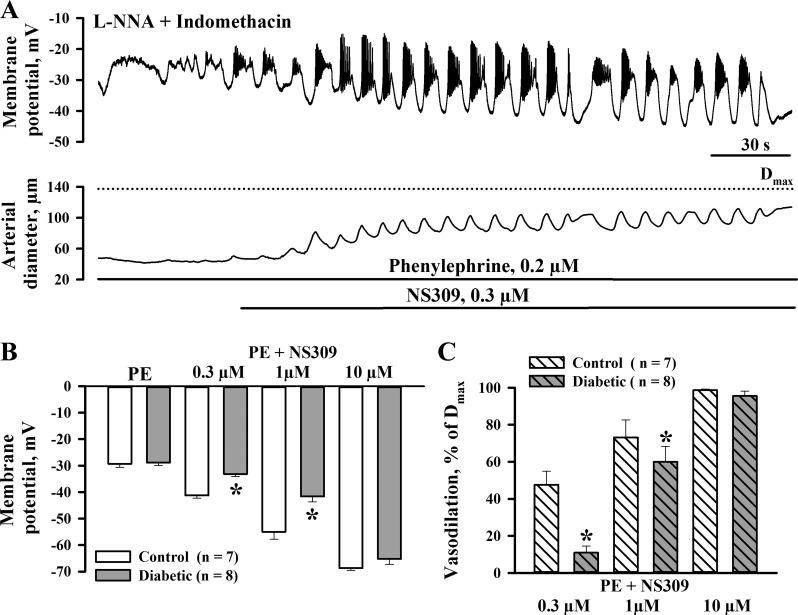
In a subset of experiments, we assessed the effects of NS309 on SMC membrane potential of pressurized arteries from control and diabetic rats. Measurements of SMC membrane potential were performed in the presence of l-NNA and indomethacin. In the presence of PE, SMC membrane potential was −29.3 ± 1.3 mV (n = 7, controls) and −28.9 ± 1.1 mV (n = 8, diabetics), which was associated with vasoconstriction of 66.4 ± 4.8% and 66.7 ± 1.7%, respectively. Subsequent administration of NS309 in concentrations of 0.3, 1, and 10 μM resulted in concentration-dependent SMC hyperpolarization to −41.3 ± 1.0, −55.0 ± 2.8, and −68.7 ± 0.9 mV, respectively, in controls (n = 7) and to −33.1 ± 0.9, −41.6 ± 2.1, and −65.3 ± 2.1 mV in diabetics (n = 8). Representative recordings of NS309-induced changes in SMC membrane potential and diameters of control arteries are shown in Fig. 4A. As evident from the summary graphs, NS309-induced hyperpolarization was significantly reduced in arteries of diabetic rats and was associated with diminished vasodilation (Fig. 4, B and C).
Fig. 4.
Diabetes in pregnancy impairs NS309-induced SMC hyperpolarization and associated dilation of uteroplacental arteries. A: representative changes in SMC membrane potential and arterial diameter in response to application of 0.3 μM NS309. The control artery was pretreated with l-NNA and indomethacin and preconstricted with phenylephrine before NS309 was tested. B and C: bar graphs summarizing the effect of diabetes on SMC hyperpolarization (B) and vasodilation (C) induced by NS309. Vasodilation is expressed as a percentage of Dmax induced by application of papaverine and diltiazem. Numbers in parentheses are numbers of tested arteries. *Significantly different from controls at P < 0.05.
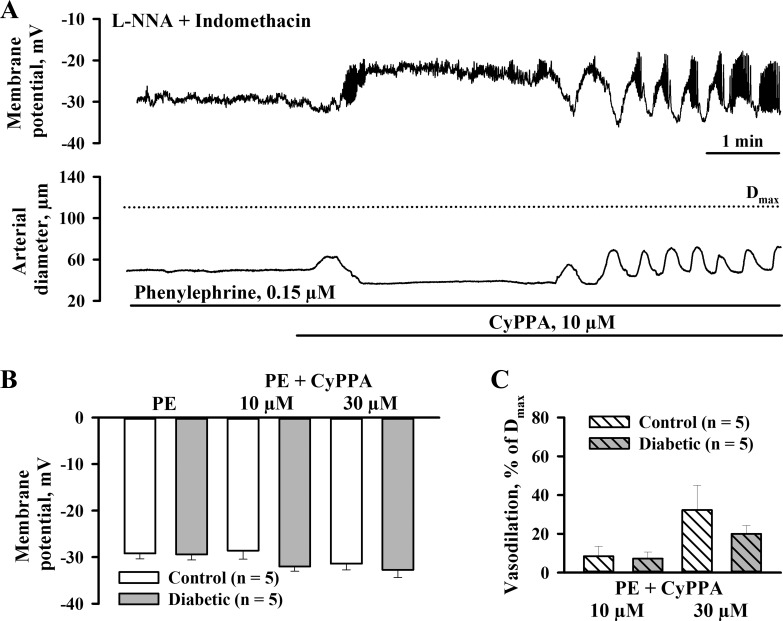
Similar electrophysiological experiments were performed to define the effect of CyPPA on SMC membrane potential in control and diabetic arteries preconstricted with PE to 59.4 ± 4.1% and 63.9 ± 2.5%, respectively. The application of CyPPA resulted in a modest hyperpolarization that was associated with a partial vasodilation (Fig. 5A). The summary graphs demonstrated no significant difference in hyperpolarizing and dilator effects of CyPPA in arteries from control and diabetic rats (Fig. 5, B and C).
Fig. 5.
Maternal diabetes does not change SKCa channel-mediated SMC hyperpolarization and vasodilation induced by CyPPA in rat uteroplacental arteries. A: representative tracings showing changes in SMC membrane potential and diameters of arteries from a diabetic rat in response to CyPPA. Arteries were pretreated with l-NNA and indomethacin and preconstricted with phenylephrine before CyPPA was tested. B and C: summary graphs demonstrating hyperpolarization (B) and vasodilation (C) to CyPPA. There were no significant differences in the responses between control and diabetic vessels. Vasodilation is expressed as a percentage of Dmax induced by application of papaverine and diltiazem. Numbers in parentheses are numbers of tested arteries.
Our data indicate that diabetic pregnancy is associated with significantly reduced responses to NS309 but not to CyPPA, suggesting that in maternal uteroplacental arteries, diabetes impairs function of endothelial IKCa rather than SKCa channels.
Functional role of BKCa channels in mediating outward K+ currents in ECs.
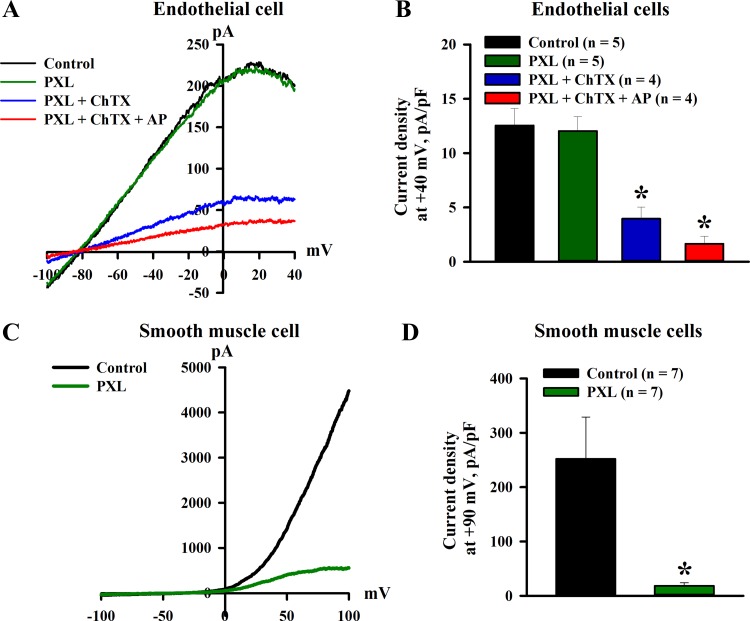
The functional role of BKCa channels in mediating outward K+ currents in ECs of uteroplacental vessels was evaluated next based on the effects of paxilline, a potent inhibitor of BKCa channels. Representative tracings of whole cell currents from an EC dialyzed with 3 μM free pipette Ca2+ in response to a voltage ramp from −100 to +40 mV are shown in Fig. 6A. As evident from this record, the application of paxilline induced no changes in the current. Subsequent addition of ChTX resulted in marked inhibition of the current, which was further reduced by apamin. A summary of current densities at +40 mV of control currents and currents in the presence of paxilline alone or in combination with ChTX as well as ChTX and apamin is shown in Fig. 6B. Similarly, paxilline did not change 3 μM Ca2+-induced currents in ECs from diabetic rats (6.7 ± 1.9 vs. 6.6 ± 2.0 pA/pF before and after paxilline application, n = 3 rats). Experiments were also performed using single SMCs dissociated from uteroplacental arteries of control pregnant rats. Paxilline markedly reduced the outward currents in SMCs dialyzed with 1 μM free Ca2+ (Fig. 6, C and D).
Fig. 6.
Lack of functional role of large-conductance Ca2+-activated K+ (BKCa) channels in the generation of Ca2+-induced K+ currents in endothelial cells (ECs) dissociated from uterine radial arteries of control pregnant rats. A: current-voltage relationship of whole cell currents evoked in an EC dialyzed with 3 μM Ca2+ (voltage-ramp protocol). The application of 500 nM paxilline (PXL) produced no changes in the control current. The subsequent addition of 300 nM charybdotoxin (ChTX) markedly inhibited the current. Apamin (AP; 300 nM) application further reduced the current. B: a summary graph showing the mean density of currents at +40 mV under control conditions, in the presence of PXL, PXL + ChTX, and PXL + ChTX + AP. C: whole cell current-voltage relationship recorded from a single SMC dialyzed with 1 μM Ca2+ (voltage-ramp protocol). The current was markedly inhibited by application of 500 nM PXL. D: bar graph summarizing the effect of PXL on Ca2+-activated currents of SMCs freshly dissociated from radial uterine arteries of control pregnant rats. Mean current densities at +90 mV are shown before and after application of paxilline. Numbers in parentheses are numbers of tested SMCs. *Significantly different from controls at P < 0.05.
Diabetes reduces IKCa channel-mediated currents in ECs from uteroplacental arteries.
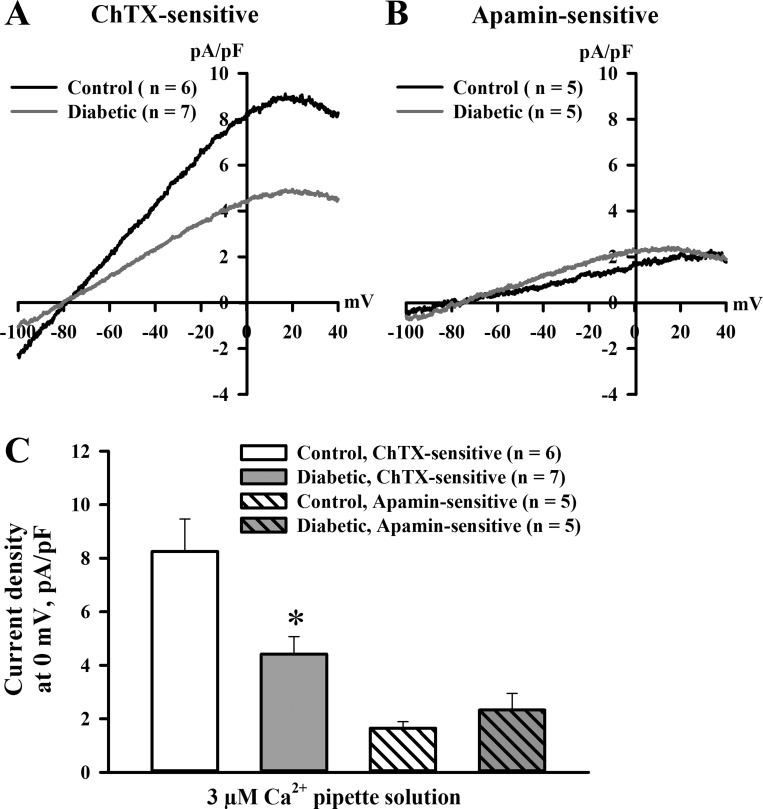
To assess the effect of diabetes on function of endothelial SKCa and IKCa channels, we studied whole cell outward ion currents in ECs freshly dissociated from radial uteroplacental arteries of control and diabetic pregnant rats. Membrane capacitance was similar in ECs isolated from control (16.7 ± 3.7 pF, n = 35) and diabetic (18.1 ± 3.7 pF, n = 29) rats. In cells dialyzed with 3 μM Ca2+ to maximally activate SKCa and IKCa channels, currents were effectively inhibited by ChTX and, to a much lesser degree, with apamin. In ECs from control rats, current densities at 0 mV for the ChTX-sensitive component were 8.1 ± 0.2 pA/pF (n = 6) and were approximately four times higher than densities of apamin-sensitive (1.8 ± 0.01 pA/pF, n = 5) currents (Fig. 7, A and B). ChTX-sensitive currents were significantly reduced in ECs from diabetic rats. We detected no differences in apamin-sensitive currents recorded from ECs of control and diabetic rats (Fig. 7C).
Fig. 7.
Diabetes inhibits ChTX-sensitive K+ currents induced by Ca2+ dialysis of ECs freshly dissociated from radial uteroplacental arteries of pregnant rats. A: current densities as a function of voltage for ChTX-sensitive whole cell currents evoked in ECs of control and diabetic rats dialyzed with 3 μM Ca2+. B: current-voltage relationships of AP-sensitive whole cell currents normalized for cell capacities evoked in ECs of control and diabetic rats. C: summary graph demonstrating the inhibitory effect of diabetes on mean densities of ChTX-sensitive currents at 0 mV. No difference was detected in mean densities of AP-sensitive currents. Numbers in parentheses are numbers of tested ECs. *Significantly different from controls at P < 0.05.
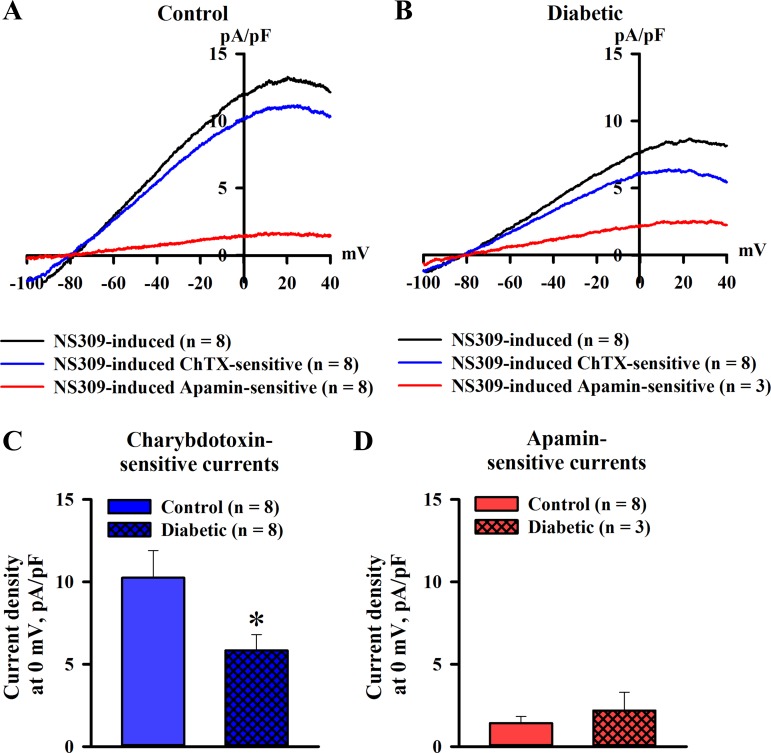
NS309-induced ion currents in ECs from uteroplacental arteries were characterized next. In these experiments, cells were dialyzed with 300 nM free Ca2+. Ion currents in response to 10 μM NS309 were studied in ECs of control and diabetic rats using the voltage-ramp protocol. Figure 8, A and B, shows NS309-induced currents and ChTX- and apamin-sensitive components of these currents normalized for membrane capacitance of ECs from control and diabetic rats. ChTX-sensitive currents were predominant in both groups of cells with a relatively minor contribution of apamin-sensitive currents. In a subset of experiments, we also used TRAM-34 to inhibit IKCa channels in ECs of control rats. NS309-induced currents (9.2 ± 1.6 pA/pF) were mostly TRAM-34 sensitive (7.5 ± 0.7 pA/pF, n = 5). These data indicate that outward currents stimulated with NS309 in ECs of small uteroplacental arteries are mainly mediated by the activation of IKCa channels. NS309-induced currents were significantly reduced by experimental diabetes with a markedly impaired ChTX-sensitive component (Fig. 8C). No significant differences were found in the apamin-sensitive component of NS309-induced currents in ECs of control and diabetic rats (Fig. 8D).
Fig. 8.
The ChTX-sensitive component of NS309-induced K+ currents in ECs of uteroplacental arteries is reduced by maternal diabetes. A and B: current-voltage relationships of ChTX (300 nM)- and AP (300 nM)-sensitive whole cell currents normalized for capacitance. Currents were activated by 10 μM NS309 in ECs dialyzed with 300 nM Ca2+. ChTX-sensitive currents were significantly greater than AP-sensitive currents in ECs from control (A) and diabetic (B) rats. C: bar graph showing the diabetes-induced reduction in mean densities of NS309-induced ChTX-sensitive currents. D: diabetes did not affect NS309-induced AP-sensitive currents. Numbers in parentheses are numbers of tested ECs. *Significantly different from controls at P < 0.05.
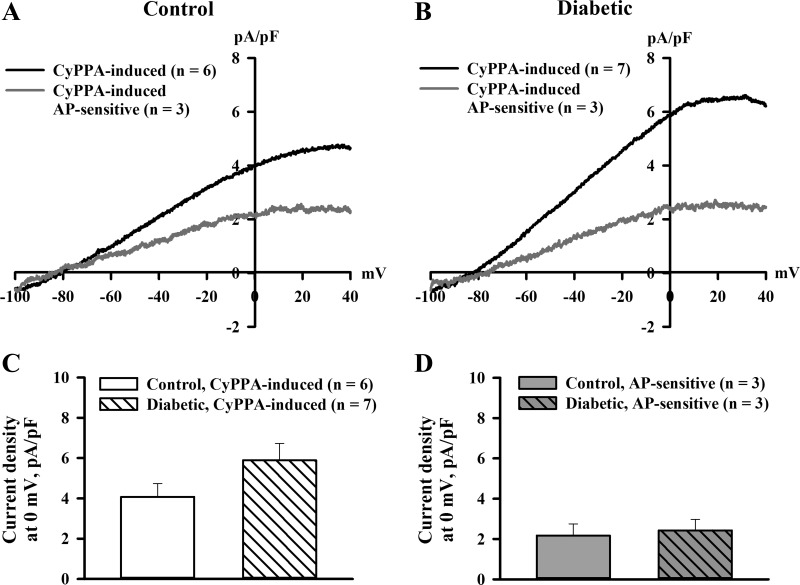
We next characterized K+ currents of ECs in response to stimulation of SKCa channels with CyPPA. As shown in Fig. 9, A and B, CyPPA-induced current densities were only about half of currents induced by NS309 (Fig. 8) and were partially blocked with apamin. CyPPA-induced currents were not significantly different between ECs from diabetic rats compared with control rats (Fig. 9, C and D).
Fig. 9.
Diabetes during rat pregnancy did not affect K+ currents generated in response to activation of SKCa channels with 30 μM CyPPA. A and B: current-voltage relationships of CyPPA-induced current densities recorded from ECs of control (A) and diabetic (B) pregnant rats. ECs were dialyzed with 300 nM Ca2+. C and D: bar graphs demonstrating the lack of differences in current densities of CyPPA-induced currents (C) and in AP-sensitive components of these currents (D) in ECs of control and diabetic rats. The concentration of AP was 300 nM. Numbers in parentheses are numbers of tested ECs.
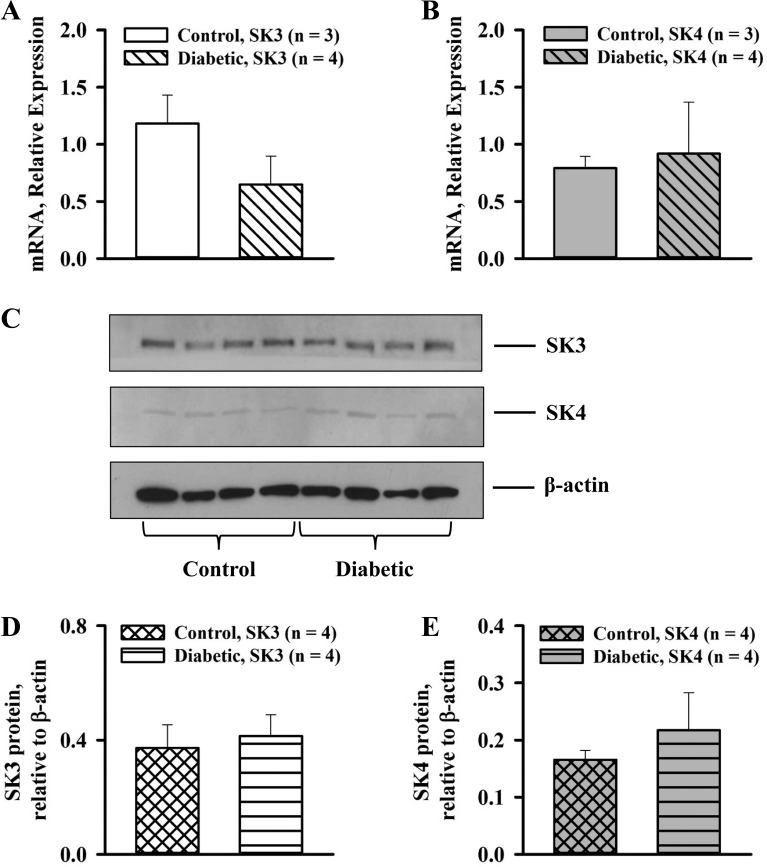
Finally, the relative expression of SKCa (SK3) and IKCa (SK4) channels was examined in small radial uteroplacental arteries of control and diabetic rats. Both SKCa and IKCa mRNA were detected in both types of vessels with real-time PCR. As shown in Fig. 10, no significant changes were found in the expression of SKCa or IKCa channels in arteries of diabetic pregnant rats. Western blot analysis revealed that maternal diabetes induced no significant changes in SKCa (SK3) and IKCa (SK4) protein levels in radial uteroplacental arteries of pregnant rats (Fig. 10, C–E).
Fig. 10.
Diabetes during rat pregnancy did not change the expression of SKCa and IKCa channels in maternal radial uteroplacental arteries. A and B: relative expression of mRNA for SKCa (SK3; A) and IKCa (SK4; B) channels in arteries of control and diabetic pregnant rats. C: Western blot analysis of SKCa (SK3) and IKCa (SK4) channel expression in arteries of control and diabetic rats. D and E: summary graphs demonstrating the lack of differences in SKCa (D) and IKCa (E) channel protein levels in arteries of control and diabetic pregnant rats. Numbers in parentheses are numbers of rats used for collection of vessels; 8–10 (real-time PCR) or 12–14 (Western blot analysis) uteroplacental radial arteries were collected from each rat.
DISCUSSION
The present study aimed to explore the role of SKCa and IKCa channels in mediating endothelium-dependent vasodilation of the uteroplacental vasculature during normal and diabetic rat pregnancy. The main findings of this study were, first, that pharmacological activation of SKCa/IKCa channels with NS309 or SKCa channels with CyPPA resulted in endothelium-dependent uterine vasodilation that was associated with SMC [Ca2+]i reduction and SMC hyperpolarization. Experimental diabetes during rat pregnancy significantly impaired vasodilation, SMC [Ca2+]i, and hyperpolarization in response to NS309 but not to CyPPA. Second, endothelial Ca2+-activated K+ currents were mediated by IKCa channels and, to a much lesser degree, by SKCa channels with no contribution of BKCa channels. Third, Ca2+- and NS309-induced currents were mostly mediated by IKCa channels and were significantly reduced by diabetes. Fourth, CyPPA-induced K+ currents were not affected by maternal diabetes. Finally, the expression of IKCa and SKCa channels was not significantly different between control and diabetic vessels. These findings support the hypothesis that impaired function of IKCa channels contributes to reduced uteroplacental vasodilation in pregnancy complicated by maternal diabetes.
Both animal and human studies have provided evidence of decreased maternal uterine blood flow in pregnancies complicated by diabetes (10, 16, 43, 50). Endothelial dysfunction is considered a key mechanism of diabetic vascular disease, as evidenced by impaired endothelium-dependent vasodilation in various vascular beds of diabetic animals and humans (12–14, 20). Previously, we demonstrated that EDHF-mediated uterine vasodilation was severely reduced by diabetic pregnancy, indicating that an impairment of the EDHF system importantly contributes to uteroplacental endothelial dysfunction (24).
In the resistance uterine vasculature of rats and humans, NO- and prostacyclin-independent vasodilation is mediated by the activation of SKCa and IKCa channels. This vasodilation can be effectively blocked by combined treatment with apamin and ChTX but not with iberiotoxin (23, 26). In the present study, we demonstrated that direct activation of these channels with NS309 resulted in uterine vasodilation that was nearly maximal at 1 μM NS309 (Fig. 1). Rat uteroplacental arteries were also almost 10 times more sensitive to NS309 than uterine arteries of pregnant sheep (54). In addition, in our study, NS309-induced vasodilation of rat arteries was abolished by endothelial denudation and was therefore mediated by SKCa/IKCa channels located on the vascular endothelium. In contrast, the relaxation in response to NS309 was preserved in denuded uterine vessels of pregnant sheep, indicating the major role of Ca2+-activated channels on vascular SMCs (54). These data suggest that cellular localization and function of SKCa/IKCa channels in the maternal uterine vasculature may be different in species with hemochorial versus epitheliochorial placentation.
NS309 is a potent activator of both SKCa and IKCa channels (27). To assess the relative contribution of SKCa versus IKCa channels in NS309-induced uterine vasodilation, we performed experiments in arteries treated with either apamin or TRAM-34, specific inhibitors of SKCa and IKCa channels, respectively. Apamin did not change but TRAM-34 abolished the vasodilation, clearly implicating the major role of IKCa channels in the NS309-induced response. On the other hand, CyPPA, a specific activator of SKCa channels, produced uterine vasodilation that was insensitive to TRAM-34 but was abolished with apamin (Fig. 3). Activation of SKCa channels with a high concentration (30 μM) of CyPPA resulted in only a partial dilation. This suggests the predominant role of IKCa over SKCa channels in the endothelium-dependent control of the uterine vasculature of late pregnant rats. Our finding support the key role of IKCa channels in bradykinin-induced responses of human myometrial arteries (23).
Our study demonstrated that NS309-induced SMC hyperpolarization, [Ca2+]i reduction, and uterine vasodilation were significantly diminished in diabetic pregnant rats. Multiple mechanisms may be involved in this inhibitory effect of maternal diabetes. Hyperpolarization of ECs is a primary mechanism triggered by SKCa/IKCa channel activation. This hyperpolarization can be electrotonically transferred to neighboring SMCs through myoendothelial gap junctions, resulting in SMC hyperpolarization, a reduction in SMC [Ca2+]i, and relaxation. In addition, K+ efflux from ECs through opened SKCa/IKCa channels can result in K+ accumulation in the extracellular space between ECs and SMCs (8, 15, 27). This localized elevation in K+ can activate the Na+-K+ pump and inward rectifier K+ (Kir) channels on ECs and SMCs, resulting in SMC hyperpolarization. Therefore, impaired function of SKCa/IKCa channels, reduced myoendothelial electrical coupling, or changes in the function of Kir channels and the Na+-K+ pump may be the underlying mechanisms of diabetes-induced inhibition of uterine vasodilation during pregnancy.
In the present study, we focused on understanding the role of SKCa, IKCa, and BKCa channels in generating K+ currents in ECs of control and diabetic uteroplacental arteries. Dialysis of ECs with 3 μM Ca2+ resulted in the generation of outward currents that were not sensitive to paxilline, a potent BKCa channel inhibitor. ChTX markedly (by ∼75%) reduced Ca2+-activated currents in the presence of paxilline, indicating that the currents are mainly mediated by activation of IKCa channels. This conclusion was also confirmed by the strong reduction of Ca2+-activated currents by TRAM-34, a specific IKCa channel inhibitor. On the other hand, apamin reduced outward currents by ∼20%, demonstrating a relatively minor role of SKCa channels in the generation of Ca2+-induced K+ currents (Fig. 6). To the best of our knowledge, this is the first report on the role of three types of Ca2+-activated channels in ECs freshly dissociated from maternal uterine arteries. The absence of any effect of paxilline on currents of ECs is consistent with the lack of functional role of endothelial BKCa channels in late pregnancy. In contrast, paxilline-sensitive BKCa channels appear to be the major contributor to Ca2+-activated outward currents in uterine vascular SMCs (Fig. 6). Our data are in agreement with reported deficiency of BKCa channels in mediating Ca2+-activated currents in ECs freshly dissociated from arteries and arterioles of healthy subjects (17, 29, 31, 46). BKCa channel function can be induced in ECs during some vascular disease states. For example, increased BKCa channel activity has been reported in ECs of gracilis resistance arteries after exposure of rats to hypoxia (31). We found no significant effect of paxilline on Ca2+-activated currents in ECs of diabetic rats. Therefore, it is unlikely that diabetes upregulates endothelial BKCa channel function in rat uterine vasculature. The role of BKCa channels in controlling endothelial function of uterine arteries of healthy and diabetic pregnant women remains to be determined.
Ion currents recorded from ECs of uteroplacental arteries dialyzed with 3 μM Ca2+ were greatly attenuated by ChTX or TRAM-34, indicating that they are mostly mediated by activation of IKCa channels. Similarly, ChTX-sensitive currents activated by NS309 were much greater than apamin-sensitive currents. The significant prevalence of IKCa channels in mediating NS309-induced vasodilation of both control and diabetic ECs was also confirmed by the strong inhibitory effect of TRAM-34 (Fig. 3). Direct stimulation of SKCa channels with CyPPA resulted in significantly smaller currents compared with those in response to NS309. Our data from patch-clamp experiments correlated well with significantly smaller uterine vasodilator responses to CyPPA than to NS309. Therefore, in the maternal uterine vasculature of pregnant rats, IKCa channels play a predominant role in mediating endothelial K+ currents and in controlling the associated vasodilation. A prevalent role of endothelial IKCa over SKCa channels in generating K+ currents was also demonstrated in rat cerebral and human mesenteric arteries (35, 39). Both IKCa and SKCa channels importantly contribute to the generation of Ca2+-activated currents in single ECs of rat parenchymal arteries (29). Collectively, the available data demonstrate significant variations in the role of IKCa and SKCa channels in mediating endothelial K+ currents and in the control of vascular tone in different microcirculatory beds.
The ChTX-sensitive component of Ca2+-activated currents was significantly attenuated in ECs from diabetic uterine vessels. On the other hand, no difference in apamin-sensitive currents in ECs of control and diabetic rats was detected. The ChTX- but not apamin-sensitive component of NS309-induced currents was significantly attenuated by diabetic pregnancy. Direct activation of SKCa channels with CyPPA resulted in currents that were not affected by diabetes. These data correlate well with the attenuated responses to NS309 but not to CyPPA in pressurized uterine vessels from diabetic rats. Our findings indicate that in the maternal uterine vasculature, diabetes impairs the function of endothelial IKCa but not SKCa channels.
There are very limited and contradictory data on the function of SKCa and IKCa channels in the vascular endothelium of diabetic animals or humans. The SKCa channel-mediated component of the EDHF response was impaired in mesenteric arteries of Zucker type 2 diabetic fatty rats without a reduction in channel gene expression (7). No significant changes in outward K+ currents were reported in ECs of male coronary arteries from type 1 diabetic rats. Impairment of EDHF-mediated responses in these vessels was associated with reduced expression of connexin40 composing myoendothelial gap junctions in the vessel wall (38). These data suggest that in the coronary microcirculation, long-term (10–12 wk) experimental diabetes attenuates electrotonic transfer of hyperpolarization from ECs to SMCs via downregulation of connexin40. In contrast, levels of mRNAs for connexin40 and connexin37 were significantly greater in mesenteric arteries of STZ-injected diabetic mice than those of control mice (40). The expression and function of connexins were not evaluated in our study. However, we did not find any effect of diabetes on SKCa channel-mediated apamin-sensitive currents induced by cell dialysis with 3 μM Ca2+ or in response to NS309. Neither CyPPA-induced currents nor vasodilator responses were different between control and diabetic vessels, indicating that the transfer of SKCa channel-mediated endothelial hyperpolarization to SMCs was not affected. The short duration of diabetes, animal sex, or pregnancy condition may explain the lack of diabetes-induced changes in myoendothelial electrical coupling in uterine versus coronary arteries.
Although IKCa function was reduced in ECs of diabetic rats, we did not detect any significant changes in the levels of IKCa or SKCa mRNA in uterine vessels. Protein expression for SKCa and IKCa channels was also not different in arteries of control and diabetic rats. In mesenteric arteries of diabetic rats, reduced SKCa channel-mediated EDHF-dependent vasodilation was associated with no change or an increase in SKCa mRNA levels (7, 40). Recently, it has been demonstrated that SKCa and IKCa channels can recycle between the cytosol and cell membrane. The dynamic of the recycling defines the number of these channels present in the plasma membrane (21). Therefore, any physiological or pathological changes in recycling of these channels may affect their function. It is possible that diabetes modulates cellular trafficking of endothelial IKCa channels and reduces their membrane expression and, in this way, impairs IKCa channel function in uterine arteries. This suggestion will require further experimental validation.
The exact casual factors responsible for the reduced IKCa function in our model of diabetic pregnancy remain unknown. Hyperglycemia is considered a major factor of diabetic vascular damage mediated by the generation of ROS, increased PKC activity, or formation of peroxynitrite (22). It remains to be defined which of these factors affects the function of IKCa channels and vasodilation in the maternal uteroplacental circulation during diabetic pregnancy.
In conclusion, using multiple experimental approaches, we demonstrated that IKCa channels play a predominant role in the control of uteroplacental vasodilatation. Impaired function of IKCa channels is an essential mechanism contributing to diabetes-induced endothelial dysfunction in the maternal uteroplacental circulation. These data suggest that therapeutic treatments restoring the function of IKCa channels may be an important new strategy in improvement of endothelial function and maternal uteroplacental blood flow in pregnancies complicated with diabetes.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-088245 (to N. I. Gokina) and P01-HL-095488 (to A. D. Bonev).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.I.G., A.D.B., J.P., A.P.G., and K.O. conception and design of research; N.I.G., J.P., A.P.G., K.V., K.O., and G.G. performed experiments; N.I.G., A.D.B., J.P., A.P.G., K.V., K.O., and G.G. analyzed data; N.I.G., A.D.B., J.P., A.P.G., K.O., and G.G. interpreted results of experiments; N.I.G., A.P.G., and K.V. prepared figures; N.I.G. and A.P.G. drafted manuscript; N.I.G., A.D.B., J.P., A.P.G., K.V., K.O., and G.G. edited and revised manuscript; N.I.G., A.D.B., J.P., A.P.G., K.V., K.O., and G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Chris Williams for excellent technical assistance and Lia Venner for help with editing of the manuscript.
REFERENCES
- 1.Anastasiou E, Lekakis JP, Alevizaki M, Papamichael CM, Megas J, Souvatzoglou A, Stamatelopoulos SF. Impaired endothelium-dependent vasodilatation in women with previous gestational diabetes. Diabetes Care 21: 2111–2115, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Ang C, Lumsden MA. Diabetes and the maternal resistance vasculature. Clin Sci (Lond) 101: 719–729, 2001. [PubMed] [Google Scholar]
- 3.Barden A, Singh R, Walters BN, Ritchie J, Roberman B, Beilin LJ. Factors predisposing to pre-eclampsia in women with gestational diabetes. J Hypertens 22: 2371–2378, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med 21: 103–113, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 158: 1148–1153, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 115: 485–491, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnham MP, Johnson IT, Weston AH. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. Br J Pharmacol 148: 434–441, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction 140: 365–371, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chartrel NC, Clabaut MT, Boismare FA, Schrub JC. Uteroplacental hemodynamic disturbances in establishment of fetal growth retardation in streptozocin-induced diabetic rats. Diabetes 39: 743–746, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Chirayath HH, Wareing M, Taggart MJ, Baker PN. Endothelial dysfunction in myometrial arteries of women with gestational diabetes. Diabetes Res Clin Pract 89: 134–140, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Cohen RA. ATVB in focus: diabetic vascular disease: pathophysiological mechanisms in the diabetic milieu and therapeutic implications. Arterioscler Thromb Vasc Biol 24: 1340–1341, 2004. [DOI] [PubMed] [Google Scholar]
- 13.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding H, Triggle CR. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflügers Arch 459: 977–994, 2010. [DOI] [PubMed] [Google Scholar]
- 15.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 396: 269–272, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson UJ, Jansson L. Diabetes in pregnancy: decreased placental blood flow and disturbed fetal development in the rat. Pediatr Res 18: 735–738, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol 156: 545–562, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture). Am J Physiol Heart Circ Physiol 291: H985–H1002, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol 26: 1215–1225, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald SM, Kemp-Harper BK, Tare M, Parkington HC. Role of endothelium-derived hyperpolarizing factor in endothelial dysfunction during diabetes. Clin Exp Pharmacol Physiol 32: 482–487, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Balut CM, Bailey MA, Patino-Lopez G, Shaw S, Devor DC. Recycling of the Ca2+-activated K+ channel, KCa2.3, is dependent upon RME-1, Rab35/EPI64C, and an N-terminal domain. J Biol Chem 285: 17938–17953, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillham JC, Myers JE, Baker PN, Taggart MJ. Regulation of endothelial-dependent relaxation in human systemic arteries by SKCa and IKCa channels. Reprod Sci 14: 43–50, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Gokina NI, Bonev AD, Gokin AP, Goloman G. Role of impaired endothelial cell Ca2+ signaling in uteroplacental vascular dysfunction during diabetic rat pregnancy. Am J Physiol Heart Circ Physiol 304: H935–H945, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gokina NI, Goecks T. Upregulation of endothelial cell Ca2+ signaling contributes to pregnancy-enhanced vasodilation of rat uteroplacental arteries. Am J Physiol Heart Circ Physiol 290: H2124–H2135, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Gokina NI, Kuzina OY, Vance AM. Augmented EDHF signaling in rat uteroplacental vasculature during late pregnancy. Am J Physiol Heart Circ Physiol 299: H1642–H1652, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grgic I, Kaistha BP, Hoyer J, Kohler R. Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses–relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol 157: 509–526, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 29.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SKCa and IKCa channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab 31: 1175–1186, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hannemann MM, Liddell WG, Shore AC, Clark PM, Tooke JE. Vascular function in women with previous gestational diabetes mellitus. J Vasc Res 39: 311–319, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JM, Riddle MA, Paffett ML, Gonzalez Bosc LV, Walker BR. Novel role of endothelial BKCa channels in altered vasoreactivity following hypoxia. Am J Physiol Heart Circ Physiol 299: H1439–H1450, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King H. Epidemiology of glucose intolerance and gestational diabetes in women of childbearing age. Diabetes Care 21, Suppl 2: B9–B13, 1998. [PubMed] [Google Scholar]
- 33.Knock GA, McCarthy AL, Lowy C, Poston L. Association of gestational diabetes with abnormal maternal vascular endothelial function. Br J Obstet Gynaecol 104: 229–234, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol 508: 199–209, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohler R, Degenhardt C, Kuhn M, Runkel N, Paul M, Hoyer J. Expression and function of endothelial Ca2+-activated K+ channels in human mesenteric artery: a single-cell reverse transcriptase-polymerase chain reaction and electrophysiological study in situ. Circ Res 87: 496–503, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Kohler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int 72: 145–150, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda) 21: 69–78, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol 295: C221–C230, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marrelli SP, Eckmann MS, Hunte MS. Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol 285: H1590–H1599, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto T, Miyamori K, Kobayashi T, Kamata K. Specific impairment of endothelium-derived hyperpolarizing factor-type relaxation in mesenteric arteries from streptozotocin-induced diabetic mice. Vasc Pharmacol 44: 450–460, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Paradisi G, Biaggi A, Ferrazzani S, De Carolis S, Caruso A. Abnormal carbohydrate metabolism during pregnancy: association with endothelial dysfunction. Diabetes Care 25: 560–564, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Pietryga M, Brazert J, Wender-Ozegowska E, Biczysko R, Dubiel M, Gudmundsson S. Abnormal uterine Doppler is related to vasculopathy in pregestational diabetes mellitus. Circulation 112: 2496–2500, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 308: 1592–1594, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Rodie VA, Freeman DJ, Sattar N, Greer IA. Pre-eclampsia and cardiovascular disease: metabolic syndrome of pregnancy? Atherosclerosis 175: 189–202, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Sandow SL, Grayson TH. Limits of isolation and culture: intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 297: H1–H7, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Sheikh AQ, Hurley JR, Huang W, Taghian T, Kogan A, Cho H, Wang Y, Narmoneva DA. Diabetes alters intracellular calcium transients in cardiac endothelial cells. PLos One 7: e36840, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanley JL, Ashton N, Taggart MJ, Davidge ST, Baker PN. Uterine artery function in a mouse model of pregnancy complicated by diabetes. Vasc Pharmacol 50: 8–13, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Stanley JL, Cheung CC, Rueda-Clausen CF, Sankaralingam S, Baker PN, Davidge ST. Effect of gestational diabetes on maternal artery function. Reprod Sci 18: 342–352, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Triggle CR, Hollenberg M, Anderson TJ, Ding H, Jiang Y, Ceroni L, Wiehler WB, Ng ES, Ellis A, Andrews K, McGuire JJ, Pannirselvam M. The endothelium in health and disease–a target for therapeutic intervention. J Smooth Muscle Res 39: 249–267, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol Reprod 82: 66–75, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol 191: 1655–1660, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Zhu R, Hu XQ, Xiao D, Yang S, Wilson SM, Longo LD, Zhang L. Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension 62: 367–374, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]