Abstract
Purpose: To investigate the association between the N-acetyltransferase 1 (NAT1) slow and rapid acetylation phenotypes with cancer risk based on a meta-analysis. Methods: Previously published case-control studies were retrieved from PubMed, Embase, and Web of Science. Odds ratios (ORs) with 95% confidence intervals (CIs) were determined to assess the relationship between NAT1 polymorphisms and cancer risk. Results: A total of 73 studies (24874 cases and 30226 controls) were included in this meta-analysis. No significant association was identified between NAT1 polymorphisms (slow acetylation versus rapid acetylation genotypes: OR = 0.978, 95% CI = 0.927-1.030, P < 0.001 for heterogeneity, I2 = 45.5%) and cancer risk, whereas a significantly reduced risk of pancreatic cancer was identified in individuals with NAT1 slow acetylation genotype (OR = 0.856, 95% CI = 0.733-0.999, P =0.509 for heterogeneity, I2 = 0). When the NAT1 slow acetylation genotype was analysed on the basis of stratified analyses of ethnicity, a significantly reduced risk of head and neck cancers was found among Asian (OR=0.281, 95% CI = 0.127-0.622). When the NAT1 slow acetylation genotype was analysed on the basis of stratified analyses of source of control, only significantly reduced risks of colorectal cancer (OR = 0.882, 95% CI = 0.798- 0.974, P = 0.212 for heterogeneity, I2 = 22.9) and pancreatic cancer (OR=0.856, 95% CI = 0.733-0.999, P = 0.509 for heterogeneity, I2 = 0) were found among hospital-based studies. Conclusions: No significant association between the NAT1 polymorphisms and the risk of cancer was found except for pancreatic cancer.
Keywords: N-acetyltransferase 1, polymorphism, cancer, meta-analysis
Introduction
Cancer, also known as malignant neoplasm, is a major public health problem worldwide. Approximately 12.7 million cancer cases and 7.6 million deaths caused by were reported by GLOBOCAN 2008 [1]. Carcinogenesis is a multistep process in which numerous genetic and environmental factors are involved [2]. It has been shown that host genetic factors contribute to carcinogenesis through modification of gene structure and protein expression [3,4]. Recent studies suggest that variants of genes encoding metabolic enzymes are significantly associated with the development of a number of cancers.
The NAT gene on chromosome 8p21.3-23.1, which encodes N-acetyltransferases (NAT; E.C.2.3.1.5) isozymes NAT1 (N-acetyltransferase 1) and NAT2 (N-acetyltransferase 2) [5] and phase II xenobiotic metabolizing enzyme, plays an essential role in detoxifying carcinogens, and their reactive intermediates are also involved in N-acetylation and O-acetylation of aromatic and heterocyclic amine carcinogens [6]. There are many systematic reviews on the association of NAT2 polymorphism and the risk of cancer. A meta-analysis conducted by Zhong [7] indicated that no association was found between NAT2 acetylation status and gastric cancer risk. No significant association was found in overall analysis between NAT2 acetylation status and lung cancer risk by Cui’s meta-analysis [8], either. A meta-analysis conducted by Gong [9] found that a statistically significant association between NAT2 polymorphism and prostate cancer appeared in Asians, but not in Caucasians. And a pooled analysis conducted by Liu [10] found that suggested that individuals with NAT2 genotype had an elevated risk of colorectal adenoma risk.
To date, 28 human NAT1 variants have been identified (http://louisville.edu/medschool/pharmacology/consensushuman-arylamine-n-acetyltransferase-gene-nomenclature/). The NAT1*4 genotype has historically been designated as “wild type” and is commonly used a reference for studying NAT1 polymorphisms. In the past decade, numerous epidemiological studies investigating the association between NAT1 polymorphisms and cancer risk have been reported, however, the results of some studies are conflicting. For example, a case–control study conducted in Norway by Zienolddiny et al. [11] found that the fast acetylator phenotype of NAT1 was significantly associated with lung cancer. However, negative association between them has also been reported [12].
In the present study, we conducted a meta-analysis to systematically study the association between NAT1 polymorphisms and cancer risk based on published studies.
Materials and methods
Selection of published studies
A systematic search in the PubMed, Embase and Web of Science databases was conducted to retrieve studies published until July 1, 2014 using the following MeSH terms and keywords: ‘NAT1’ or ‘N-acetyltransferase 1’, ‘polymorphism’ or ‘variant’, and ‘cancer’ or ‘carcinoma’. The references of retrieved studies were also scanned to identify eligible studies. Studies included in the present meta-analysis have to meet the following criteria: (i) articles investigating the association between NAT1 polymorphisms and cancer risk; (ii) case-control studies; (iii) available genotype frequency for computing odds ratios (ORs) with 95% confidence intervals (CIs); (iv) studies with full-text article. Criteria for excluding studies were (i) only case population; (ii) outcome comparison not available or not able to be determined; (iii) duplicated publications; (iv) benign tumor or precancerous lesions.
Data extraction
Two investigators (Zhang KY and Gao LJ independently screened the titles, abstracts and full texts using a standardized extraction form. Agreement was reached to resolve conflicting evaluation based on consensus and discussion. For each study, the following results were collected: first author’s name, year of publication, country of origin, ethnicity, cancer type, genotyping method, source of controls (population-based [PB] or hospital-based [HB] controls), total number of cases and controls, and genotype distributions in cases and controls. No minimum number of patients was defined in the present meta-analysis. In accordance with most studies, individuals with at least one of the high-activity NAT1 alleles (NAT1*10, NAT1*21, NAT1*24, and NAT1*25) were defined as rapid acetylators, whereas individuals carrying two low-activity NAT1 alleles(others except the high-activity alleles ) were considered as slow acetylators.
Statistical analysis
Statistical analyses were performed using the STATA software (version 12.0; Stata Corporation, College Station, Texas). Statistical significance was evaluated using two-tailed test and a P value less than 0.05 was considered as statistical significance unless stated otherwise. Hardy-Weinberg equilibrium (HWE) in controls was assessed by chi-squared test and a P value less than 0.05 was considered as significant disequilibrium. If HWE disequilibrium was identified (P < 0.05), or equilibrium evaluation was not possible, sensitivity analysis was performed. The strength of the association between NAT1 polymorphisms and cancer risk was evaluated on the basis of ORs with 95% confidence intervals (CIs). The chi-square-based Q statistic was used to test heterogeneities among the studies included in the present meta-analysis [13]. A fixed-effect model with Mantel–Haenszel method was used to calculate the pooled odds ratios if Q-test P value was ≥ 0.1 [14]. Otherwise, a random-effect model with inverse variance method was used. The risks (ORs) of cancer associated with the NAT1 slow/rapid acetylation polymorphisms were estimated for each study. One-way sensitivity analysis was performed to assess the stability of the results. Specifically, each study was sequentially removed from the meta-analysis to evaluate its influence on pooled ORs. Begg and Mazumdar [15] adjusted rank correlation test and the Egger regression asymmetry test [16] were used to identify publication bias.
Results
Characteristics of the studies
A total of 207 articles were retrieved from PubMed, Embase, and Web of Science. Among them, 76 case-control studies including 24874 cases and 30226 controls in73 articles met the inclusion criteria. Three articles reported two independent studies that were considered separately. The characteristics of each study were listed in Table 1. In general, there were 5 lung cancer studies [17-20], 23 colorectal cancer studies [21-41], 5 head and neck cancer studies [42-46], 3 pancreatic cancer studies [47-49], 5 non-Hodgkin’s lymphoma studies [50-54], 12 bladder cancer studies [55-66], 8 prostate cancer studies [67-73], 4 gastric cancer studies [34,74,75], 5 breast cancer studies [76-80] and 6 other cancers studies [74,81-85]. Thirty-nine, 11, 22, and 4 studies were on Caucasian, Asian, and Mixed population, and other population, respectively. There were 48 hospital-based studies and 28 population-based studies.
Table 1.
Characteristics of the studies included in the meta-analysis
| First Author | Year | Country | Ethnicity | Cancer type | Genotyping Method | Source of control | Case | Control |
|---|---|---|---|---|---|---|---|---|
| lung cancer | ||||||||
| Abdel-Rahman | 1998 | USA | Mixed | lung cancer | PCR-RFLP | HB | 45 | 47 |
| Bouchardy, C. | 1998 | France | Caucasian | lung cancer | PCR-RFLP | HB | 150 | 172 |
| Ishibe | 1998 | USA | Mixed | lung cancer | PCR-RFLP | HB | 174 | 319 |
| Wikman, H | 2001 | Germany | Caucasian | lung cancer | PCR-RFLP | HB | 392 | 351 |
| Zienolddiny, S. | 2008 | Norway | Caucasian | lung cancer | Sequecing | PB | 390 | 186 |
| colorectal cancer | ||||||||
| Eichholzer | 2012 | Switzerland | Caucasian | colorectal cancer | MassArray | PB | 399 | 776 |
| Cleary | 2010 | Canada | Caucasian | colorectal cancer | TaqMan | HB | 1159 | 1284 |
| Yeh | 2009 | China | Asian | colorectal cancer | PCR–RFLP | HB | 722 | 733 |
| Nothlings | 2009 | USA | Mixed | colorectal cancer | TaqMan/Sequence Detection System | PB | 844 | 1345 |
| Sorensen | 2008 | Denmark | Caucasian | colorectal cancer | TaqMan/Sequence Detection System | HB | 377 | 766 |
| Butler | 2008 | USA | others | colorectal cancer | PCR–RFLP/(AS)-PCR | HB | 208 | 299 |
| Butler | 2008 | USA | Caucasian | colorectal cancer | PCR–RFLP/(AS)-PCR | HB | 282 | 528 |
| Mahid | 2007 | USA | Mixed | colorectal cancer | TaqMan | HB | 123 | 223 |
| Lilla | 2006 | Germany | Caucasian | colorectal cancer | Fluorescence-based melting curve | PB | 605 | 604 |
| Landi | 2005 | Italy | Caucasian | colorectal cancer | Sequence Detection System | HB | 359 | 321 |
| Chen | 2006 | China | Asian | colorectal cancer | PCR–RFLP | PB | 138 | 343 |
| Kiss | 2004 | Hungary | Caucasian | colorectal cancer | PCR–RFLP | HB | 500 | 500 |
| Van Der Hel | 2003 | Netherlands | Caucasian | colorectal cancer | PCR–RFLP | PB | 218 | 804 |
| Zhang | 2002 | China | Asian | colorectal cancer | PCR–RFLP | HB | 104 | 101 |
| Tiemersma | 2002 | Netherlands | Caucasian | colorectal cancer | Allele-specific hybridization | PB | 102 | 536 |
| Le Marchand | 2001 | USA | Mixed | colorectal cancer | PCR–RFLP | PB | 539 | 649 |
| Katoh | 2000 | Japan | Asian | colorectal cancer | PCR–RFLP/(AS)-PCR | HB | 103 | 122 |
| Kampman | 1999 | USA | Mixed | colorectal cancer | Oligonucleotide ligation assay | PB | 1624 | 1963 |
| Chen | 1998 | USA | Mixed | colorectal cancer | PCR–RFLP | PB | 212 | 221 |
| Bell | 1995 | UK | Caucasian | colorectal cancer | PCR–RFLP | HB | 202 | 112 |
| Moslehi | 2006 | USA | Mixed | colorectal cancer | TaqMan | PB | 636 | 636 |
| Ishibe | 2002 | USA | Mixed | colorectal cancer | PCR–RFLP | HB | 132 | 192 |
| Probst-Hensch | 1996 | USA | Mixed | colorectal cancer | PCR–RFLP | HB | 441 | 484 |
| head and neck cancer | ||||||||
| Demokan | 2010 | Turkey | others | head and neck cancer | PCR–RFLP | HB | 95 | 93 |
| Fronhoffs | 2001 | Fronhoffs | Caucasian | head and neck cancer | PCR | HB | 291 | 300 |
| Olshan | 2000 | USA | Mixed | head and neck cancer | PCR–RFLP | HB | 171 | 193 |
| Majumder | 2012 | India | others | head and neck cancer | TaqMan | HB | 299 | 381 |
| Katoh | 1998 | Japan | Asian | head and neck cancer | PCR–RFLP | HB | 62 | 122 |
| pancreatic cancer | ||||||||
| Suzuki | 2008 | USA | Mixed | pancreatic cancer | PCR–RFLP | HB | 649 | 585 |
| Li | 2006 | USA | Mixed | pancreatic cancer | TaqMan | HB | 304 | 322 |
| Jiao | 2007 | USA | Caucasian | pancreatic cancer | TaqMan | HB | 501 | 548 |
| non-Hodgkin’s lymphoma | ||||||||
| Chiu | 2005 | USA | Mixed | non-Hodgkin’s lymphoma | PCR–RFLP | PB | 267 | 543 |
| Kilfoy | 2010 | USA | Mixed | non-Hodgkin’s lymphoma | TaqMan | PB | 453 | 522 |
| Morton | 2006 | USA | Mixed | non-Hodgkin’s lymphoma | TaqMan | PB | 916 | 746 |
| Aschebrook-Kilfoy | 2012 | USA | Mixed | non-Hodgkin’s lymphoma | PCR–RFLP | PB | 328 | 447 |
| Kerridge | 2002 | Australia | Caucasian | non-Hodgkin’s lymphoma | PCR–RFLP | HB | 164 | 193 |
| bladder cancer | ||||||||
| Koutros | 2011 | USA | Caucasian | bladder cancer | TaqMan | PB | 247 | 324 |
| Covolo | 2008 | Italy | Caucasian | bladder cancer | PCR–RFLP | HB | 197 | 211 |
| McGrath | 2006 | USA | Caucasian | bladder cancer | TaqMan | PB | 193 | 479 |
| Gu | 2005 | USA | Caucasian | bladder cancer | PCR–RFLP | HB | 490 | 491 |
| Garcia-Closas | 2005 | Spain | Caucasian | bladder cancer | TaqMan | HB | 965 | 942 |
| Hung, R | 2004 | Italy | Caucasian | bladder cancer | PCR–RFLP | HB | 201 | 214 |
| Schroeder | 2003 | USA | Mixed | bladder cancer | PCR–RFLP | HB | 234 | 207 |
| Stern | 2002 | USA | Mixed | bladder cancer | PCR–RFLP | HB | 225 | 200 |
| Cascorbi | 2001 | Germany | Caucasian | bladder cancer | PCR–RFLP | HB | 425 | 343 |
| Hsieh | 1999 | China | Asian | bladder cancer | PCR–RFLP | HB | 65 | 171 |
| Taylor | 1998 | USA | Mixed | bladder cancer | PCR–RFLP | HB | 230 | 203 |
| Okkels | 1997 | Denmark | Caucasian | bladder cancer | PCR–RFLP | HB | 248 | 223 |
| prostate cancer | ||||||||
| Sharma | 2010 | Canada | Mixed | prostate cancer | TaqMan | PB | 1685 | 1642 |
| Sharma | 2010 | Canada | Caucasian | prostate cancer | TaqMan | PB | 421 | 421 |
| Kidd | 2011 | USA | Caucasian | prostate cancer | mass spectrometry | PB | 200 | 184 |
| Iguchi | 2009 | USA | Caucasian | prostate cancer | TaqMan | PB | 179 | 170 |
| Hein | 2002 | USA | Caucasian | prostate cancer | PCR–RFLP | HB | 47 | 121 |
| Costa | 2005 | Portugal | Caucasian | prostate cancer | PCR–RFLP | PB | 127 | 145 |
| Rovito | 2005 | USA | Caucasian | prostate cancer | TaqMan | PB | 139 | 146 |
| Fukutome | 1999 | Japan | Asian | prostate cancer | PCR–RFLP | HB | 101 | 97 |
| gastri cancer | ||||||||
| Wideroff | 2007 | USA | Caucasian | gastri cancer | TaqMan | PB | 116 | 211 |
| Katoh | 2000 | Japan | Asian | gastri cancer | PCR–RFLP/(AS)-PCR | HB | 140 | 122 |
| BOISSY | 2000 | USA | Caucasian | gastri cancer | PCR-RFLP | HB | 94 | 112 |
| Lang | 2003 | Poland | Caucasian | gastri cancer | PCR-RFLP | HB | 292 | 410 |
| breast cancer | ||||||||
| Van Der Hel | 2003 | Netherlands | Caucasian | breast cancer | PCR-RFLP | PB | 228 | 264 |
| Lee | 2003 | Korea | Asian | breast cancer | TaqMan | PB | 245 | 275 |
| Krajinovic | 2001 | Canada | Caucasian | breast cancer | PCR allele-specific-oligonucleotide (ASO) hybridization assays | HB | 125 | 182 |
| Millikan | 2000 | USA | Mixed | breast cancer | PCR-RFLP | HB | 490 | 469 |
| other cancers | ||||||||
| Muller | 2008 | Germany | others | acute myeloid leukemia | TaqMan | HB | 132 | 208 |
| Krajinovic | 2000 | Canada | Caucasian | acute myeloid leukemia | PCR-RFLP | HB | 155 | 306 |
| Wideroff | 2007 | USA | Caucasian | Esophageal adenocarcinoma | TaqMan | PB | 67 | 211 |
| Zhang | 2005 | China | Asian | hepatocellular carcinoma | PCR-RFLP | HB | 96 | 173 |
| Yu | 2000 | China | Asian | hepatocellular carcinoma | PCR-RFLP | HB | 151 | 211 |
| Lincz | 2004 | Australia | Caucasian | multiple myeloma | PCR-RFLP | HB | 90 | 198 |
PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism; PB: population-based case control study; HB: hospital-based case control.
Meta-analysis
The strength of the association between NAT1 polymorphisms (slow acetylation versus rapid acetylation genotypes) and the susceptibility to cancers were shown in Table 2. Overall, the NAT1 acetylation phenotype was not significantly associated with cancer risk compared with the NAT1 rapid acetylation phenotype. The forest plot of overall comparison between slow and rapid acetylation genotypes was shown in Figure 1. The pooled OR was 0.978 (95% CI = 0.927-1.030, P < 0.001 for heterogeneity, I2 = 45.5%). Substantial heterogeneity was identified among these studies.
Table 2.
Pooled ORs and 95% CIs of stratified meta-analysis
| Variables | N | OR | 95% CIs | I2 (%) | P for Heterogeneity |
|---|---|---|---|---|---|
| Total | 76 | 0.978 | 0.927-1.030 | 45.5 | < 0.001 |
| Cancer type | |||||
| Lung cancer | 5 | 0.867 | 0.592-1.269 | 73.3 | 0.005 |
| Colorectal cancer | 23 | 0.961 | 0.880-1.050 | 54.8 | 0.001 |
| Head and neck cancer | 5 | 0.826 | 0.595-1.146 | 63.5 | 0.027 |
| Pancreatic cancer | 3 | 0.856 | 0.733-0.999 | 0 | 0.509 |
| Non-Hodgkin’s lymph | 5 | 1.007 | 0.892-1.136 | 0 | 0.863 |
| Bladder cancer | 12 | 1.068 | 0.929-1.227 | 47.2 | 0.035 |
| Prostate cancer | 8 | 1.019 | 0.892-1.164 | 9.3 | 0.358 |
| Gastri cancer | 4 | 0.913 | 0.532-1.567 | 81.0 | 0.001 |
| Breast cancer | 5 | 0.967 | 0.826-1.132 | 0 | 0.791 |
| other cancers | 6 | 1.102 | 0.906-1.339 | 0 | 0.641 |
| Source of control | |||||
| PB | 28 | 0.978 | 0.927-1.030 | 27.0 | 0.096 |
| HB | 48 | 0.941 | 0.872-1.016 | 51.6 | < 0.001 |
| Ethnicity | |||||
| Caucasian | 39 | 0.981 | 0.906-1.061 | 49.3 | < 0.001 |
| Asian | 11 | 0.887 | 0.730-1.076 | 44.6 | 0.054 |
| Mixed | 22 | 0.996 | 0.918-1.080 | 46.6 | 0.009 |
| Others | 4 | 1.028 | 0.843-1.253 | 0 | 0.532 |
N: involved studies’ number; OR, odds ratio; PB: population-based case control study; HB: hospital-based case control. Random model was chosen for data pooling when P-value < 0.10 and /or I2 > 50%; otherwise fixed model was used; The numbers in bold indicated statistically significant values.
Figure 1.
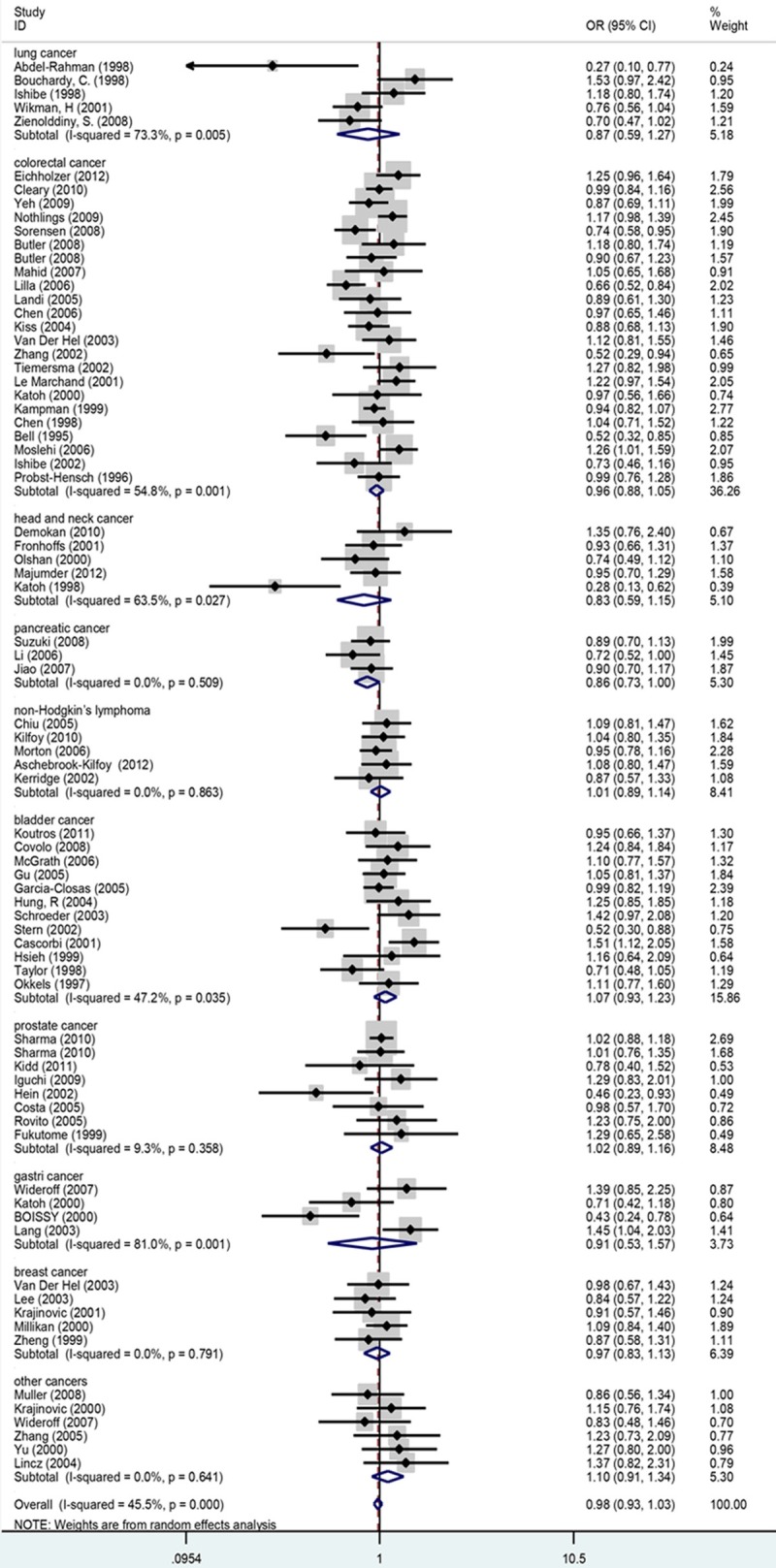
Meta-analysis of the association between NAT1 polymorphisms (slow and rapid acetylation genotypes) and susceptibility to cancer. The sizes of the symbols are proportional to the study.
In the subgroup analyses by ethnicity, no significant risks were found in Caucasian (OR = 0.981, 95% CI = 0.906-1.061, P < 0.001 for heterogeneity, I2 = 49.3%), Asian (OR = 0.887, 95% CI = 0.730-1.076, P < 0.001 for heterogeneity, I2 = 44.6%), Mixed population(OR = 0.996, 95% CI = 0.918-1.080, P < 0.001 for heterogeneity, I2 = 46.6%) and Others (OR = 1.028, 95% CI = 0.843-1.253, P = 0.532 for heterogeneity, I2 = 0). In addition, no significantly increased risk was detected in different source of controls (for hospital-based studies: OR = 0.941, 95% CI = 0.872-1.016, P < 0.001 for heterogeneity, I2 = 51.6%); for population-based studies: OR = 0.978, 95% CI = 0.927-1.030, P = 0.096 for heterogeneity, I2 = 27.0%). In stratified analyses by cancer types, significant associations were found only for pancreatic cancer (OR = 0.856, 95% CI = 0.733-0.999, P = 0.509 for heterogeneity, I2 = 0) (Table 2). We also performed analyses based on different cancer types in different ethnicities. The results showed that significantly reduced risk of slow acetylation genotype of head and neck cancers was found in Asian (OR = 0.281, 95% CI = 0.127-0.622). However, no significant association between NAT1 polymorphisms and risks of other types of cancers was detected in both Asian and Caucasian (Table 3). In addition, we conducted analyses based on different cancer types among source of control and found significantly reduced risks of both colorectal cancer (OR = 0.882, 95% CI = 0.798- 0.974, P = 0.212 for heterogeneity, I2 = 22.9) and pancreatic cancer (OR = 0.856, 95% CI = 0.733-0.999, P = 0.509 for heterogeneity, I2 = 0) among hospital-based population. Similarly, no significant association between NAT1 polymorphisms and the risks of other different types of cancers was found in both hospital-based studies and population-based studies (Table 4).
Table 3.
Stratified analyses of NAT1 polymorphisms on cancer risk by ethnicity
| Variables | N | OR | 95% CIs | I2 (%) | P for Heterogeneity |
|---|---|---|---|---|---|
| Total | 76 | 0.978 | 0.927-1.030 | 45.5 | < 0.001 |
| Caucasian | |||||
| Lung cancer | 3 | 0.912 | 0.592-1.404 | 74.5 | 0.020 |
| Colorectal cancer | 10 | 0.899 | 0.773-1.044 | 63.8 | 0.003 |
| Head and neck cancer | 1 | 0.927 | 0.656-1.309 | _ | _ |
| Pancreatic cancer | 1 | 0.905 | 0.700-1.169 | _ | _ |
| Non-Hodgkin’s lymph | 1 | 0.874 | 0.575-1.328 | _ | _ |
| Bladder cancer | 8 | 1.104 | 0.993-1.227 | 1.5 | 0.418 |
| Prostate cancer | 6 | 1.003 | 0.834-1.207 | 30.8 | 0.204 |
| Gastric cancer | 3 | 0.985 | 0.498-1.948 | 84.6 | 0.002 |
| Breast cancer | 3 | 0.925 | 0.728-1.174 | 0 | 0.916 |
| other cancers | 6 | 1.102 | 0.906-1.339 | 0 | 0.641 |
| Asian | |||||
| Colorectal cancer | 4 | 0.855 | 0.699-1.046 | 9.8 | 0.344 |
| Head and neck cancer | 1 | 0.281 | 0.127-0.622 | _ | _ |
| Bladder cancer | 1 | 1.156 | 0.641-2.086 | _ | _ |
| Prostate cancer | 1 | 1.294 | 0.648-2.584 | _ | _ |
| Gastric cancer | 1 | 0.708 | 0.423-1.184 | _ | _ |
| Breast cancer | 1 | 0.836 | 0.575-1.216 | _ | _ |
| Mixed | |||||
| Lung cancer | 2 | 0.616 | 0.147-2.578 | 85.1 | 0.010 |
| Colorectal cancer | 8 | 1.065 | 0.954-1.188 | 36.7 | 0.136 |
| Head and neck cancer | 1 | 0.742 | 0.491-1.122 | _ | _ |
| Pancreatic cancer | 2 | 0.829 | 0.683-1.006 | 6.6 | 0.301 |
| Non-Hodgkin’s lymph | 4 | 1.020 | 0.899-1.157 | 0 | 0.846 |
| Bladder cancer | 3 | 0.822 | 0.461-1.466 | 81.6 | 0.004 |
| Prostate cancer | 1 | 1.019 | 0.884-1.176 | _ | _ |
| Breast cancer | 1 | 1.086 | 0.843- 1.399 | _ | _ |
| others | |||||
| Colorectal cancer | 1 | 1.183 | 0.803- 1.742 | _ | _ |
| Head and neck cancer | 2 | 1.034 | 0.770- 1.388 | 9.2 | 0.294 |
N: involved studies’ number; OR, odds ratio; PB: population-based case control study; HB: hospital-based case control. Random model was chosen for data pooling when P-value < 0.10 and /or I2 > 50%; otherwise fixed model was used; The numbers in bold indicated statistically significant values.
Table 4.
Stratified analyses of NAT1 polymorphisms on cancer risk by source of control
| Variables | N | OR | 95% CIs | Tau-squared | I2 (%) | P for Heterogeneity |
|---|---|---|---|---|---|---|
| Total | 76 | 0.978 | 0.927-1.030 | 0.0215 | 45.5 | < 0.001 |
| PB | ||||||
| Lung cancer | 1 | 0.695 | 0.474-1.020 | 0.1279 | _ | _ |
| Colorectal cancer | 10 | 1.063 | 0.929-1.217 | 0.0225 | 65.0 | 0.002 |
| Non-Hodgkin’s lymph | 4 | 1.020 | 0.899-1.157 | 0 | 0.846 | |
| Bladder cancer | 2 | 1.022 | 0.792-1.318 | 0.0265 | 0 | 0.587 |
| Prostate cancer | 6 | 1.035 | 0.923-1.161 | 0 | 0 | 0.818 |
| Gastric cancer | 1 | 1.385 | 0.853-2.249 | 0.2425 | _ | _ |
| Breast cancer | 3 | 0.896 | 0.717-1.118 | 0 | 0 | 0.832 |
| other cancers | 1 | 0.834 | 0.476-1.459 | _ | _ | _ |
| HB | ||||||
| Lung cancer | 4 | 0.911 | 0.564-1.471 | 0.0074 | 76.7 | 0.005 |
| Colorectal cancer | 13 | 0.882 | 0.798- 0.974 | 0.0334 | 22.9 | 0.212 |
| Head and neck cancer | 5 | 0.826 | 0.595-1.146 | 0.0836 | 63.5 | 0.027 |
| Pancreatic cancer | 3 | 0.856 | 0.733-0.999 | 0 | 0 | 0.509 |
| Non-Hodgkin’s lymph | 1 | 0.874 | 0.575-1.328 | 0 | _ | _ |
| Bladder cancer | 10 | 1.075 | 0.911-1.269 | 0.0152 | 55.9 | 0.015 |
| Prostate cancer | 2 | 0.784 | 0.483-1.272 | 0 | 76.4 | 0.040 |
| Gastric cancer | 3 | 0.786 | 0.379-1.629 | 85.9 | 0.001 | |
| Breast cancer | 2 | 1.044 | 0.835-1.306 | 0 | 0.517 | |
| other cancers | 5 | 1.146 | 0.930-1.411 | 0 | 0.641 | |
N: involved studies’ number; OR, odds ratio; PB: population-based case control study; HB: hospital-based case control. Random model was chosen for data pooling when P-value < 0.10 and /or I2 > 50%; otherwise fixed model was used; The numbers in bold indicated statistically significant values.
Heterogeneity and sensitivity analyses
Significant heterogeneities was detected between studies. Then the source of heterogeneity was evaluated by cancer types (lung cancer, colorectal cancer, head and neck cancer, pancreatic cancer, non-Hodgkin’s lymphoma, bladder cancer, prostate cancer, gastric cancer, breast cancer and other types of cancers), ethnicity (Caucasian, Asian, Mixed and Others) and source of controls (population-based and hospital-based case controls). The results suggested that cancer types (χ2 = 42.158, df = 9, P < 0.001) and ethnicity (χ2 = 36.737, df = 3, P < 0.001), but not the source of controls (χ2 = 0.615, df = 1, P = 0.433) contributed substantially to heterogeneity. Sensitivity analysis through sequentially removal of individual study demonstrated that no study significantly affected the overall OR (the 95% CIs always overlap one unit).
Publication bias
As shown in Figures 2 and 3, the symmetrical funnel plots suggested no publication bias (P = 0.260). The Egger’s test further supported no publication bias in the present meta-analysis (P = 0.150).
Figure 2.
No significant publication bias was found based on the Begg’s funnel plots. Each point represents an individual study for the indicated association. Log (OR), natural logarithm of OR. Horizontal line, mean effect size.
Figure 3.
No significant publication bias was found on the basis of Egger’s funnel plots. Each point represents an individual study for the indicated association. Log (OR), natural logarithm of OR. Horizontal line, mean effect size.
Discussion
To date, many epidemiological studies have evaluated the association of NAT1 polymorphism with the risk of cancer such as (lung cancer [11,17-19], colorectal cancer [22-27], head and neck cancer [43,45,46], pancreatic cancer [47-49] non-Hodgkin’s lymphoma [50-52], bladder cancer [55-59], prostate cancer [67,69,70], gastric cancer [34,74,75], breast cancer [76-79], but the results remain contradictory. Meta-analysis is a powerful method for the evaluation of effect size of numerous independent epidemiological studies based on statistical analysis, providing more reliable results than single study. To the best of our knowledge, this study is the first meta-analysis to date including the largest and most comprehensive assessments of the relationship between the NAT1 polymorphisms and cancer risk. No significant association between the NAT1 polymorphisms and cancer risk was identified in the present meta-analysis of 73 case-control studies including 24874 and 30226 control cases. In the stratified analysis by cancer types, no significant associations were found among studies on lung cancer, colorectal cancer, head and neck cancer, non-Hodgkin’s lymphoma, bladder cancer, prostate cancer, gastric cancer and breast cancer. However, we observed an increase risk in pancreatic cancer among the NAT1 rapid acetylator compared to the slow one. Our results are consistent with five previously pooled analysis on colorectal cancer [86,87], prostate cancer [88] and bladder cancer [89,90], in which no significant association was found between NAT1 polymorphisms and cancer risk. Inconsistent results among different studies on various cancers may be explained by the distinct role of NAT1 in different cell types and tissues. However, no significant association between the NAT1 phenotypes and cancer risk was detected in the present meta-analysis even when stratifying for race and study design.
Interestingly, analyses based on various cancer types in different ethnicities revealed that a significantly reduced risk of a head and neck cancer study among Asian (OR = 0.281, 95% CI = 0.127-0.622) was found. However, given the limited sample size, the result should be carefully interpreted and further validation in larger well-designed studies are highlighted. To date, numerous studies have been conducted to detect the overall effects of NAT1 polymorphisms on cancer susceptibilities. However, many studies generated conflicting results. Although negative association between NAT1 polymorphisms and cancer risk [12] has been reported, two independent studies [18,19] have observed a significant association of the NAT1 polymorphism with lung cancer risk. However these studies should be interpreted cautiously because these do not agree on the NAT1 risk genotype. Given that chemical compounds in tobacco are inactivated by phase II enzymes, it has been proposed that head and neck cancer risk could be modified by NAT genotypes. Head and neck cancer are strongly associated with smoking, and a few studies have explored the role of NAT1 polymorphisms in the risk of developing head and neck cancer in smokers. However, these findings are inconsistent. Either a decreased risk in carriers with the variant NAT1*10 [91] or a lack of association between NAT1 polymorphisms and the risk of head and neck cancer have been reported [43]. The NAT1*10 variant was associated with increased risk of breast cancer among women who consumed well-done meat [78]. The other study, however, reported that no significant association of NAT polymorphisms and breast cancer risk was identified [92]. First, ethnic differences of NAT1 polymorphisms may contribute to the discrepancy of these results. In addition, the influence of genetic variants may be masked by other as-yet-unidentified causal genes involved in carcinogenesis, because gene-to-gene and gene-to-environment interactions have been of great interest to evaluate the exact roles of genetic polymorphisms in carcinogenesis. However, lack of the original data limited our further evaluation of potential gene-to-gene and gene-to-environment interactions and to validate the influence of ethnic differences on the effects of functional polymorphism on cancer risk.
In addition, analysis based on cancer types stratified by the source of controls indicated only significantly reduced risk of colorectal cancer and pancreatic cancer in studies using hospital-based controls. However, these hospital-based studies may have biases because certain benign diseases that have different risk of developing malignancy can be included in such controls and they are not the best representative of general population. Thus, the use of a proper and representative cancer-free control subjects is critically important for reducing study biases in such case-control studies.
The present meta-analysis has some limitations. First, lack of the original data of the reviewed studies limited our evaluation on the potential both gene-gene and gene-environment interactions. Second, the controls were not uniformly defined. Some studies employed a healthy population as the reference group, whereas others used hospital patients without gastric cancer as the reference group. Thus, the controls may not always truly represent the underlying source populations. In addition, our meta-analysis was based on unadjusted OR estimates because not all published studies were presented with adjusted ORs. ORs were provided in some other studies, however, the ORs were not adjusted by the same potential confounders. Fourth, we only considered the NAT1 metabolic enzyme. Because NAT2 enzyme is involved in the bioactivation and detoxification of heterocyclic amine, it may also play a role in modifying cancer risk, this may increase the misclassification of measured variables. Therefore, these results should be interpreted cautiously.
In summary, the present meta-analysis suggests no significant association between NAT1 slow genotype and cancer risk except for pancreatic cancer. However, we observed a reduce risk in pancreatic cancer among the NAT1 slow acetylators. Further studies evaluating the effects of gene-gene and gene-environment interactions may eventually lead to a better and more comprehensive understanding of the association between NAT1 genotypes and cancer risk.
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bredberg A. Cancer: more of polygenic disease and less of multiple mutations? A quantitative viewpoint. Cancer. 2011;117:440–445. doi: 10.1002/cncr.25440. [DOI] [PubMed] [Google Scholar]
- 3.Lin BK, Clyne M, Walsh M, Gomez O, Yu W, Gwinn M, Khoury MJ. Tracking the epidemiology of human genes in the literature: the HuGE Published Literature database. Am J Epidemiol. 2006;164:1–4. doi: 10.1093/aje/kwj175. [DOI] [PubMed] [Google Scholar]
- 4.Hudson TJ. Genome variation and personalized cancer medicine. J Intern Med. 2013;274:440–450. doi: 10.1111/joim.12097. [DOI] [PubMed] [Google Scholar]
- 5.Blum M, Grant DM, McBride W, Heim M, Meyer UA. Human arylamine N-acetyltransferase genes: isolation, chromosomal localization, and functional expression. Dna Cell Biol. 1990;9:193–203. doi: 10.1089/dna.1990.9.193. [DOI] [PubMed] [Google Scholar]
- 6.Hein DW. Molecular genetics and function of NAT1 and NAT2: role in aromatic amine metabolism and carcinogenesis. Mutat Res. 2002;506-507:65–77. doi: 10.1016/s0027-5107(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhong X, Hui C, Xiao-Ling W, Yan L, Na L. NAT2 polymorphism and gastric cancer susceptibility: a meta-analysis. Arch Med Res. 2010;41:275–280. doi: 10.1016/j.arcmed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Cui D, Wang Z, Zhao E, Ma J, Lu W. NAT2 polymorphism and lung cancer risk: a meta-analysis. Lung Cancer-J Iaslc. 2011;73:153–157. doi: 10.1016/j.lungcan.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Gong C, Hu X, Gao Y, Cao Y, Gao F, Mo Z. A meta-analysis of the NAT1 and NAT2 polymorphisms and prostate cancer: a huge review. Med Oncol. 2011;28:365–376. doi: 10.1007/s12032-010-9423-5. [DOI] [PubMed] [Google Scholar]
- 10.Liu J, Ding D, Wang X, Chen Y, Li R, Zhang Y, Luo R. N-acetyltransferase polymorphism and risk of colorectal adenoma and cancer: a pooled analysis of variations from 59 studies. PLoS One. 2012;7:e42797. doi: 10.1371/journal.pone.0042797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zienolddiny S, Campa D, Lind H, Ryberg D, Skaug V, Stangeland LB, Canzian F, Haugen A. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of non-small cell lung cancer in smokers. Carcinogenesis. 2008;29:1164–1169. doi: 10.1093/carcin/bgn020. [DOI] [PubMed] [Google Scholar]
- 12.Perera FP, Tang D, Brandt-Rauf P, Santella RM, Mooney LV, Tu YH, Bendkowska I, Bell DA. Lack of associations among cancer and albumin adducts, ras p21 oncoprotein levels, and CYP1A1, CYP2D6, NAT1, and NAT2 in a nested case-control study of lung cancer within the physicians’ health study. Cancer Epidemiol Biomarkers Prev. 2006;15:1417–1419. doi: 10.1158/1055-9965.EPI-05-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petitti DB. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. Oxford University Press; 1999. [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 16.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdel-Rahman SZ, El-Zein RA, Zwischenberger JB, Au WW. Association of the NAT1*10 genotype with increased chromosome aberrations and higher lung cancer risk in cigarette smokers. Mutat Res. 1998;398:43–54. doi: 10.1016/s0027-5107(97)00238-8. [DOI] [PubMed] [Google Scholar]
- 18.Bouchardy C, Mitrunen K, Wikman H, Husgafvel-Pursiainen K, Dayer P, Benhamou S, Hirvonen A. N-acetyltransferase NAT1 and NAT2 genotypes and lung cancer risk. Pharmacogenetics. 1998;8:291–298. doi: 10.1097/00008571-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Wikman H, Thiel S, Jager B, Schmezer P, Spiegelhalder B, Edler L, Dienemann H, Kayser K, Schulz V, Drings P, Bartsch H, Risch A. Relevance of N-acetyltransferase 1 and 2 (NAT1, NAT2) genetic polymorphisms in non-small cell lung cancer susceptibility. Pharmacogenetics. 2001;11:157–168. doi: 10.1097/00008571-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Ishibe N, Wiencke JK, Zuo ZF, McMillan A, Spitz MR, Kelsey KT. Polymorphisms in the N acetyltransferase 1 NAT1 gene and lung cancer risk in a minority population. Biomarkers. 1998;3:219–226. doi: 10.1080/135475098231237. [DOI] [PubMed] [Google Scholar]
- 21.Eichholzer M, Rohrmann S, Barbir A, Hermann S, Teucher B, Kaaks R, Linseisen J. Polymorphisms in heterocyclic aromatic amines metabolism-related genes are associated with colorectal adenoma risk. Int J Mol Epidemiol Genet. 2012;3:96–106. [PMC free article] [PubMed] [Google Scholar]
- 22.Cleary SP, Cotterchio M, Shi E, Gallinger S, Harper P. Cigarette smoking, genetic variants in carcinogen-metabolizing enzymes, and colorectal cancer risk. Am J Epidemiol. 2010;172:1000–1014. doi: 10.1093/aje/kwq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nothlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, Henderson BE, Kolonel LN, Le Marchand L. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2098–2106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen M, Autrup H, Olsen A, Tjonneland A, Overvad K, Raaschou-Nielsen O. Prospective study of NAT1 and NAT2 polymorphisms, tobacco smoking and meat consumption and risk of colorectal cancer. Cancer Lett. 2008;266:186–193. doi: 10.1016/j.canlet.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Butler LM, Millikan RC, Sinha R, Keku TO, Winkel S, Harlan B, Eaton A, Gammon MD, Sandler RS. Modification by N-acetyltransferase 1 genotype on the association between dietary heterocyclic amines and colon cancer in a multiethnic study. Mutat Res. 2008;638:162–174. doi: 10.1016/j.mrfmmm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahid SS, Colliver DW, Crawford NP, Martini BD, Doll MA, Hein DW, Cobbs GA, Petras RE, Galandiuk S. Characterization of N-acetyltransferase 1 and 2 polymorphisms and haplotype analysis for inflammatory bowel disease and sporadic colorectal carcinoma. BMC Med Genet. 2007;8:28. doi: 10.1186/1471-2350-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lilla C, Verla-Tebit E, Risch A, Jager B, Hoffmeister M, Brenner H, Chang-Claude J. Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer Epidemiol Biomarkers Prev. 2006;15:99–107. doi: 10.1158/1055-9965.EPI-05-0618. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Jiang QT, He HQ. Relationship between metabolic enzyme polymorphism and colorectal cancer. World J Gastroenterol. 2005;11:331–335. doi: 10.3748/wjg.v11.i3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landi S, Gemignani F, Moreno V, Gioia-Patricola L, Chabrier A, Guino E, Navarro M, de Oca J, Capella G, Canzian F. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;15:535–546. doi: 10.1097/01.fpc.0000165904.48994.3d. [DOI] [PubMed] [Google Scholar]
- 30.Kiss I, Nemeth A, Bogner B, Pajkos G, Orsos Z, Sandor J, Csejtey A, Faluhelyi Z, Rodler I, Ember I. Polymorphisms of glutathione-S-transferase and arylamine N-acetyltransferase enzymes and susceptibility to colorectal cancer. Anticancer Res. 2004;24:3965–3970. [PubMed] [Google Scholar]
- 31.van der Hel OL, Bueno DMH, Roest M, Slothouber B, van Gils C, van Noord PA, Grobbee DE, Peeters PH. No modifying effect of NAT1, GSTM1, and GSTT1 on the relation between smoking and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:681–682. [PubMed] [Google Scholar]
- 32.Zhang Y, Shi Z, Deng C, Zhu Y, Zhou Y. [The research the relationship between N-acetyltransferase 1 gene polymorphisms and genetic susceptibility to sporadic colorectal adenocarcinoma] . Zhonghua Nei Ke Za Zhi. 2002;41:746–749. [PubMed] [Google Scholar]
- 33.Tiemersma EW, Kampman E, Bueno DM, Bunschoten A, van Schothorst EM, Kok FJ, Kromhout D. Meat consumption, cigarette smoking, and genetic susceptibility in the etiology of colorectal cancer: results from a Dutch prospective study. Cancer Causes Control. 2002;13:383–393. doi: 10.1023/a:1015236701054. [DOI] [PubMed] [Google Scholar]
- 34.Katoh T, Boissy R, Nagata N, Kitagawa K, Kuroda Y, Itoh H, Kawamoto T, Bell DA. Inherited polymorphism in the N-acetyltransferase 1 (NAT1) and 2 (NAT2) genes and susceptibility to gastric and colorectal adenocarcinoma. Int J Cancer. 2000;85:46–49. doi: 10.1002/(sici)1097-0215(20000101)85:1<46::aid-ijc8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Yeh CC, Sung FC, Tang R, Chang-Chieh CR, Hsieh LL. Association between polymorphisms of biotransformation and DNA-repair genes and risk of colorectal cancer in Taiwan. J Biomed Sci. 2007;14:183–193. doi: 10.1007/s11373-006-9139-x. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Stampfer MJ, Hough HL, Garcia-Closas M, Willett WC, Hennekens CH, Kelsey KT, Hunter DJ. A prospective study of N-acetyltransferase genotype, red meat intake, and risk of colorectal cancer. Cancer Res. 1998;58:3307–3311. [PubMed] [Google Scholar]
- 37.Moslehi R, Chatterjee N, Church TR, Chen J, Yeager M, Weissfeld J, Hein DW, Hayes RB. Cigarette smoking, N-acetyltransferase genes and the risk of advanced colorectal adenoma. Pharmacogenomics. 2006;7:819–829. doi: 10.2217/14622416.7.6.819. [DOI] [PubMed] [Google Scholar]
- 38.Ishibe N, Sinha R, Hein DW, Kulldorff M, Strickland P, Fretland AJ, Chow WH, Kadlubar FF, Lang NP, Rothman N. Genetic polymorphisms in heterocyclic amine metabolism and risk of colorectal adenomas. Pharmacogenetics. 2002;12:145–150. doi: 10.1097/00008571-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Probst-Hensch NM, Haile RW, Li DS, Sakamoto GT, Louie AD, Lin BK, Frankl HD, Lee ER, Lin HJ. Lack of association between the polyadenylation polymorphism in the NAT1 (acetyltransferase 1) gene and colorectal adenomas. Carcinogenesis. 1996;17:2125–2129. doi: 10.1093/carcin/17.10.2125. [DOI] [PubMed] [Google Scholar]
- 40.Le Marchand L, Hankin JH, Wilkens LR, Pierce LM, Franke A, Kolonel LN, Seifried A, Custer LJ, Chang W, Lum-Jones A, Donlon T. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1259–1266. [PubMed] [Google Scholar]
- 41.Kampman E, Slattery ML, Bigler J, Leppert M, Samowitz W, Caan BJ, Potter JD. Meat consumption, genetic susceptibility, and colon cancer risk: a United States multicenter case-control study. Cancer Epidemiol Biomarkers Prev. 1999;8:15–24. [PubMed] [Google Scholar]
- 42.Demokan S, Suoglu Y, Gozeler M, Demir D, Dalay N. N-acetyltransferase 1 and 2 gene sequence variants and risk of head and neck cancer. Mol Biol Rep. 2010;37:3217–3226. doi: 10.1007/s11033-009-9905-8. [DOI] [PubMed] [Google Scholar]
- 43.Fronhoffs S, Bruning T, Ortiz-Pallardo E, Brode P, Koch B, Harth V, Sachinidis A, Bolt HM, Herberhold C, Vetter H, Ko Y. Real-time PCR analysis of the N-acetyltransferase NAT1 allele *3, *4, *10, *11, *14 and *17 polymorphism in squamous cell cancer of head and neck. Carcinogenesis. 2001;22:1405–1412. doi: 10.1093/carcin/22.9.1405. [DOI] [PubMed] [Google Scholar]
- 44.Olshan AF, Weissler MC, Watson MA, Bell DA. GSTM1, GSTT1, GSTP1, CYP1A1, and NAT1 polymorphisms, tobacco use, and the risk of head and neck cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:185–191. [PubMed] [Google Scholar]
- 45.Majumder M, Ghosh S, Roy B. Association between polymorphisms at N-acetyltransferase 1 (NAT1) & risk of oral leukoplakia & cancer. Indian J Med Res. 2012;136:605–613. [PMC free article] [PubMed] [Google Scholar]
- 46.Katoh T, Kaneko S, Boissy R, Watson M, Ikemura K, Bell DA. A pilot study testing the association between N-acetyltransferases 1 and 2 and risk of oral squamous cell carcinoma in Japanese people. Carcinogenesis. 1998;19:1803–1807. doi: 10.1093/carcin/19.10.1803. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki H, Morris JS, Li Y, Doll MA, Hein DW, Liu J, Jiao L, Hassan MM, Day RS, Bondy ML, Abbruzzese JL, Li D. Interaction of the cytochrome P4501A2, SULT1A1 and NAT gene polymorphisms with smoking and dietary mutagen intake in modification of the risk of pancreatic cancer. Carcinogenesis. 2008;29:1184–1191. doi: 10.1093/carcin/bgn085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li D, Jiao L, Li Y, Doll MA, Hein DW, Bondy ML, Evans DB, Wolff RA, Lenzi R, Pisters PW, Abbruzzese JL, Hassan MM. Polymorphisms of cytochrome P4501A2 and N-acetyltransferase genes, smoking, and risk of pancreatic cancer. Carcinogenesis. 2006;27:103–111. doi: 10.1093/carcin/bgi171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiao L, Doll MA, Hein DW, Bondy ML, Hassan MM, Hixson JE, Abbruzzese JL, Li D. Haplotype of N-acetyltransferase 1 and 2 and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2379–2386. doi: 10.1158/1055-9965.EPI-06-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiu BC, Kolar C, Gapstur SM, Lawson T, Anderson JR, Weisenburger DD. Association of NAT and GST polymorphisms with non-Hodgkin’s lymphoma: a population-based case-control study. Br J Haematol. 2005;128:610–615. doi: 10.1111/j.1365-2141.2004.05358.x. [DOI] [PubMed] [Google Scholar]
- 51.Kilfoy BA, Zheng T, Lan Q, Han X, Holford T, Hein DW, Qin Q, Leaderer B, Morton LM, Yeager M, Boyle P, Zhao P, Chanock S, Rothman N, Zhang Y. Genetic variation in N- acetyltransferases 1 and 2, cigarette smoking, and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2010;21:127–133. doi: 10.1007/s10552-009-9442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morton LM, Schenk M, Hein DW, Davis S, Zahm SH, Cozen W, Cerhan JR, Hartge P, Welch R, Chanock SJ, Rothman N, Wang SS. Genetic variation in N-acetyltransferase 1 (NAT1) and 2 (NAT2) and risk of non-Hodgkin lymphoma. Pharmacogenet Genomics. 2006;16:537–545. doi: 10.1097/01.fpc.0000215071.59836.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerridge I, Lincz L, Scorgie F, Hickey D, Granter N, Spencer A. Association between xenobiotic gene polymorphisms and non-Hodgkin’s lymphoma risk. Br J Haematol. 2002;118:477–481. doi: 10.1046/j.1365-2141.2002.03606.x. [DOI] [PubMed] [Google Scholar]
- 54.Aschebrook-Kilfoy B, Ollberding NJ, Kolar C, Lawson TA, Smith SM, Weisenburger DD, Chiu BC. Meat intake and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2012;23:1681–1692. doi: 10.1007/s10552-012-0047-2. [DOI] [PubMed] [Google Scholar]
- 55.Koutros S, Silverman DT, Baris D, Zahm SH, Morton LM, Colt JS, Hein DW, Moore LE, Johnson A, Schwenn M, Cherala S, Schned A, Doll MA, Rothman N, Karagas MR. Hair dye use and risk of bladder cancer in the New England bladder cancer study. Int J Cancer. 2011;129:2894–2904. doi: 10.1002/ijc.26245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Covolo L, Placidi D, Gelatti U, Carta A, Scotto DCA, Lodetti P, Picciche A, Orizio G, Campagna M, Arici C, Porru S. Bladder cancer, GSTs, NAT1, NAT2, SULT1A1, XRCC1, XRCC3, XPD genetic polymorphisms and coffee consumption: a case-control study. Eur J Epidemiol. 2008;23:355–362. doi: 10.1007/s10654-008-9238-2. [DOI] [PubMed] [Google Scholar]
- 57.McGrath M, Michaud D, De Vivo I. Polymorphisms in GSTT1, GSTM1, NAT1 and NAT2 genes and bladder cancer risk in men and women. Bmc Cancer. 2006;6:239. doi: 10.1186/1471-2407-6-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu J, Liang D, Wang Y, Lu C, Wu X. Effects of N-acetyl transferase 1 and 2 polymorphisms on bladder cancer risk in Caucasians. Mutat Res. 2005;581:97–104. doi: 10.1016/j.mrgentox.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Closas M, Malats N, Silverman D, Dosemeci M, Kogevinas M, Hein DW, Tardon A, Serra C, Carrato A, Garcia-Closas R, Lloreta J, Castano-Vinyals G, Yeager M, Welch R, Chanock S, Chatterjee N, Wacholder S, Samanic C, Tora M, Fernandez F, Real FX, Rothman N. NAT2 slow acetylation, GSTM1 null genotype, and risk of bladder cancer: results from the Spanish Bladder Cancer Study and meta-analyses. Lancet. 2005;366:649–659. doi: 10.1016/S0140-6736(05)67137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung RJ, Boffetta P, Brennan P, Malaveille C, Hautefeuille A, Donato F, Gelatti U, Spaliviero M, Placidi D, Carta A, Scotto DCA, Porru S. GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer. 2004;110:598–604. doi: 10.1002/ijc.20157. [DOI] [PubMed] [Google Scholar]
- 61.Schroeder JC, Conway K, Li Y, Mistry K, Bell DA, Taylor JA. p53 mutations in bladder cancer: evidence for exogenous versus endogenous risk factors. Cancer Res. 2003;63:7530–7538. [PubMed] [Google Scholar]
- 62.Stern MC, Johnson LR, Bell DA, Taylor JA. XPD codon 751 polymorphism, metabolism genes, smoking, and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2002;11:1004–1011. [PubMed] [Google Scholar]
- 63.Cascorbi I, Roots I, Brockmoller J. Association of NAT1 and NAT2 polymorphisms to urinary bladder cancer: significantly reduced risk in subjects with NAT1*10. Cancer Res. 2001;61:5051–5056. [PubMed] [Google Scholar]
- 64.Hsieh FI, Pu YS, Chern HD, Hsu LI, Chiou HY, Chen CJ. Genetic polymorphisms of N-acetyltransferase 1 and 2 and risk of cigarette smoking-related bladder cancer. Br J Cancer. 1999;81:537–541. doi: 10.1038/sj.bjc.6690727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor JA, Umbach DM, Stephens E, Castranio T, Paulson D, Robertson C, Mohler JL, Bell DA. The role of N-acetylation polymorphisms in smoking-associated bladder cancer: evidence of a gene-gene-exposure three-way interaction. Cancer Res. 1998;58:3603–3610. [PubMed] [Google Scholar]
- 66.Okkels H, Sigsgaard T, Wolf H, Autrup H. Arylamine N-acetyltransferase 1 (NAT1) and 2 (NAT2) polymorphisms in susceptibility to bladder cancer: the influence of smoking. Cancer Epidemiol Biomarkers Prev. 1997;6:225–231. [PubMed] [Google Scholar]
- 67.Kidd LR, Hein DW, Woodson K, Taylor PR, Albanes D, Virtamo J, Tangrea JA. Lack of association of the N-acetyltransferase NAT1*10 allele with prostate cancer incidence, grade, or stage among smokers in Finland. Biochem Genet. 2011;49:73–82. doi: 10.1007/s10528-010-9386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma S, Cao X, Wilkens LR, Yamamoto J, Lum-Jones A, Henderson BE, Kolonel LN, Le Marchand L. Well-done meat consumption, NAT1 and NAT2 acetylator genotypes and prostate cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2010;19:1866–1870. doi: 10.1158/1055-9965.EPI-10-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iguchi T, Sugita S, Wang CY, Newman NB, Nakatani T, Haas GP. MnSOD genotype and prostate cancer risk as a function of NAT genotype and smoking status. In Vivo. 2009;23:7–12. [PMC free article] [PubMed] [Google Scholar]
- 70.Hein DW, Leff MA, Ishibe N, Sinha R, Frazier HA, Doll MA, Xiao GH, Weinrich MC, Caporaso NE. Association of prostate cancer with rapid N-acetyltransferase 1 (NAT1*10) in combination with slow N-acetyltransferase 2 acetylator genotypes in a pilot case-control study. Environ Mol Mutagen. 2002;40:161–167. doi: 10.1002/em.10103. [DOI] [PubMed] [Google Scholar]
- 71.Fukutome K, Watanabe M, Shiraishi T, Murata M, Uemura H, Kubota Y, Kawamura J, Ito H, Yatani R. N-acetyltransferase 1 genetic polymorphism influences the risk of prostate cancer development. Cancer Lett. 1999;136:83–87. doi: 10.1016/s0304-3835(98)00311-5. [DOI] [PubMed] [Google Scholar]
- 72.Costa S, Pinto D, Morais A, Vasconcelos A, Oliveira J, Lopes C, Medeiros R. Acetylation genotype and the genetic susceptibility to prostate cancer in a southern European population. Prostate. 2005;64:246–252. doi: 10.1002/pros.20241. [DOI] [PubMed] [Google Scholar]
- 73.Rovito PJ, Morse PD, Spinek K, Newman N, Jones RF, Wang CY, Haas GP. Heterocyclic amines and genotype of N-acetyltransferases as risk factors for prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:69–74. doi: 10.1038/sj.pcan.4500780. [DOI] [PubMed] [Google Scholar]
- 74.Wideroff L, Vaughan TL, Farin FM, Gammon MD, Risch H, Stanford JL, Chow WH. GST, NAT1, CYP1A1 polymorphisms and risk of esophageal and gastric adenocarcinomas. Cancer Detect Prev. 2007;31:233–236. doi: 10.1016/j.cdp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boissy RJ, Watson MA, Umbach DM, Deakin M, Elder J, Strange RC, Bell DA. A pilot study investigating the role of NAT1 and NAT2 polymorphisms in gastric adenocarcinoma. Int J Cancer. 2000;87:507–511. [PubMed] [Google Scholar]
- 76.van der Hel OL, Peeters PH, Hein DW, Doll MA, Grobbee DE, Kromhout D, Bueno DMH. NAT2 slow acetylation and GSTM1 null genotypes may increase postmenopausal breast cancer risk in long-term smoking women. Pharmacogenetics. 2003;13:399–407. doi: 10.1097/00008571-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 77.Lee KM, Park SK, Kim SU, Doll MA, Yoo KY, Ahn SH, Noh DY, Hirvonen A, Hein DW, Kang D. N-acetyltransferase (NAT1, NAT2) and glutathione S-transferase (GSTM1, GSTT1) polymorphisms in breast cancer. Cancer Lett. 2003;196:179–186. doi: 10.1016/s0304-3835(03)00311-2. [DOI] [PubMed] [Google Scholar]
- 78.Krajinovic M, Ghadirian P, Richer C, Sinnett H, Gandini S, Perret C, Lacroix A, Labuda D, Sinnett D. Genetic susceptibility to breast cancer in French-Canadians: role of carcinogen-metabolizing enzymes and gene-environment interactions. Int J Cancer. 2001;92:220–225. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1184>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 79.Millikan RC. NAT1*10 and NAT1*11 polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:217–219. [PubMed] [Google Scholar]
- 80.Zheng W, Deitz AC, Campbell DR, Wen WQ, Cerhan JR, Sellers TA, Folsom AR, Hein DW. N-acetyltransferase 1 genetic polymorphism, cigarette smoking, well-done meat intake, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1999;8:233–239. [PubMed] [Google Scholar]
- 81.Muller P, Asher N, Heled M, Cohen SB, Risch A, Rund D. Polymorphisms in transporter and phase II metabolism genes as potential modifiers of the predisposition to and treatment outcome of de novo acute myeloid leukemia in Israeli ethnic groups. Leuk Res. 2008;32:919–929. doi: 10.1016/j.leukres.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 82.Krajinovic M, Richer C, Sinnett H, Labuda D, Sinnett D. Genetic polymorphisms of N-acetyltransferases 1 and 2 and gene-gene interaction in the susceptibility to childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev. 2000;9:557–562. [PubMed] [Google Scholar]
- 83.Zhang XF, Bian JC, Zhang XY, Zhang ZM, Jiang F, Wang QM, Wang QJ, Cao YY, Tang BM. Are polymorphisms of N-acetyltransferase genes susceptible to primary liver cancer in Luoyang, China? World J Gastroenterol. 2005;11:1457–1462. doi: 10.3748/wjg.v11.i10.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu MW, Pai CI, Yang SY, Hsiao TJ, Chang HC, Lin SM, Liaw YF, Chen PJ, Chen CJ. Role of N-acetyltransferase polymorphisms in hepatitis B related hepatocellular carcinoma: impact of smoking on risk. Gut. 2000;47:703–709. doi: 10.1136/gut.47.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lincz LF, Kerridge I, Scorgie FE, Bailey M, Enno A, Spencer A. Xenobiotic gene polymorphisms and susceptibility to multiple myeloma. Haematologica. 2004;89:628–629. [PubMed] [Google Scholar]
- 86.Liu J, Ding D, Wang X, Chen Y, Li R, Zhang Y, Luo R. N-acetyltransferase polymorphism and risk of colorectal adenoma and cancer: a pooled analysis of variations from 59 studies. PLoS One. 2012;7:e42797. doi: 10.1371/journal.pone.0042797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cai J, Zhao Y, Zhu CL, Li J, Huang ZH. The association of NAT1 polymorphisms and colorectal carcinoma risk: evidence from 20,000 subjects. Mol Biol Rep. 2012;39:7497–7503. doi: 10.1007/s11033-012-1583-2. [DOI] [PubMed] [Google Scholar]
- 88.Gong C, Hu X, Gao Y, Cao Y, Gao F, Mo Z. A meta-analysis of the NAT1 and NAT2 polymorphisms and prostate cancer: a huge review. Med Oncol. 2011;28:365–376. doi: 10.1007/s12032-010-9423-5. [DOI] [PubMed] [Google Scholar]
- 89.Wu K, Wang X, Xie Z, Liu Z, Lu Y. N-acetyltransferase 1 polymorphism and bladder cancer susceptibility: a meta-analysis of epidemiological studies. J Int Med Res. 2013;41:31–37. doi: 10.1177/0300060513476988. [DOI] [PubMed] [Google Scholar]
- 90.Sanderson S, Salanti G, Higgins J. Joint effects of the N-acetyltransferase 1 and 2 (NAT1 and NAT2) genes and smoking on bladder carcinogenesis: a literature-based systematic HuGE review and evidence synthesis. Am J Epidemiol. 2007;166:741–751. doi: 10.1093/aje/kwm167. [DOI] [PubMed] [Google Scholar]
- 91.McKay JD, Hashibe M, Hung RJ, Wakefield J, Gaborieau V, Szeszenia-Dabrowska N, Zaridze D, Lissowska J, Rudnai P, Fabianova E, Mates D, Foretova L, Janout V, Bencko V, Chabrier A, Hall J, Boffetta P, Canzian F, Brennan P. Sequence variants of NAT1 and NAT2 and other xenometabolic genes and risk of lung and aerodigestive tract cancers in Central Europe. Cancer Epidemiol Biomarkers Prev. 2008;17:141–147. doi: 10.1158/1055-9965.EPI-07-0553. [DOI] [PubMed] [Google Scholar]
- 92.Agundez JA. Polymorphisms of human N-acetyltransferases and cancer risk. Curr Drug Metab. 2008;9:520–531. doi: 10.2174/138920008784892083. [DOI] [PubMed] [Google Scholar]


