Exaggerated activation of the sympathetic nervous system and/or the hypothalamic-pituitary-adrenal (HPA) axis increases cardiovascular disease risk. The present study demonstrates that macrophage migration inhibitory factor (MIF) in paraventricular nucleus (PVN) neurons is a novel mechanism mediating attenuation of both the sympathetic and HPA axis responses to acute stress.
Keywords: psychological stress, blood pressure, brain, angiotensin ii, hypertension
Abstract
Macrophage migration inhibitory factor (MIF) counteracts pressor effects of angiotensin II (ANG II) in the paraventricular nucleus of the hypothalamus (PVN) in normotensive rats, but this mechanism is absent in spontaneously hypertensive rats (SHRs) due to a lack of MIF in PVN neurons. Since endogenous ANG II in the PVN modulates stress reactivity, we tested the hypothesis that replacement of MIF in PVN neurons would reduce baseline blood pressure and inhibit stress-induced increases in blood pressure and plasma corticosterone in adult male SHRs. Radiotelemetry transmitters were implanted to measure blood pressure, and then an adeno-associated viral vector expressing either enhanced green fluorescent protein (GFP) or MIF was injected bilaterally into the PVN. Cardiovascular responses to a 15-min water stress (1-cm deep, 25°C) and a 60-min restraint stress were evaluated 3–4 wk later. MIF treatment in the PVN attenuated average restraint-induced increases in blood pressure (37.4 ± 2.0 and 27.6 ± 3.5 mmHg in GFP and MIF groups, respectively, P < 0.05) and corticosterone (42 ± 2 and 36 ± 3 μg/dl in GFP and MIF groups, respectively, P < 0.05). MIF treatment in the PVN also reduced stress-induced elevations in the number of c-Fos-positive cells in the rostral ventrolateral medulla (71 ± 5 in GFP and 47 ± 5 in MIF SHRs, P < 0.01) and corticotropin-releasing factor mRNA expression in the PVN. However, MIF had no significant effects on the cardiovascular responses to water stress in SHRs or to either stress in Sprague-Dawley rats. Therefore, viral vector-mediated restoration of MIF in PVN neurons of SHRs attenuates blood pressure and hypothalamic pituitary adrenal axis responses to stress.
NEW & NOTEWORTHY
Exaggerated activation of the sympathetic nervous system and/or the hypothalamic-pituitary-adrenal (HPA) axis increases cardiovascular disease risk. The present study demonstrates that macrophage migration inhibitory factor (MIF) in paraventricular nucleus (PVN) neurons is a novel mechanism mediating attenuation of both the sympathetic and HPA axis responses to acute stress.
several lines of evidence suggest that the amplitude of blood pressure increases in response to psychological or physical stressors in young and middle-aged normotensive adults elevates the risk of developing hypertension and other cardiovascular diseases such as coronary artery disease, myocardial ischemia, and stroke later in their life (8, 20, 39, 41, 42, 44, 50, 60). Larger variations in blood pressure in response to repeated everyday stressors may lead to accelerated damage of the kidneys and vasculature. Altered central mechanisms that mediate augmented blood pressure responses to stress could also be directly involved in maintaining elevated sympathetic tone and resting blood pressure. Stress also activates the hypothalamic-pituitary-adrenal (HPA) axis, leading to elevated plasma glucocorticoid concentrations (cortisol in humans, corticosterone in rats), and elevated glucocorticoids confer additional risk for hypertension and related cardiovascular disease (49, 61, 62). Despite the important influence stress-induced increases in blood pressure and HPA axis activity have on cardiovascular health, the central mechanisms influencing the magnitude of these responses are incompletely understood.
The paraventricular nucleus of the hypothalamus (PVN) plays a central role in integrating the physiological response to stress, and angiotensin II (ANG II) signaling within the PVN has been shown to contribute to both the activation of the hypothalamic-pituitary-adrenal (HPA) axis and elevations in blood pressure and heart rate during stress (1, 6, 14, 31, 51). Previous work from our laboratory demonstrated that within the brain macrophage migration inhibitory factor (MIF) is an important intracellular counterregulator of ANG II in PVN neurons and that this action is mediated by the intrinsic thiol-protein oxidoreductase (TPOR) activity that is exerted by a C-A-L-C motif at residues 57–60 of MIF (40, 57, 58). Activation of ANG II type 1 receptors (AT1R) increases MIF expression via NADPH oxidase-mediated production of reactive oxygen species (ROS) in PVN neurons (19, 27, 59). MIF in turn scavenges ROS by its TPOR activity, thus providing negative feedback for neuronal actions of ANG II (27). Taken in summary, these previous findings suggest that MIF within the PVN could be an endogenous regulator of the cardiovascular and HPA axis responses to stress.
The spontaneously hypertensive rat (SHR) is a commonly used experimental model for essential hypertension. These animals are characterized by elevated baseline blood pressure and plasma corticosterone concentration and exhibit augmented blood pressure and HPA axis responses to acute stress (16, 43). Interestingly, SHRs lack MIF expression within PVN neurons, and central ANG II injection also fails to elevate MIF levels in the PVN of these rats (37), indicating that the MIF-mediated counterregulation of ANG II signaling may be dysfunctional. Furthermore, restoration of MIF expression in PVN neurons of young, prehypertensive, SHRs attenuates the subsequent development of hypertension (37). Therefore, we tested the hypothesis that restoring MIF expression in PVN neurons of adult SHR with established hypertension, using viral vector-mediated gene transduction, would reduce baseline blood pressure and attenuate cardiovascular and HPA axis responses elicited by acute stressors. To establish a basis for comparison, we initially tested the hypothesis that augmenting MIF expression in the PVN of normotensive adult Sprague-Dawley rats would attenuate the cardiovascular responses to acute stress.
To investigate potential mechanisms mediating actions of PVN MIF expression on blood pressure regulation and HPA axis activity in SHRs, stress-induced neuronal activation (as indicated by immunohistochemical detection of c-Fos) and expression of stress-related genes in the PVN (using RT-qPCR) were also measured. Acute stressors induce c-Fos expression in several brain regions including the PVN and the rostral ventrolateral medulla (RVLM) (23, 34). Since the PVN projects to the RVLM, and both the PVN and RVLM are important sites for regulation of sympathetic nerve activity (26), the effect of PVN MIF transduction on stress-induced c-Fos expression was analyzed in both these regions. In other experiments, the effect of MIF transduction on the expression of mRNA for several stress-related neurotransmitters within the PVN was determined. Specifically, corticotropin-releasing factor (CRF) and arginine vasopressin (AVP) expression was measured since these are key mediators of HPA axis activation and can also contribute to central mechanisms of sympathoexcitation (2, 33, 55). Dopamine-β-hydroxylase (DβH) expression was measured as a marker of catecholaminergic activity, as catecholaminergic neurotransmission within the PVN contributes to the HPA axis response to acute stress (21) and influences blood pressure regulation (64). Brain-derived neurotrophic factor (BDNF) expression was also measured since BDNF within the PVN can stimulate HPA activity, heart rate, and indexes of sympathetic activity, and ANG II can stimulate BDNF expression (10, 18, 30).
All cardiovascular measurements were made in conscious rats using radiotelemetry, and blood samples for plasma corticosterone were obtained from a separate cohort of conscious rats using indwelling arterial catheters. Immunohistochemistry and RT-qPCR were performed on brain tissue obtained from some of these animals and from additional rats as well.
METHODS
Animals
Male Sprague-Dawley rats and SHRs were obtained from Charles River (Wilmington, MA) at 10 wk of age. Animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the University of Florida Institutional Animal Care and Use Committee approved all procedures and protocols before their implementation. Rats were housed individually with a 12:12-h light-dark cycle (0600 to 1800).
Surgical Procedures
All surgeries were performed using aseptic techniques under continuous isoflurane anesthesia (5% induction, 2–3% maintenance) delivered in oxygen. The depth of anesthesia was assured by lack of a reflex response to pinch of the hind paw. All incisions were closed in layers. Carprofen (5 mg·kg−1·day−1 sc), used for postsurgical analgesia, was administered at the beginning of surgery and for 2 days after surgery.
Radiotelemetry transmitters.
Radiotelemetry transmitters (model PA-C40; Data Sciences International, St. Paul, MN) were implanted into the descending aorta under isoflurane anesthesia for the measurement of blood pressure and heart rate as previously described (13). The abdominal aorta was isolated from a midline incision and briefly occluded, and the tip of the catheter was inserted using a 21-gauge needle. Surgical glue (3M Vetbond Tissue Adhesive) and a nitrocellulose patch were applied to secure the catheter in place. The transducer was sutured to the abdominal muscle.
Viral vectors and gene transfer into the PVN.
Adeno-associated viral vectors (AAV2) to elicit the expression of enhanced green fluorescent protein (GFP) or MIF were constructed as described previously (37). The expression of GFP and MIF was driven by a chicken-β-actin (CBA) promoter with human cytomegalovirus enhancer, and a woodchuck posttranscriptional regulatory element enhancing the expression of transgenes was present downstream of GFP and MIF. In vivo, these vectors elicit expression of MIF and GFP primarily within neurons, and not in astrocytes, as we have demonstrated previously (37, 63). AAV2-CBA-GFP or AAV2-CBA-MIF was injected bilaterally into the PVN under isoflurane anesthesia using a stereotaxic frame and pipettes pulled from thin walled borosilicate glass capillary tubes (OD: 1 mm; ID: 0.58 mm) with a tip diameter of ∼25 μm using the following stereotactic coordinates: 1.8 mm posterior from bregma, 0.3–0.35 mm lateral from midline, and 7.8 mm ventral from the dura mater. Virus stocks (109 viral particles/ml; 200 nl/side) were injected over 5 min using a pneumatic pico pump (World Precision Instruments, Sarasota, FL). The pipette was left in place for an additional 3 min before being withdrawn. PVN injections of AAV2-CBA-GFP and AAV2-CBA-MIF resulted in marked expression of GFP and MIF in the PVN neurons as reported previously (12, 37).
Arterial catheters.
Teflon-tipped arterial catheters were used to obtain blood samples to measure plasma corticosterone. The catheters were implanted into the femoral artery and the tip advanced to the descending aorta below the renal arteries as previously described (3). Catheters were flushed daily.
Experimental Procedures and Protocols
Stress procedures.
To perform the water stress, rats were placed in standard rat cages filled with 1 cm deep water (room temperature, ∼25°C) for 15 min. After 15 min they were removed from the water, their feet were patted dry, and they were returned to their home cage. Restraint stress was performed as previously described (13) by placing animals in vented cylindrical plastic restrainers within their home cage. The restrainer minimized their movement, but they could easily breath. At the end of 60 min of restraint, the animals were released into their home cage.
Blood sampling.
On the day of an experiment, the catheter was attached to a piece of sterile extension tubing so that samples could be obtained without touching the animal, as previously described (13). The rats remained in their home cages and at least 2 h elapsed from the time the extension tubing was attached to the withdrawal of the first blood sample. Each 200-μl sample was obtained using sterile technique after removal of the dead space volume. The dead space volume was then returned to the animal, and the catheter was flushed with heparinized saline. The blood sample was added to a tube containing 5 μl of heparin and placed on ice until being centrifuged at 4°C. The plasma was then removed and stored at −80°C until being assayed for plasma corticosterone concentration.
Experimental Design
Protocols 1 and 2.
Protocol 1 tested the hypothesis that augmenting MIF expression in the PVN of normotensive rats would attenuate the cardiovascular responses to acute stress. Protocol 2 tested the hypothesis that replacing MIF expression in the PVN of SHRs would attenuate their cardiovascular responses to acute stress. Sprague-Dawley rats (protocol 1) and SHRs (protocol 2) received radiotelemetry transducer implantation at 11 wk of age. After a 2-wk postoperative recovery period, baseline blood pressure and heart rate were recorded every 10 min for 15 s for a week and then rats received bilateral vector injections into the PVN. Three weeks after the vector injections, at which time MIF and GFP have reached peak levels of expression in the PVN (37), rats were subject to an acute water stress procedure followed by an acute restraint stress procedure a week later as described below. Stress procedures were performed between 8 AM and 12 PM and were started after continuous (500 Hz) baseline blood pressure and heart rate recordings were obtained for a minimum of 30 min; in most animals 60 min of baseline data were obtained. Animals were then subjected to 60 min of restraint stress or 15 min of water stress. After animals were returned to their home cages, blood pressure and heart rate were recorded for an additional 30-min poststress recovery period. Animals were euthanized a week after restraint stress (except for 4 animals euthanized 60 min after stress) by transcardiac perfusion under deep isoflurane (5%) anesthesia. The site of injection was visualized in each animal using immunohistochemistry (see details below). For experiment 1, 12 rats were injected with AAV2-CBA-GFP and 13 were injected with AAV2-CBA-MIF. Of these, five GFP-injected and eight MIF-injected rats displayed bilateral vector expression in the PVN. One GFP-injected rat had unilateral expression and was also included in the study. The cardiovascular data obtained from this animal with unilateral expression were not different from data obtained from rats with bilateral GFP expression. For experiment 2, 17 rats were injected with AAV2-CBA-GFP and 19 were injected with AAV2-CBA-MIF; 1 MIF-injected rat died following surgery. Of the remaining rats, eight GFP-injected and seven MIF-injected rats displayed bilateral vector expression in the PVN. One GFP-injected rat had unilateral expression and was also included in the study since the cardiovascular data were not different from animals with bilateral injections. Blood pressure could not be recorded during restraint stress in one SHR rat from the GFP-injected cohort due to failure of the telemetry transmitter.
Protocol 3.
This protocol tested the hypothesis that restoring MIF expression in PVN neurons of SHRs would attenuate the expression of c-Fos in the PVN and RVLM. To test this hypothesis, 20 SHRs were injected with AAV2-CBA-GFP or AAV2-CBA-MIF at 14 wk of age. Three weeks after the microinjection procedure, animals were subjected to a 60-min restraint stress. After an additional 60 min following the end of the restraint stress, rats were then euthanized using transcardiac perfusion under deep isoflurane (5%) anesthesia. Eight rats were injected with AAV2-CBA-GFP, out of which seven had bilateral GFP expression, while eight rats were injected with AAV2-CBA-MIF, out of which seven had bilateral MIF expression. In addition, the two GFP-injected and two MIF-injected SHRs from experiment 2 that were killed 1 h after the end of restraint stress were included in the c-Fos expression analysis.
Protocol 4.
This protocol tested the hypothesis that restoring MIF expression in PVN neurons of SHRs would attenuate the corticosterone response to acute stress. Thirty-seven male SHRs were injected with AAV2-CBA-GFP (n = 16) or AAV2-CBA-MIF (n = 21) at 14 wk of age. Indwelling arterial catheters were implanted ∼2.5 wk later. After a 3-day recovery period rats were subjected to the water stress and then 1 wk after that they were subjected to the restraint stress or used in time control experiments. A baseline blood sample was obtained 10–15 min before the initiation of the stress, and then additional samples were taken at 5, 10, 15, 25, 45, and 60 min after the start of the 15-min water stress and at 5, 10, 15, 25, 45, and 60 min after the start of the 60-min restraint stress. The samples were immediately placed on ice and then centrifuged at 4°C. The plasma was pipetted from the sample tube and then placed in a clean tube and stored at −80°C until being assayed for plasma corticosterone concentration. In some animals the catheter malfunctioned during the restraint and/or water stress; data are only included from experiments in which all blood samples could be obtained. At the end of the restraint stress, the rats were deeply anesthetized by placing them in a chamber with 5% isoflurane in oxygen and then they were rapidly decapitated. The brains were removed, blocked at the rostral margin of the cerebellum, rapidly frozen, and stored at −80°C until subsequent processing for the measurement of mRNA expression.
Analysis of Radiotelemetry Data
Baseline blood pressure and heart rate data were analyzed using Dataquest A.R.T. analysis software (Data Sciences International). Data were recorded for 15 s every 10 min; data collected during week 3 after vector injections between 8 AM and 4 PM were averaged to calculate daytime values, whereas data collected between 8 PM and 4 AM were averaged to calculate nighttime values for each animal.
Spontaneous baroreflex sensitivity, heart rate variability (HRV), and blood pressure variability (BPV) data analyses was performed using the freely available HemoLab software (http://www.haraldstauss.com/HemoLab/HemoLab.html). Blood pressure was recorded continuously at a 500-Hz sampling rate for 3 h between 9 AM and 12 PM 18–21 days after vector injections before the animals were subjected to acute stress. The sampling rate of the datasets was increased to 1,500 Hz using spline interpolation. The gain of the baroreceptor reflex was determined using the sequence technique. Sequences were defined as a minimum of three consecutive (beat-by-beat) increases or decreases in systolic blood pressure accompanied by likewise increases or decreases in pulse interval. Sequences with increases and decreases in systolic blood pressure were pooled. No time delay between systolic blood pressure and pulse interval and no thresholds for changes in systolic blood pressure or pulse interval were used. Only sequences with a correlation coefficient (R) for the linear correlation between systolic blood pressure and pulse interval of >0.8 were included in the analysis, and the slope of the linear correlation was taken as the gain of baroreceptor reflex (5). For HRV and BPV analysis, blood pressure datasets were visually inspected, and three 5- to 10-min-long artifact-free segments per animal were extracted. Beat-by-beat pulse interval and systolic blood pressure time series were spline interpolated to an equidistant sampling rate of 15-Hz from the 1,500-Hz datasets. Power spectra were computed by the fast Fourier transform using the full length of each time series with 50% overlapping segments of 2,048 data points. Low-frequency (HRV-LF, 0.2–0.6 Hz) and high-frequency (HRV-HF, 1.0–3.0 Hz) spectral powers of pulse intervals and low-frequency (0.2–0.6 Hz) spectral power of systolic blood pressure (BPV-LF) were calculated as the area under the curve of the respective power spectra, and HRV-HF was used as an index for cardiac parasympathetic tone, HRV-LF/HRV-HF ratio was used as an index for cardiac sympathetic tone, and BPV-LF was used as an index of vascular sympathetic tone (5). Parameters obtained from the three datasets were averaged for each animal.
Blood pressure and heart rate data were exported using Dataquest A.R.T. analysis software as 1-min moving averages calculated from continuously recorded blood pressure data. Baseline values were calculated by averaging the baseline period after removing physical activity-related peaks from blood pressure and heart rate datasets. The data were then averaged in 5 min bins, and changes from prestress baseline values were calculated. The average changes in blood pressure and heart rate during the stress and recovery periods were also calculated.
Immunohistochemistry and Analysis of Stress-Induced c-Fos Expression
Animals were deeply anesthetized with 5% isoflurane and then perfused through the ascending aorta with 400 ml cold phosphate-buffered saline followed by 400 ml cold 4% paraformaldehyde in phosphate-buffered saline. Brains were postfixed for 2 h in 4% paraformaldehyde and then equilibrated in 30% sucrose solution at 4°C. Coronal sections (40 μm) were cut on a microtome (Microm HM 450; Thermo Scientific) and mounted on Fischer Superfrost Plus slides. The following primary antibodies were used: rabbit anti-MIF (1:500; Torrey Pines Biolabs, East Orange, NJ; at this dilution the MIF antibody does not detect endogenous MIF but will detect the MIF immunoreactivity produced by AAV2-CBA-MIF; Ref. 22), rabbit anti-c-Fos (1:1,000; EMD Millipore, Temecula, CA), mouse anti-DβH (1:1,000; EMD Millipore), guinea pig anti-(Arg8)-vasopressin (1:250; Bachem, Torrance, CA). For c-Fos detection, floating brain sections were incubated 48 h at 4°C, and for all other primary antibodies, mounted brain sections were incubated overnight at 4°C. The secondary antibodies were anti-rabbit AF488, anti-mouse AF488, and anti-rabbit AF546 (Invitrogen, Carlsbad, CA) and anti-guinea pig orange-Cy3 (Jackson Immunoresearch, West Grove, PA). All secondary antibodies were used at a 1:200 dilution with a 2-h incubation at room temperature. Immunofluorescence and GFP were detected and imaged using a fluorescent microscope (Olympus BX41 with Olympus DP71 digital camera).
The number of c-Fos-positive neurons was determined in the PVN at three rostral-caudal levels, ∼1.60 mm (level 1), 1.88 mm (level 2), and 2.12 mm (level 3) posterior from bregma (48) as described by Stocker et al. (56). Identification of each level was aided by colabeling with an anti-arginine vasopressin (AVP) antibody. c-Fos-positive nuclei were counted in the following subnuclei of the PVN: dorsal parvocellular (levels 1 and 2), medial parvocellular (levels 1–3), ventrolateral parvocellular (level 2), lateral parvocellular (level 3), and posterior magnocellular (level 1–2). The RVLM was identified in brain sections 12.1–12.5 mm posterior from bregma (48) with the aid of colabeling with an anti-DβH antibody.
Image files of the PVN and RVLM were deidentified by assigning a random number file name before determining the number of c-Fos-positive neurons using the “Cell Counter” plugin for the ImageJ software (http://imagej.nih.gov/ij). The random identification numbers were broken after histological examination.
Plasma Corticosterone Assay
Plasma corticosterone concentration was measured in duplicate using double antibody I125 radioimmunoassay kits purchased from MP Biomedicals (Solon, OH) as previously described (13, 52).
RT-qPCR
RT-qPCR was used to analyze mRNA expression for CRF, AVP, BDNF, and DβH within the PVN. Forebrain sections (1-mm thick) were obtained using a brain matrix (Harvard Apparatus), and then bilateral 1.5-mm punches of PVN tissue were removed and processed for mRNA expression. RNAeasy kits (Qiagen) were used to isolate total RNA. DNAse I treatment was then used to remove genomic DNA. RT-qPCR was performed using OneStep RT-PCR kits (Qiagen). Specific oligonucleotide primers and Taqman probes were purchased from Applied Biosystems. Data were normalized to the mRNA expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are graphed as 2−ΔΔCT values, while statistical analyses were performed on the cycle threshold (ΔCT) values. PVN tissue was not obtained for mRNA analysis from one GFP time control animal.
Statistical Analysis
The average changes in blood pressure and heart rate during the stress and recovery periods were calculated and analyzed by two-way analysis of variance (ANOVA; time and treatment effects). Other comparisons for cardiovascular data were made using unpaired t-tests. Overall average baseline plasma corticosterone concentration for all rats used in the blood sampling protocol was determined by first determining an average value for each rat, then finding the overall average for GFP and MIF rats. Experiments (n = 3) in which baseline corticosterone exceeded 20 μg/dl were excluded, since such a high baseline level indicates the animals were stressed. Plasma corticosterone responses to stress were analyzed as absolute values and as average changes from baseline by one- and two-way ANOVA. For RT-qPCR, ΔCT values were analyzed using a 2 × 2 between subjects factorial ANOVA (main effects of GFP vs. MIF treatment and time control vs. stress). When ANOVA detected significant between-factor effects, additional analyses were performed. One MIF time control value for CRF mRNA was removed as an outlier using the interquartile range rule. Results are expressed as means ± SE, and a value of P ≤ 0.05 was considered significant.
RESULTS
Viral-Mediated Expression of GFP and MIF in the PVN
The representative fluorescence micrographs presented in Fig. 1 show GFP (top) and MIF (bottom) in the PVN of SHR at ∼1.8 mm posterior from bregma, 4 wk after microinjection of AAV2-CBA-GFP or AAV2-CBA-MIF. These fluorescence micrographs indicate that the vast majority of expressed GFP and MIF is contained within the confines of the PVN.
Fig. 1.
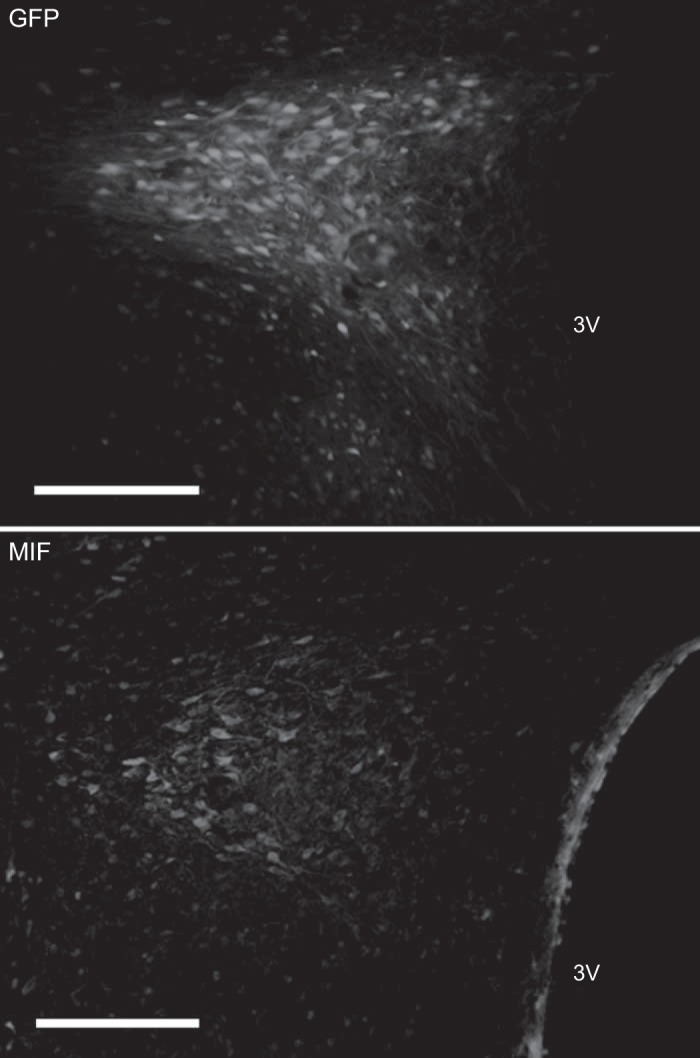
Viral-mediated transduction of green fluorescent protein (GFP) and migration inhibitory factor (MIF) into the paraventricular nucleus of the hypothalamus (PVN). Representative low-power fluorescence micrographs taken from coronal sections of the forebrain in spontaneously hypertensive rats (SHRs) that received microinjections of either AAV2-CBA-GFP or AAV2-CBA-MIF into the PVN as described in methods. Top: GFP. Bottom: MIF. Bar = 200 μm. Note that the MIF antibody dilution used here (1:500) does not detect endogenous MIF, and so the MIF staining shown is largely from virally transduced MIF (22).
Protocol 1 (Sprague-Dawley Rats)
Baseline parameters.
Enhanced MIF expression in the PVN of Sprague-Dawley rats had no effect on baseline mean arterial pressure (MAP) either during daytime (GFP: 94.9 ± 1.8 mmHg; MIF: 95.3 ± 1.9) or nighttime (GFP: 98.0 ± 2.2 mmHg; MIF: 100.3 ± 2.1). Heart rate was also similar between the two groups both during daytime (GFP: 325 ± 7 beats/min; MIF: 320 ± 5 beats/min) and nighttime (GFP: 369 ± 6 beats/min; MIF: 369 ± 5). However, spontaneous baroreflex sensitivity decreased in the MIF group compared with the GFP rats (GFP: 2.51 ± 0.018 mmHg; MIF: 2.00 ± 0.09, P < 0.05). HRV analysis showed no significant change in cardiac parasympathetic tone as indicated by HRV-HF power (GFP: 15.0 ± 2.3 ms2 vs. MIF: 12.1 ± 2.9 ms2; P = 0.50). The ratio of HRV LF and HF power, an indicator of cardiac sympathetic tone, tended to be higher in the MIF group (0.70 ± 0.08) compared with GFP (0.44 ± 0.08), but the difference was not statistically significant (P = 0.07). Increased MIF expression in the PVN also failed to affect BPV LF power, an index of vascular sympathetic tone (GFP: 1.11 ± 0.22 mmHg2 vs. MIF: 1.32 ± 0.11 mmHg2; P = 0.36).
Acute stress.
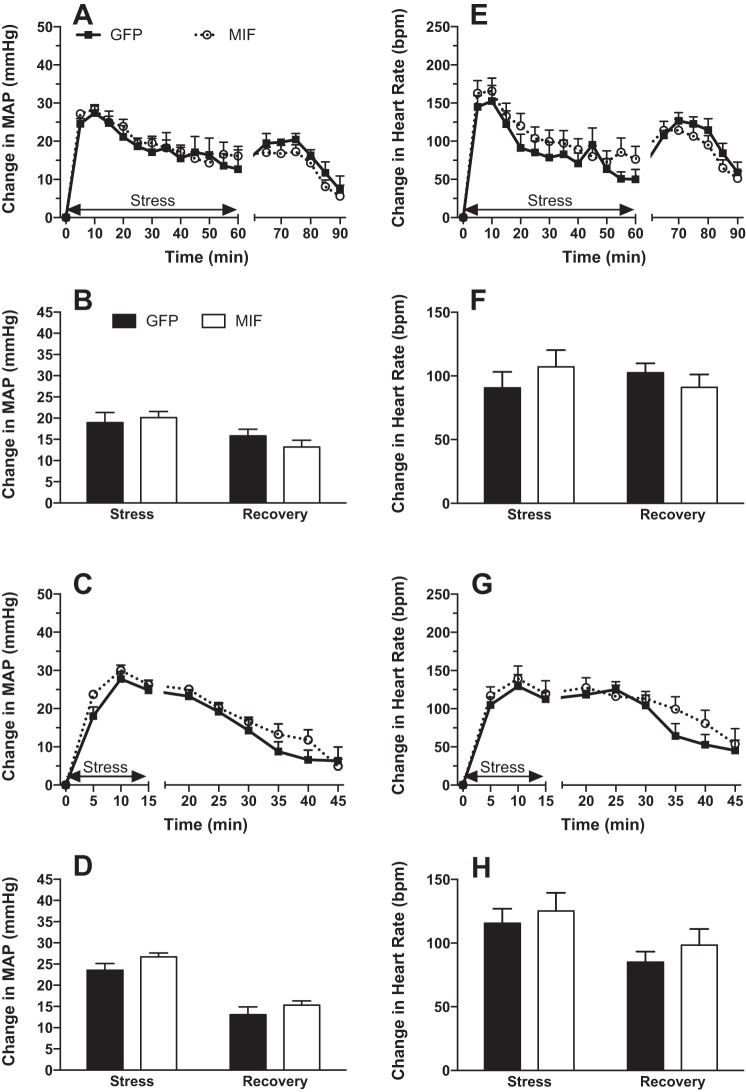
Increases in MAP (Fig. 2, A–D) and heart rate (Fig. 2, E–H) induced by restraint stress (Fig. 2, A, B, E, and F) or water stress (Fig. 2, C, D, G, and H) were unaffected by MIF treatment in Sprague-Dawley animals both during stress and during the poststress recovery phase.
Fig. 2.
Mean arterial pressure (MAP; A–D) and heart rate (HR; E–H) responses to 60 min of restraint stress (A, B, E, and F) and 15 min of water stress (C, D, G, and H) in GFP (solid squares or bars)- and MIF-treated (open circles or bars) Sprague-Dawley rats. Stress begins at time = 0. Bar graphs represent values averaged for the durations of stress and recovery. There were no significant effects of MIF on the stress responses in these rats. MIF treatment also had no significant effect on baseline values for MAP and HR, and data are expressed as changes from baseline values. Baseline MAP and HR values recorded before restraint stress were 95 ± 4 mmHg and 314 ± 7 beats/min in GFP (n = 6) and 100 ± 2 mmHg and 309 ± 6 beats/min in MIF rats (n = 8). Baseline MAP and HR values recorded before water stress were 95 ± 2 mmHg and 305 ± 8 beats/min in GFP (n = 6) and 99 ± 2 mmHg and 299 ± 7 beats/min in MIF rats (n = 8).
Protocol 2 (SHRs)
Baseline parameters.
Daytime baseline MAP (Fig. 3A), heart rate (Fig. 3B) and spontaneous baroreflex sensitivity (Fig. 3C) were unaffected by increased MIF expression in the PVN of adult SHRs. Nighttime MAP (157 ± 1 and 153 ± 2 mmHg in GFP and MIF rats, respectively) and heart rate (335 ± 7 and 337 ± 7 beats/min in GFP and MIF rats, respectively) were also unaffected by MIF treatment. HRV analysis revealed no significant change in cardiac sympathetic tone indicated by the ratio of HRV-LF to -HF power (GFP: 0.33 ± 0.04 vs. MIF: 0.46 ± 0.1; P = 0.23, Fig. 3D). There were also no significant effects of MIF on cardiac parasympathetic tone as indicated by HRV-HF power (GFP: 11.7 ± 5.7 ms2 vs. MIF: 8.8 ± 3.0 ms2; P = 0.69) or on LF power of BPV (GFP: 1.55 ± 0.24 mmHg2 vs. MIF: 1.68 ± 0.36 mmHg2; P = 0.75).
Fig. 3.
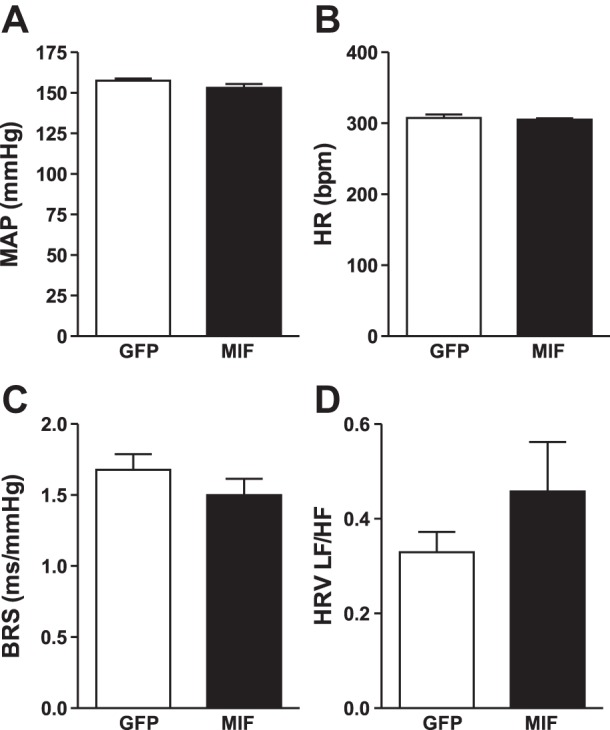
Daytime MAP (A), HR (B), spontaneous baroreflex sensitivity (BRS; C) and heart rate variability-low frequency/high frequency (HRV-LF/HF) ratio (D) in GFP- (n = 9 for MAP and HR; n = 5 BRS and HRV analysis) and MIF-treated SHRs (n = 7) averaged for the 3rd wk after PVN injections. There were no significant effects of MIF treatment on these variables.
Acute stress.
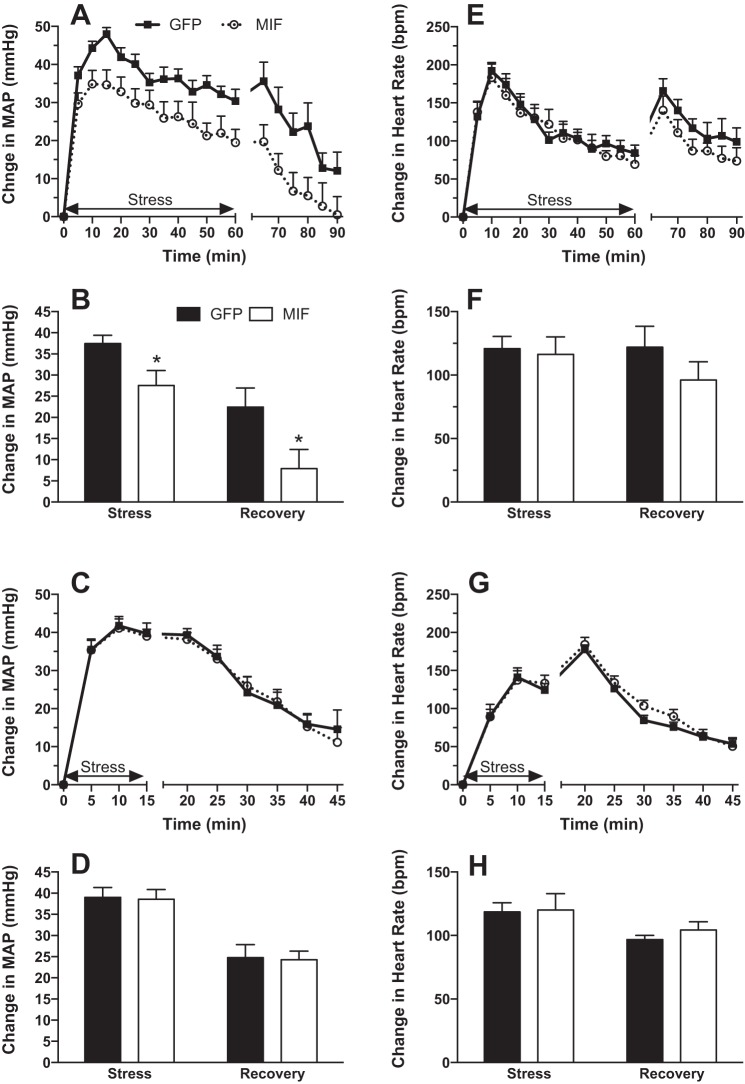
Restraint stress-induced increases in MAP were significantly attenuated by elevated MIF expression in the PVN of SHRs (Fig. 4, A and B). Average increases in MAP for the 60 min of restraint were 37.4 ± 2.0 mmHg in GFP and 27.6 ± 3.5 mmHg in MIF animals (P < 0.05), while the average increases in MAP during the 30-min recovery were 22.4 ± 4.5 mmHg in the GFP and 7.9 ± 4.5 mmHg in the MIF group (P < 0.05). On the other hand, increases in heart rate were not affected by MIF treatment either during stress or recovery (Fig. 4, E and F).
Fig. 4.
MAP (A–D) and HR (E–H) responses to 60 min of restraint stress (A, B, E, and F) and 15 min of water stress (C, D, G, and H) in GFP (solid squares or bars)- and MIF-treated (open circles or bars) SHRs. Stress begins at time = 0. Data are expressed as changes from baseline values, and bar graphs represent values averaged for the durations of stress and recovery. MIF treatment significantly attenuated the arterial pressure response to restraint stress during both the stress and recovery periods but had no significant effect on baseline values for MAP and HR. Baseline MAP and HR values recorded before restraint stress were 95 ± 4 mmHg and 314 ± 7 beats/min in GFP (n = 8) and 100 ± 2 mmHg and 309 ± 6 beats/min in MIF rats (n = 7). Baseline MAP and HR values recorded before water stress were 95 ± 2 mmHg and 305 ± 8 beats/min in GFP (n = 9) and 99 ± 2 mmHg and 299 ± 7 beats/min in MIF rats (n = 8). *P < 0.05 MIF vs. GFP.
In contrast to restraint stress, water stress-induced MAP (Fig. 4, C and D) and heart rate (Fig. 4, G and H) elevations were unaffected by MIF treatment.
Protocol 3 (SHRs)
Since MIF treatment significantly attenuated the acute restraint stress-induced increase in MAP in SHRs, we set out to determine whether this reduction in the pressor response is associated with attenuated neuronal activation in the PVN and RVLM as indicated by c-Fos expression. We analyzed c-Fos expression in SHRs at 120 min following the initiation of the 60-min restraint stress (allowing for 60 min of recovery time) in three rostral-caudal levels of the PVN (∼1.60, 1.88, and 2.12 mm posterior from the bregma) and in the RVLM in brain sections 12.1–12.5 mm posterior from bregma (48) identified with the aid of AVP and DβH staining, respectively.
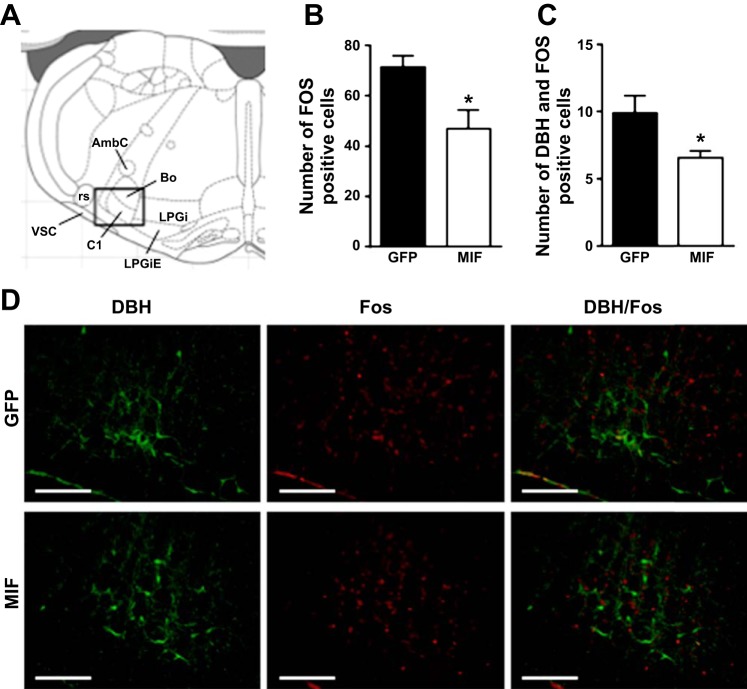
We found that increased MIF levels in the PVN did not significantly affect stress-induced c-Fos expression in any subnuclei of the PVN. The number of c-Fos-positive cells in GFP and MIF rats were 12 ± 1 and 15 ± 2 in the dorsal parvocellular subnucleus; 59 ± 6 and 62 ± 6 in the medial parvocellular subnucleus; 3 ± 1 and 4 ± 1 in the ventrolateral parvocellular subnucleus; 26 ± 4 and 34 ± 4 in the lateral parvocellular subnucleus, and 12 ± 3 and 15 ± 3 in the posterior magnocellular subnucleus. In contrast, the number of c-Fos-positive cells in the RVLM was significantly reduced in MIF-treated SHRs (47 ± 7) compared with GFP-treated SHRs (71 ± 5; P < 0.05; Fig. 5B). In addition, the number of cells double labeled with anti-c-Fos and anti-DβH antibodies were also significantly reduced in the MIF group (6.6 ± 0.5) compared with the GFP group (9.9 ± 1.3; P < 0.05; Fig. 5C).
Fig. 5.
A: diagram of a coronal section of the brainstem 12.24 mm posterior from bregma taken from Paxinos and Watson (48). Rectangle indicates the location of images taken to analyze c-Fos expression. Numbers of c-Fos-positive (B) and DβH/c-Fos double labeled (C) cells in the RVLM of GFP (n = 9)- and MIF-treated (n = 9) SHRs. Rats were euthanized and the tissue collected 120 min after the onset of the 60-min restraint stress. Representative fluorescence photomicrographs (D) showing c-Fos (red) and dopamine-β-hydroxylase (DβH; green) expression in the RVLM of GFP- and MIF-treated SHRs in coronal brain sections at 12.2–12.4 mm posterior from bregma. Scale bars = 200 μm. AmbC, ambiguous nucleus, compact part; Bo, Botzinger complex; C1, C1 adrenalin cells; LPGi, lateral paragigantocellular nucleus; LPGiE, lateral paragigantocellular nucleus, external part; rs, rubrospinal tract; vsc, ventral spinocerebellar tract. Results are represented as means ± SE; *P < 0.05 MIF vs. GFP.
Protocol 4 (SHRs)
Plasma corticosterone.
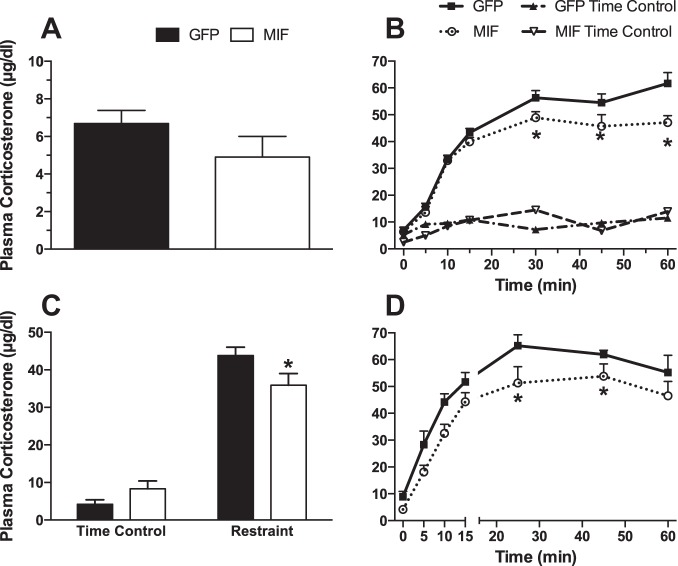
Increased expression of MIF in PVN neurons in SHRs did not significantly reduce baseline plasma corticosterone (Fig. 6A). During 60 min of restraint stress, plasma corticosterone was significantly lower in MIF-treated compared with GFP-treated rats at 30, 45, and 60 min of stress (Fig. 6B). There were no significant changes in plasma corticosterone in the corresponding time control groups. The average change in plasma corticosterone from baseline in response to restraint stress was also significantly reduced by MIF (Fig. 6C). MIF treatment had no significant effect on plasma corticosterone during the 15 min of water stress but did reduce plasma corticosterone during the subsequent recovery period (Fig. 6D). The average change in plasma corticosterone from baseline in response to water stress was not significantly reduced by MIF (6.4 ± 0.7 vs. 5.5 ± 0.4 μg/dl for GFP vs. MIF during stress and 3.4 ± 0.2 vs. 3.1 ± 0.3 μg/dl for GFP vs. MIF during the recovery period).
Fig. 6.
A: average baseline plasma corticosterone concentration in all GFP (n = 15)- and MIF (n = 16)-treated rats. B: plasma corticosterone concentration at baseline (time 0) and after 10, 15, 30, 45 and 60 min of restraint stress (n = 9 per group) or in time control experiments (n = 5–6 per group). C: average change from baseline in plasma corticosterone in response to restraint stress and during time control experiments calculated from data in B. D: plasma corticosterone concentration at baseline (time 0) and after 10, 15, 30, 45, and 60 min of water stress (n = 7 for GFP and 9 for MIF treatment.). *P < 0.05 MIF vs. GFP.
RT-qPCR.
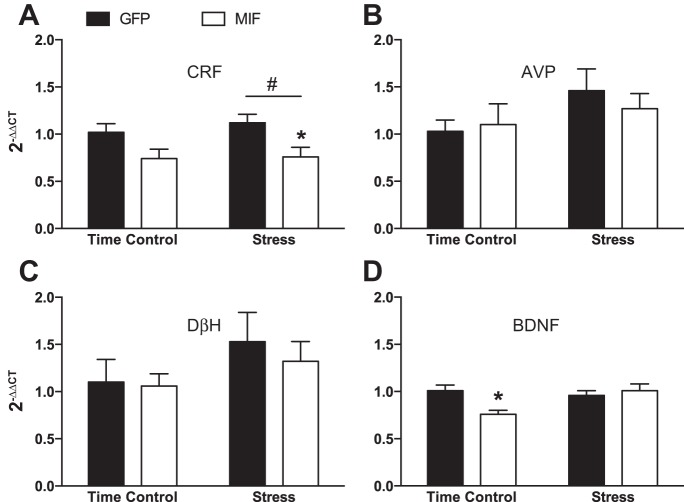
Stress significantly increased expression of CRF mRNA in the PVN, and restoration of MIF in PVN neurons of SHR attenuated the enhanced expression (Fig. 7A). There were no significant main effects of stress on mRNA expression for AVP (Fig. 7B), DβH (Fig. 7C), or BDNF (Fig. 7D). MIF significantly reduced BDNF mRNA expression in time control animals, but had no other significant effects on AVP or DβH mRNA expression.
Fig. 7.
Effects of restraint stress and MIF treatment on PVN mRNA expression for corticotropin releasing factor (CRF; A), arginine vasopressin (AVP; B), DβH (C), and brain derived neurotrophic factor (BDNF; D). GFP time control, n = 6; MIF time control, n = 7–8; GFP stress, n = 9; MIF stress, n = 13. *P < 0.05 MIF vs. GFP, #P < 0.05 stress vs. time control.
DISCUSSION
The primary responses to psychological stress are an increase in sympathetic nerve activity and activation of the HPA axis, and both limbs of the response can increase cardiovascular disease risk (8, 24, 49, 50, 61, 62). The results of this study indicate that restoration of MIF expression in PVN neurons in SHRs attenuates both limbs of the stress response. Specifically, restored expression of MIF in the PVN of adult SHRs with established hypertension attenuated restraint stress-induced elevations in MAP, inhibited restraint stress-induced neuronal activation in the RVLM as indicated by c-Fos expression, reduced the corticosterone response to restraint stress, and attenuated stress-induced CRF expression in the PVN. In contrast, increased PVN MIF expression failed to decrease resting blood pressure, affect water stress-induced elevations in blood pressure or plasma corticosterone in SHRs, and also failed to attenuate pressor responses to both water and restraint stress in normotensive Sprague-Dawley rats.
The present experiments utilized an AAV2-based viral vector construct to restore MIF expression to PVN neurons in SHRs and to augment MIF expression in PVN neurons of normotensive controls. This vector construct has been fully validated and shown to transduce primarily neurons in both the PVN and nucleus tractus solitaries in rats (22, 37). The volume (200 nl) and titer (109 viral particles/ml) were selected based on our experience and published information (17) indicating that the use of these parameters would limit vector expression primarily to the PVN. Immunoshistochemical verification of the injection sites confirmed that, when the PVN was accurately targeted by the microinjection, expression outside the PVN was limited to a few scattered cells (Fig. 1); animals with significant MIF or GFP expression outside of the PVN were considered to be “misses” and were excluded from the study.
Our previous investigations established that ANG II increases MIF expression in hypothalamic neurons from normotensive rats and that MIF counteracts the neuroexcitatory actions of ANG II (58). ANG II exerts its sympathoexcitatory and pressor effects by activating AT1Rs, increasing NADPH oxidase-mediated production of ROS leading to ROS-mediated inhibition of delayed rectifier potassium channels and increased neuronal firing rate (14, 15). Besides their effect on neuronal activity, ROS also upregulate MIF in PVN neurons (27). MIF in turn, scavenges ROS via its TPOR activity, and when injected in the PVN of normotensive rats, MIF inhibits ANG II-induced dipsogenic and pressor responses (36, 40). However, unlike in normotensive rats, ANG II fails to upregulate MIF expression in hypothalamic neurons from SHRs, and the lack of MIF-mediated inhibition is responsible for the augmented excitatory actions of ANG II in SHR neurons compared with neurons from normotensive rats (57). These results were confirmed by in vivo experiments indicating that central injections of ANG II in SHRs failed to increase MIF levels in the PVN, and while baseline MIF mRNA and protein levels were found to be similar in the PVN of normotensive rats and SHRs, immunohistochemistry revealed that MIF is mostly expressed in neurons in the PVN of normotensive rats, whereas in SHRs MIF is mainly expressed in astrocytes (37). In addition, when vector-mediated elevation of MIF expression in PVN neurons was initiated in 8-wk-old SHRs, the development of hypertension was attenuated (37). Thus these previous studies established that neuronal activation induced by ANG II in the PVN is augmented in SHRs due to the lack of MIF-dependent elimination of ROS produced as downstream intracellular mediators of ANG II.
ANG II signaling in the PVN plays an important role in mediating both the HPA axis and autonomic responses to stress (4, 6, 9, 11, 51). Moreover, PVN AT1R expression is increased in SHRs and in normotensive rats following repeated restraint and 24-h isolation stress (51), and systemic administration of an AT1R antagonist blunts the sympathoadrenal response to stress in SHRs (25). Results from this study suggest that the lack of MIF negative feedback on ANG II-mediated neuronal activation within the PVN could contribute to enhanced stress sensitivity in SHRs. Restoring MIF in PVN neurons of SHRs attenuated restraint stress-induced increases in blood pressure, PVN CRF mRNA expression, and plasma corticosterone. However, the same treatment failed to affect blood pressure increases during water stress. These differences in MIF effects may have been caused by the activation of different neural circuitry in response to different types of stressors, to the shorter duration of water stress relative to our restraint stress protocol, or possibly due to the influence of significant physical activity on cardiovascular regulation during water stress as the animals continuously attempted to escape from the water. The last possibility seems unlikely since MIF expression did not affect activity, as measured by telemetry, during the water stress (data not shown). Restraint stress is a standard psychological stressor in rats (15), whereas the water stress protocol is unique. Future experiments are needed to determine if PVN MIF modulates the arterial pressure and HPA axis responses to other standard psychological and physical stressors in SHRs.
In contrast with SHRs, where the vector treatment restored baseline neuronal MIF expression, in Sprague-Dawley rats, the same treatment resulted in an elevated baseline MIF level in the PVN, but this increased expression of MIF above normal MIF levels had no effect on stress responses, suggesting that the endogenous MIF-mediated inhibition on ANG II signaling could not be further enhanced. In fact, increasing MIF levels in the PVN of Sprague-Dawley rats had the seemingly adverse effect of reducing baroreflex sensitivity. The PVN exerts tonic inhibition on baroreflex function (46); however, the mechanism accounting for the effect of excess MIF to inhibit baroreflex function in Sprague-Dawley rats cannot be determined from the present experiments. Nonetheless, the results in the SHRs indicate that normal levels of MIF probably have no effect on baroreflex control of heart rate, since restoring MIF expression in these rats had no effect on the spontaneous baroreceptor reflex. Although Wistar-Kyoto (WKY) rats are commonly used as a control strain for SHRs, we chose to perform the control experiments in Sprague-Dawley rats, since WKY rats have elevated HPA axis activity and are used as an animal model of depression (54). That being said, there is minimal difference in the arterial pressure responses to acute stress between WKY and Sprague-Dawley rats (43). Also, our previous work demonstrated that WKY rats (unlike SHR) already express MIF in PVN neurons and that MIF overexpression (using the same construct) had no effect on baseline blood pressure in young WKY rats, while reducing baseline blood pressure in young SHRs (37). Therefore, it seems unlikely that the choice of control rat altered the basic conclusions of the study.
Heart rate responses were unaffected by MIF treatment in either Sprague-Dawley rats or SHRs, even though administration of exogenous ANG II into the PVN increases both arterial pressure and heart rate (38). It could be that with stress the increase in ANG II within the PVN is not global and that ANG II levels are not elevated in synaptic regions that can influence heart rate. This explanation is supported by a recent study by Busnardo et al. (6) that demonstrated that PVN injections of AT1R or angiotensin-converting enzyme inhibitors diminished restraint stress-induced pressor responses without affecting tachycardic responses.
Our previous studies highlighted the importance of MIF in ANG II signaling in the PVN; however, MIF may exert regulatory effects independent of ANG II that could also impact stress responses. MIF has been shown to induce inhibition of glucocorticoid receptor function (32) and to counterregulate anti-inflammatory actions of glucocorticoids by reducing the inhibitory effect of glucocorticoids on the release of proinflammatory cytokines (7). While these effects of MIF have not been demonstrated in the PVN, there is a possibility that increasing MIF levels in the PVN by viral vector-mediated transduction affected stress responses by inhibiting glucocorticoid receptor function. However, such an effect would likely lead to exaggerated as opposed to diminished stress responses by abolishing glucocorticoid-mediated negative feedback on corticotrophin-releasing hormone production in the PVN or by increasing the level of proinflammatory cytokines that also exert pressor effects in the PVN (53). Thus the attenuated blood pressure increases during and after restraint stress are unlikely to be caused by modulation of glucocorticoid signaling. Instead, these results suggest that the effects of MIF to inhibit corticosterone secretion could act synergistically with the antiglucocorticoid actions of systemic MIF to reduce peripheral glucocorticoid activity.
Based on our previous studies demonstrating that MIF reduces chronotropic actions of ANG II in PVN neurons (40), we expected that MIF treatment would reduce stress-induced neuronal activation in the PVN of SHRs as indicated by c-Fos expression. However, our results indicated that following restraint stress PVN c-Fos immunoreactivity was similar in GFP- and MIF-treated SHRs. On the other hand, the number of c-Fos-positive nuclei in the RVLM was markedly reduced in MIF-treated compared with GFP-injected SHRs, suggesting a role for reduced sympathetic tone in mediating the effect of PVN MIF to attenuate the blood pressure response to restraint stress. A similar discrepancy between PVN and RVLM c-Fos expression was observed by Palmer and Printz (47) when comparing the pattern of c-Fos expression in response to air-puff stress in WKY rats and SHRs; SHRs had a larger c-Fos response in the RVLM, but there was no between-strain difference in c-Fos expression in the PVN. c-Fos is widely used as an indicator of neuronal activation in response to stress, since its expression is increased only when neurons are stimulated with “unusual” inputs such as those induced by systemic or neurogenic stressors and not by normal neuronal activity (34). However, there are several limitations of using c-Fos as a marker for neuronal activation, and a lack of suppression of the number of c-Fos-positive nuclei in the PVN does not rule out a reduction in total neuronal output from PVN to RVLM projecting neurons in the PVN MIF-treated rats. First, counting neurons that are positive for c-Fos expression indicates the number of depolarized neurons, but cannot determine if neuronal activity was attenuated in individual neurons. If MIF treatment in the PVN reduced overall neuronal output to the RVLM without reducing the number of neurons that were depolarized, this change would not be detected by counting the number of c-Fos-positive neurons. Second, induction of c-Fos is mediated by multiple mechanisms, including depolarization, increases in intracellular and intranuclear Ca2+ concentrations, neurotrophic factors, and neurotransmitters and activation of various signal transduction pathways including protein kinase A, protein kinase C, Ca2+/calmodulin-dependent protein kinase, and mitogen-activated protein kinase (34). The relative contribution of these pathways to induction of c-Fos may vary in different neuronal cell types and may result in different threshold levels for c-Fos expression. It is possible that even with the inhibitory effect of elevated MIF levels on neuronal firing rate, activity levels of PVN neurons were still above the threshold for inducing c-Fos expression following restraint stress. However, RVLM neurons may have a higher threshold for c-Fos activation; therefore, any reduced input from descending PVN projections in MIF-treated SHRs resulted in a large enough decrease in RVLM neuronal activity that prevented c-Fos expression in a significant number of neurons in this important regulatory site of sympathetic activity. We previously demonstrated that restoration of MIF expression in PVN neurons of young, prehypertensive, SHRs attenuated the development of hypertension (37). However, in contrast to our hypothesis, restoration of MIF expression in PVN neurons in adult SHRs with established hypertension failed to lower resting blood pressure. In the current study, SHRs were injected with the MIF vector at the age of 14 wk, whereas in our previous study rats were injected at 8 wk of age (37). Considering that blood pressure rises rapidly between 5 and 10 wk of age in SHRs, and that the rate of blood pressure increase slows down considerably after 15 wk of age (45), it seems that by injecting the MIF vector at this older age, we missed the window of opportunity to induce a long-term reduction in blood pressure, and restoring MIF-mediated blood pressure regulatory mechanisms in the PVN apparently could not reverse either the multiple central mechanisms that contribute to established hypertension in SHR, and/or the potential adverse peripheral effects such as hypertension-related vascular dysfunction or changes in renal function (29).
Perspectives
The current results together with our previous findings indicate that the lack of MIF-mediated inhibition on ANG II signaling in the PVN of SHRs significantly contributes to impaired blood pressure regulation, resulting in augmented pressor and HPA responses to restraint stress as well as augmented blood pressure elevations to central ANG II injections and progressive development of hypertension in SHRs. An important objective of future studies will be to investigate whether a dysfunction in MIF-mediated inhibitory mechanisms in the PVN is an important contributor to the development of chronic stress-induced hypertension. In addition, as it has recently been shown that MIF modulates ANG II signaling not only in the PVN, but in other brain regions as well, such as the nucleus of the solitary tract (22), so further investigations are needed to determine whether MIF-dependent mechanisms affect acute or chronic stress-related blood pressure elevations outside of the PVN. Importantly, human MIF gene variants are already associated with the risk for atherosclerosis and type II diabetes (28, 35), so future studies are also needed to determine if gene variants leading to diminished MIF-mediated inhibition of ANG II signaling is one of the mechanisms responsible for the association between cardiovascular stress sensitivity and the risk of hypertension in humans.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-093186.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: B.E., R.R.C., M.L., H.L., M.L.M., and D.A.S. performed experiments; B.E., M.L., and D.A.S. analyzed data; B.E., M.L., H.L., C.S., and D.A.S. interpreted results of experiments; B.E. and D.A.S. prepared figures; B.E. and D.A.S. drafted manuscript; B.E., M.L., H.L., M.L.M., C.S., and D.A.S. edited and revised manuscript; B.E., R.R.C., M.L., H.L., M.L.M., C.S., and D.A.S. approved final version of manuscript; C.S. and D.A.S. conception and design of research.
ACKNOWLEDGMENTS
Present address of B. Erdos: Department of Pharmacology, University of Vermont, Burlington, VT, 05405.
Present address of H. Li: School of Biotechnology, Southern Medical University, Guangzhou, China.
REFERENCES
- 1.Armando I, Carranza A, Nishimura Y, Hoe KL, Barontini M, Terron JA, Falcon-Neri A, Ito T, Juorio AV, Saavedra JM. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology 142: 3880–3889, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Bardgett ME, Sharpe AL, Toney GM. Activation of corticotropin-releasing factor receptors in the rostral ventrolateral medulla is required for glucose-induced sympathoexcitation. Am J Physiol Endocrinol Metab 307: E944–E953, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechtold AG, Scheuer DA. Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate. Am J Physiol Regul Integr Comp Physiol 290: R1003–R1011, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clin Auton Res 15: 254–263, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia V, Rarick KR, Stauss HM. Effect of the data sampling rate on accuracy of indices for heart rate and blood pressure variability and baroreflex function in resting rats and mice. Physiol Meas 31: 1185–1201, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Busnardo C, Tavares RF, Correa FM. Angiotensinergic neurotransmission in the paraventricular nucleus of the hypothalamus modulates the pressor response to acute restraint stress in rats. Neuroscience 270: 12–19, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 377: 68–71, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Carroll D, Smith GD, Shipley MJ, Steptoe A, Brunner EJ, Marmot MG. Blood pressure reactions to acute psychological stress and future blood pressure status: a 10-year follow-up of men in the Whitehall II study. Psychosom Med 63: 737–743, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Cato MJ, Toney GM. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 93: 403–413, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan SH, Wu CW, Chang AY, Hsu KS, Chan JY. Transcriptional upregulation of brain-derived neurotrophic factor in rostral ventrolateral medulla by angiotensin II: significance in superoxide homeostasis and neural regulation of arterial pressure. Circ Res 107: 1127–1139, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Chen QH, Toney GM. AT1-receptor blockade in the hypothalamic PVN reduces central hyperosmolality-induced renal sympathoexcitation. Am J Physiol Regul Integr Comp Physiol 281: R1844–R1853, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Colombari E, Colombari DS, Li H, Shi P, Dong Y, Jiang N, Raizada MK, Sumners C, Murphy D, Paton JF. Macrophage migration inhibitory factor in the paraventricular nucleus plays a major role in the sympathoexcitatory response to salt. Hypertension 56: 956–963, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daubert DL, Looney BM, Clifton RR, Cho JN, Scheuer DA. Elevated corticosterone in the dorsal hindbrain increases plasma norepinephrine and neuropeptide Y and recruits a vasopressin response to stress. Am J Physiol Regul Integr Comp Physiol 307: R212–R224, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davern PJ, Chen D, Head GA, Chavez CA, Walther T, Mayorov DN. Role of angiotensin II type 1A receptors in cardiovascular reactivity and neuronal activation after aversive stress in mice. Hypertension 54: 1262–1268, 2009. [DOI] [PubMed] [Google Scholar]
- 15.Dayas CV, Buller KM, Crane JW, Xu Y, Day TA. Stressor categorization: acute physical and psychological stressors elicit distinctive recruitment patterns in the amygdala and in medullary noradrenergic cell groups. Eur J Neurosci 14: 1143–1152, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Djordjevic J, Vuckovic T, Jasnic N, Cvijic G. Effect of various stressors on the blood ACTH and corticosterone concentration in normotensive Wistar and spontaneously hypertensive Wistar-Kyoto rats. Gen Comp Endocrinol 153: 217–220, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Doherty FC, Schaack JB, Sladek CD. Comparison of the efficacy of four viral vectors for transducing hypothalamic magnocellular neurosecretory neurons in the rat supraoptic nucleus. J Neurosci Methods 197: 238–248, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erdos B, Backes I, McCowan ML, Hayward LF, Scheuer DA. Brain-derived neurotrophic factor modulates angiotensin signaling in the hypothalamus to increase blood pressure in rats. Am J Physiol Heart Circ Physiol 308: H612–H622, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdos B, Broxson CS, King MA, Scarpace PJ, Tumer N. Acute pressor effect of central angiotensin II is mediated by NAD(P)H-oxidase-dependent production of superoxide in the hypothalamic cardiovascular regulatory nuclei. J Hypertens 24: 109–116, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Everson SA, Kaplan GA, Goldberg DE, Salonen JT. Anticipatory blood pressure response to exercise predicts future high blood pressure in middle-aged men. Hypertension 27: 1059–1064, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci 39: 1903–1911, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freiria-Oliveira AH, Blanch GT, Li H, Colombari E, Colombari DS, Sumners C. Macrophage migration inhibitory factor in the nucleus of solitary tract decreases blood pressure in SHRs. Cardiovasc Res 97: 153–160, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlong TM, McDowall LM, Horiuchi J, Polson JW, Dampney RA. The effect of air puff stress on c-Fos expression in rat hypothalamus and brainstem: central circuitry mediating sympathoexcitation and baroreflex resetting. Eur J Neurosci 39: 1429–1438, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res 116: 976–990, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundt A, Grundt C, Gorbey S, Thomas MA, Lemmer B. Strain-dependent differences of restraint stress-induced hypertension in WKY and SHR. Physiol Behav 97: 341–346, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Harrison RA, Sumners C. Redox regulation of macrophage migration inhibitory factor expression in rat neurons. Biochem Biophys Res Commun 390: 171–175, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herder C, Klopp N, Baumert J, Muller M, Khuseyinova N, Meisinger C, Martin S, Illig T, Koenig W, Thorand B. Effect of macrophage migration inhibitory factor (MIF) gene variants and MIF serum concentrations in the risk of type 2 diabetes: results from the MONICA/KORA Augsburg Case-Cohort study, 1984–2002. Diabetologia 51: 276–284, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Hultstrom M. Development of structural kidney damage in spontaneously hypertensive rats. J Hypertens 30: 1087–1091, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Jeanneteau FD, Lambert WM, Ismaili N, Bath KG, Lee FS, Garabedian MJ, Chao MV. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc Natl Acad Sci USA 109: 1305–1310, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jezova D, Ochedalski T, Kiss A, Aguilera G. Brain angiotensin II modulates sympathoadrenal and hypothalamic pituitary adrenocortical activation during stress. J Neuroendocrinol 10: 67–72, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Jing L, Bu M. Role of macrophage migration inhibitory factor in glucocorticoid release and glucocorticoid receptor function in rats. Ann Clin Lab Sci 41: 14–19, 2011. [PubMed] [Google Scholar]
- 33.Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kovacs KJ. c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33: 287–297, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Lan MY, Chang YY, Tseng YL, Lin HS, Lai SL, Liu JS. Association between MIF gene polymorphisms and carotid artery atherosclerosis. Biochem Biophys Res Commun 453: 319–322, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Li H, Gao Y, Freire CD, Raizada MK, Toney GM, Sumners C. Macrophage migration inhibitory factor in the PVN attenuates the central pressor and dipsogenic actions of angiotensin II. FASEB J 20: 1748–1750, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Gao Y, Qi Y, Katovich MJ, Jiang N, Braseth LN, Scheuer DA, Shi P, Sumners C. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. FASEB J 22: 3175–3185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YF, Wang W, Mayhan WG, Patel KP. Angiotensin-mediated increase in renal sympathetic nerve discharge within the PVN: role of nitric oxide. Am J Physiol Regul Integr Comp Physiol 290: R1035–R1043, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Markovitz JH, Raczynski JM, Wallace D, Chettur V, Chesney MA. Cardiovascular reactivity to video game predicts subsequent blood pressure increases in young men: The CARDIA study. Psychosom Med 60: 186–191, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Matsuura T, Sun C, Leng L, Kapurniotu A, Bernhagen J, Bucala R, Martynyuk AE, Sumners C. Macrophage migration inhibitory factor increases neuronal delayed rectifier K+ current. J Neurophysiol 95: 1042–1048, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the CARDIA study. Circulation 110: 74–78, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Matthews KA, Woodall KL, Allen MT. Cardiovascular reactivity to stress predicts future blood pressure status. Hypertension 22: 479–485, 1993. [DOI] [PubMed] [Google Scholar]
- 43.McDougall SJ, Lawrence AJ, Widdop RE. Differential cardiovascular responses to stressors in hypertensive and normotensive rats. Exp Physiol 90: 141–150, 2005. [DOI] [PubMed] [Google Scholar]
- 44.O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S; INTERSTROKE investigators. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 376: 112–123, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27: 282–293, 1963. [DOI] [PubMed] [Google Scholar]
- 46.Page MC, Cassaglia PA, Brooks VL. GABA in the paraventricular nucleus tonically suppresses baroreflex function: alterations during pregnancy. Am J Physiol Regul Integr Comp Physiol 300: R1452–R1458, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmer AA, Printz MP. Strain differences in Fos expression following airpuff startle in spontaneously hypertensive and Wistar Kyoto rats. Neuroscience 89: 965–978, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic, 2007. [Google Scholar]
- 49.Rossi R, Tauchmanova L, Luciano A, Di Martino M, Battista C, Del Viscova LN, Lombardi G. Subclinical Cushing's syndrome in patients with adrenal incidentaloma: clinical and biochemical features. J Clin Endocrinol Metab 85: 1440–1448, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99: 2192–2217, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Jezova M, Zhou J. Brain angiotensin II, an important stress hormone: regulatory sites and therapeutic opportunities. Ann NY Acad Sci 1018: 76–84, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Scheuer DA, Mifflin SW. Chronic corticosterone treatment increases myocardial infarct size in rats with ischemia-reperfusion injury. Am J Physiol Regul Integr Comp Physiol 272: R2017–R2024, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Shi Z, Gan XB, Fan ZD, Zhang F, Zhou YB, Gao XY, De W, Zhu GQ. Inflammatory cytokines in paraventricular nucleus modulate sympathetic activity and cardiac sympathetic afferent reflex in rats. Acta Physiol (Oxf) 203: 289–297, 2011. [DOI] [PubMed] [Google Scholar]
- 54.Solberg LC, Olson SL, Turek FW, Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol 281: R786–R794, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Spiga F, Walker JJ, Terry JR, Lightman SL. HPA axis-rhythms. Compr Physiol 4: 1273–1298, 2014. [DOI] [PubMed] [Google Scholar]
- 56.Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol 287: R1172–R1183, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Sun C, Li H, Gao Y, Matsuura T, Upchurch PA, Raizada MK, Sumners C. Lack of macrophage migration inhibitory factor regulation is linked to the increased chronotropic action of angiotensin II in SHR neurons. Hypertension 49: 528–534, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci 24: 9944–9952, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res 96: 659–666, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Treiber FA, Turner JR, Davis H, Strong WB. Prediction of resting cardiovascular functioning in youth with family histories of essential hypertension: a 5-year follow-up. Int J Behav Med 4: 278–291, 1997. [DOI] [PubMed] [Google Scholar]
- 61.van der Hooft CS, Heeringa J, Brusselle GG, Hofman A, Witteman JCM, Kingma JH, Sturkenboom MC, Stricker BH. Corticosteroids and the risk of atrial fibrilation. Arch Intern Med 165: 1016–1020, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol 157: 545–559, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Gao Y, Speth RC, Jiang N, Mao Y, Sumners C, Li H. Adenoviral and adeno-associated viral vectors-mediated neuronal gene transfer to cardiovascular control regions of the rat brain. Int J Med Sci 10: 607–616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang ZH, Felder RB. Hypothalamic corticotrophin-releasing factor and norepinephrine mediate sympathetic and cardiovascular responses to acute intracarotid injection of tumour necrosis factor-alpha in the rat. J Neuroendocrinol 20: 978–987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]