Abstract
TWIST, an epithelial-mesenchymal transition inducer, has been thought to play a critical role in the progression of a number of malignancies. Published studies reporting the association of TWIST expression with oral carcinoma risk has yielded conflicting results. Thus, we conducted a meta-analysis to address this controversy. After rigorous searching and screening, a total of seven studies were included. The results showed that the TWIST positive expression rate in oral cancer tissues was higher than that in the normal tissues. TWIST expression might have a correlation with clinical features such as low differentiation, advanced clinical stage, presence of lymph node metastasis and local recurrence, but not age, gender, T stage and smoking and drinking. The data suggested that TWIST might play critical roles in the cancer progression and act as a prognostic factor in oral cancer patients.
Keywords: TWIST, expression, metastasis, oral carcinoma, meta-analysis
Introduction
Oral carcinoma is a common malignancy of the upper respiratory tract that severely affects the life quality of patients, with compromise of ability to talk, drink and eat [1]. Complex interactions between many genetic and environmental factors might contribute to oral cancer risk. Previously, cigarette smoking, alcohol consumption [2], betel quid chewing, infection of human papilloma virus [3], diet low in nutritional value lacking vegetables and fruits and low socioeconomic status [4] are probably important etiological factors contributing to oral carcinoma. Besides, genetic variations also play important roles in the genesis of oral cancer [5]. Thus, the etiological factors for this cancer are complicated. To find new biomarkers for predicting the prognosis of oral cancer patients is required.
Previously, epithelial-mesenchymal transition (EMT), a key event of embryogenesis, has been shown to play a role in the development and progression of tumors [6]. EMT is an essential step for the formation of different organs during the process of embryonic development, while it may be inhibited for maintaining epithelial integrity and homeostasis in adult tissues [7]. Aberrant activation of EMT in epithelial tumors usually has been shown to have a correlation with tumor genesis. Several factors might be critical in the process of EMT. TWIST, a basic helix-loop-helix transcription factor, has been regarded as one of the important EMT inducers. In recent years, over-expression of TWIST has been detected in a number of cancers, such as gastric cancer, breast cancer and nasopharyngeal cancer, and might be associated with the development and unfavorable prognosis of these cancers [8-10]. On the basis of this understanding, TWIST has been regarded as a potential target for cancer therapy [11].
A number of studies have been devoted to the expression and significance of TWIST in oral carcinoma. However, the results were inconclusive. Thus, in the present study we conducted a quantitative meta-analysis containing published data up to Mar 2015 that increased statistical power to address this controversy.
Materials and methods
Literature search strategy
A systematic search was carried out in the databases such as Medline, EMBASE, and CNKI without a language limitation, covering all papers published up to Mar 2015. A combination of the following keywords was used: TWIST, EMT, neoplasm, tumor, cancer, head and neck, and oral. All searched studies were retrieved and the bibliographies were checked for other relevant publications. Review articles and bibliographies of other relevant studies identified were hand searched to find additional eligible studies.
Inclusion criteria
The following criteria were used for the literature selection: first, studies focused on the correlation between TWIST expression in primary oral cancer tissues and pathological features; second, papers stated detailed clinical data of cancer cases who were not subjected to radiotherapy or chemotherapy prior to selection; third, papers regarding TWIST, TWIST1 or TWIST2 were involved as TWIST expression. Fourth, only studies using immunohistochemistry were selected.
Accordingly, the exclusion criteria were used as follows: first, papers showed an inconsistent judgment standard for positive TWIST expression or TWIST expression in non-primary tumor tissues including those detected from the blood circulation of patients; second, studies concerned animal experiments or cell line cultures; third, reviews and duplicate publications. After rigorous searching, we reviewed all papers in accordance with the criteria defined above for further analysis.
Data extraction
Data were carefully extracted from all eligible publications independently by two of the authors according to the inclusion criteria mentioned above. For conflicting evaluations, an agreement was reached following a discussion. If a consensus could not be reached, another author was consulted to resolve the dispute and then a final decision was made by the majority of the votes. Extracted information was entered into a database.
Statistical analysis
The association of TWIST expression with clinical features was evaluated by the pooled odd ratio (ORs) and their 95% confidence interval (CIs). Hazard ratio (HRs) and their 95% CIs were used to evaluate the correlation between TWIST expression and the prognosis of patients with oral carcinoma. An HR > 1 was associated with poor outcome. HRs were estimated directly by the available data or estimated from the Kaplan-Meier curves according to the method raised by Tierney et al. [12] if they were not reported in the primary literature. A chi-square based Q statistic test was performed to assess heterogeneity. If a P value for a given Q-test was found to be more than 0.1, ORs were pooled according to a fixed-effect model (Mantel-Haenszel) [13]. Otherwise, a random-effect model (DerSimonian and laird) was used [14]. Publication bias was assessed by visual inspection of funnel plots [15], an asymmetrical plot indicated possible publication bias. Symmetry of the funnel plot was further evaluated by Egger’s linear regression test [16]. Statistical analysis was carried out using the program STATA 11.0 software (Stata Corporation, Texas).
Results
Study characteristics
Publications relevant to the key words were retrieved and screened originally. A total of forty-five publications were searched, of which twenty-nine irrelevant papers were excluded. Sixteen publications were preliminary eligible. Then, three review articles [17-19] and one study in which IHC was not used [20] were discarded. Thus, twelve articles were selected for data extraction. Afterwards, five papers were further excluded because they provided insufficient information [21-25]. Lastly, seven studies were selected for data extraction and assessment [26-32] (Figure 1).
Figure 1.
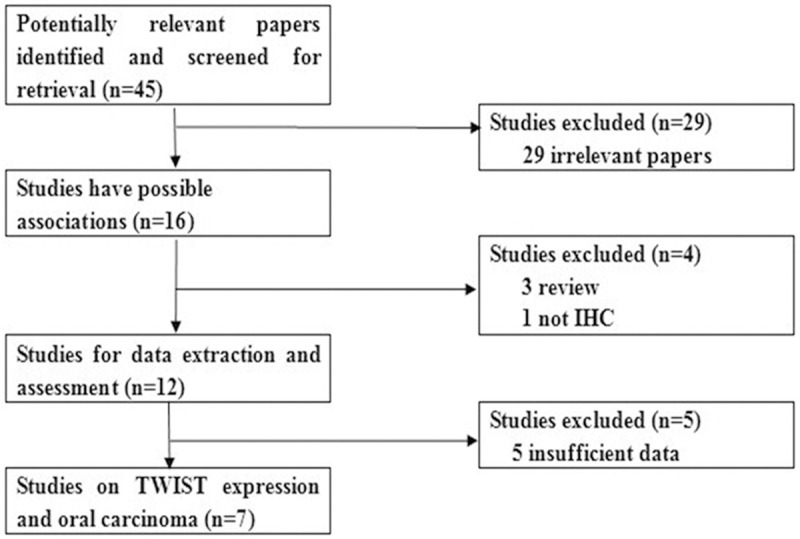
The flow diagram of included/excluded studies.
Of the seven studies, four were written in English [26,28-30] while the remaining three were in Chinese [27,31,32]. All the studies were conducted on Chinese population except for one study on Brazilians [30]. No studies on other ethnicities were included because relevant reports could not be found in our searching process. The relevant information was listed in Table 1. According to the lists, the first author and the number and characteristics of cases for each study as well as other necessary information were presented. Notably, information about HR was reported in two studies [26,29], while in another two studies [28,30], HRs were estimated from the Kaplan-Meier curves according to the method reported by Tierney et al. [12].
Table 1.
Characteristics of studies included in the present meta-analysis
| First Author | Year | Number of Patients | Measurement method | Cut-off of IHC | Method of quantification | Hazard ratio (95% CI) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| total | TWIST negative or low | TWIST positive or high | |||||||
| Liang | 2011 | 89 | 49 | 40 | IHC | ≥ 5% | Percentage of staining | 3.06 (1.36-6.89)* | (1) (2) (3) (4) (5) (8) (9) (10) (11) |
| Gong | 2012 | 62 | 14 | 48 | IHC | ≥ 5% | Percentage of staining | - | (1) (2) (4) (5) (6) (7) |
| Wang | 2012 | 60 | 30 | 30 | IHC | ≥ 4 | Sum of percentage and intensity | - | (1) (2) (3) (4) (5) |
| Wushou | 2012 | 60 | 18 | 42 | IHC | ≥ 3 | Sum of percentage and intensity | 4.65 (1.06-20.45) | (1) (2) (3) (4) (5) (6) (7) (8) (9) (11) |
| Zheng | 2012 | 69 | 20 | 49 | IHC | ≥ 1 | Sum of percentage and intensity | - | (1) (2) (4) (5) (6) (7) |
| Fan | 2013 | 114 | 40 | 74 | IHC | ≥ 2 | Sum of percentage and intensity | 0.89 (0.52-1.51) | (1) (2) (3) (4) (5) (6) (7) (8) (9) (11) |
| da Silva | 2014 | 52 | 30 | 22 | IHC | > 2 | Extent of staining | 4.28 (1.03-61.12)* | (3) (4) (5) (10) (11) |
Clinical features: (1) Age; (2) Gender; (3) T stage; (4) Differentiation; (5) Lymph node metastasis; (6) Clinical stage; (7) The control benign tissue; (8) Smoking; (9) Drinking. (10) Local recurrence; (11) Survival analysis.
Estimated from the Kaplan-Meier curves in the text.
Meta-analysis results
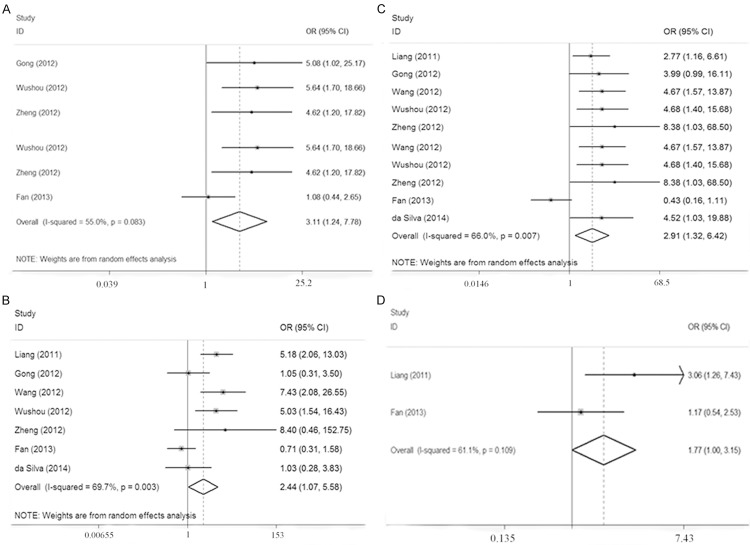
Table 2 lists the main results of the meta-analysis. Positive expression of TWIST in oral cancer tissues were significantly higher than that in the normal tissues (OR = 51.61, 95% CI = 13.72-194.15). No correlation was found between TWIST expression and several clinicopathological features, such as age, gender, smoking, drinking and T stage. Nevertheless, as shown in Figure 2, TWIST over-expression was correlated with clinical stage (III + IV vs I + II, OR = 3.11, 95% CI = 1.24-7.78) and differentiation (Low vs Moderate + High, OR = 2.44, 95% CI = 1.07-5.58), indicating that TWIST might have an association with the elevated levels of malignancy. In addition, TWIST over-expression has a correlation with lymph node metastasis (Yes vs No, OR = 2.91, 95% CI = 1.32-6.42), and local recurrence (Yes vs No, OR = 1.77, 95% CI = 1.00-3.15), suggesting that TWIST might contribute to cancer progression.
Table 2.
Main results of the meta-analysis
| Clinical features | Overall OR (95% CI) | P | Heterogeneity test | Number of studies | Egger test | Model | ||
|
|
|
|||||||
| Q | P | t | P | |||||
|
| ||||||||
| TWIST expression (Cancer vs Normal) | 51.61 (13.72-194.15) | < 0.05 | 0.32 | 0.956 | 4 | 2.70 | 0.114 | Fixed-effect |
| Age (≥ 60 vs < 60) | 1.00 (0.68-1.49) | > 0.05 | 6.38 | 0.271 | 6 | -0.30 | 0.778 | Fixed-effect |
| Gender (Male vs Female) | 1.15 (0.74-1.79) | > 0.05 | 2.44 | 0.786 | 6 | -1.03 | 0.361 | Fixed-effect |
| T stage (T3+T4 vs T1+T2) | 0.95 (0.48-1.85) | > 0.05 | 9.63 | 0.247 | 5 | 0.03 | 0.979 | Random-effect |
| Differentiation (Low vs Moderate+High) | 2.44 (1.07-5.58) | < 0.05 | 19.83 | 0.003 | 7 | 0.88 | 0.420 | Random-effect |
| Lymph node metastasis (Yes vs No) | 2.91 (1.32-6.42) | < 0.05 | 17.66 | 0.007 | 7 | 1.33 | 0.240 | Random-effect |
| Clinical stage (III+IV vs I+II) | 3.11 (1.24-7.78) | < 0.05 | 6.66 | 0.083 | 4 | 2.35 | 0.143 | Random-effect |
| Local recurrence (Yes vs No) | 1.77 (1.00-3.15) | < 0.05 | 2.57 | 0.109 | 2 | - | - | Fixed-effect |
| Smoking (Yes vs No) | 1.03 (0.62-1.72) | > 0.05 | 3.12 | 0.210 | 3 | - | - | Fixed-effect |
| Drinking (Yes vs No) | 0.86 (0.51-1.47) | > 0.05 | 0.85 | 0.655 | 3 | - | - | Fixed-effect |
|
| ||||||||
| Survival analysis | Overall HR (95%CI) | P | Heterogeneity test | Number of studies | Egger test | Model | ||
|
|
|
|||||||
| Q | P | t | P | |||||
|
| ||||||||
| TWIST (+) vs TWIST (-) | 2.25 (0.88-5.76)* | > 0.05 | 9.91 | 0.019 | 4 | 1.92 | 0.195 | Random-effect |
| 3.46 (1.77-6.77) | < 0.05 | 0.28 | 0.868 | 3 | - | - | Fixed-effect | |
The data were discarded after the sensitivity analysis.
Figure 2.
Forest plots showed that TWIST over-expression was associated with clinical stage (A), differentiation (B), lymph node metastasis (C) and local recurrence (D).
With respect to the prognostic value of TWIST for oral carcinoma, HRs for the overall survival were pooled. As shown in Table 2, the overall HR was 2.25 (95% CI = 0.88-5.76), with the data insignificant and the presence of evident heterogeneity (P = 0.019). However, when the sensitivity analysis was conducted, we found that HR from Fan et al [26] contributed to the unsteady of the overall results, and thus, the data was further omitted. Then, the pooled HR for overall survival was recalculated and the value was 3.46 (95% CI 1.77-6.77) with no heterogeneity (P = 0.868), implying that over-expression was a prognostic factor for oral carcinoma (Figure 3).
Figure 3.
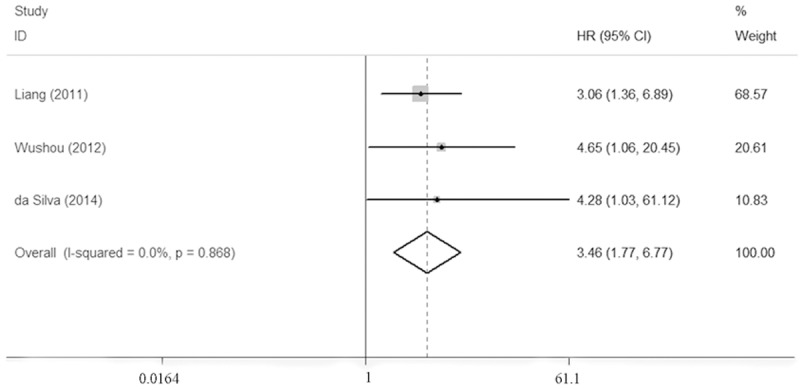
Forest plots showed that TWIST over-expression indicate a poor prognosis of patients with oral carcinoma.
One-way sensitivity analysis [33] was carried out to determine the stability of the above comparisons. The statistical significance of the results was not altered when any single study was deleted (data not shown), confirming the stability and credibility of the results.
Bias diagnostics
Funnel plots were created to detect possible publication bias. Then, Egger’s linear regression tests were used to assess the symmetries of the plots. For comparisons with limited number of included studies (< 4), the publication bias was not evaluated. As shown in Table 2, the Egger’s tests indicated that the potential publication bias was not significant for all comparisons, indicating that the publication bias might not have an evident effect on the results.
Discussion
In the present study, the results showed that TWIST over-expression might have an association with low differentiation, advanced clinical stage, presence of lymph node metastasis and local recurrence, indicating that TWIST expression might contribute to the development and progression of oral carcinoma. Moreover, TWIST might act as a prognostic factor for oral cancer.
Oral carcinoma can affect the life quality of patients because this cancer may directly influence the patients’ speaking and eating due to its specific site. The underlying mechanisms of oral cancer development are not clear. Recently, EMT has attracted much attention because this term describes a process in which epithelial cells lose their cell polarity and in the meantime gain mesenchymal characteristics, and thus, cancer cells become more malignant. This process can be induced in hypoxic microenvironment that is common to cancer cells [34], usually accompanied by a loss of cell-cell cohesiveness and enhanced cell migratory capacity [35]. Evidence suggests EMT as a key event in the development of head and neck cancer including oral carcinoma [19]. Therefore, the EMT pathway is regarded as a novel target for anti-cancer therapeutics [36].
In the present study, over-expression of TWIST was shown in cancerous tissues compared with normal tissues, indicating that TWIST might have a relation with the oral cancerigenesis. The results also showed that TWIST might contribute to the development of oral cancer because significant differences could be observed in the groups regarding low differentiation, advanced clinical stage, presence of lymph node metastasis and local recurrence, respectively. However, the mechanisms of TWIST in cancer progression have not been fully defined. Evidence indicated that TWIST can regulate gene expression and promote loss of cell-cell adhesion, thus leading to a shift in cytoskeletal dynamics and a change from epithelial morphology and physiology to the mesenchymal phenotype [37], and hence, the cancer cells acquired elevated malignant abilities. Moreover, Twist can also promote angiogenesis through recruitment of stromal macrophages [38] and up-regulation of MMP-9 expression [39]. The understandings might help clarify the possible relations of TWIST expression with advanced oral cancer stages. However, since the progression of oral cancer is a multi-step complicated process, future studies are needed to clarify the exact mechanisms.
Previous reports indicated that benzo(a)pyrene in tobacco might modulate TWIST expression and promote the migration and invasion of cancer cells [40]. In a bladder cancer study, tobacco use has been shown to correlate with up-regulated TWIST expression [41]. Thus, three of the included studies assessed the association of smoking with TWIST expression [26,28,29]. Nevertheless, no associations were found in this comparison, probably due to the limited sample sizes. The relationship between alcohol exposure and TWIST expression has rarely been reported in the literature. The results of the present meta-analysis failed to reveal a significant association between them. Another important epidemiological factor, betel quid chewing, has been shown to increase oral cancer risk, particularly in some regions of Asia. However, of the included studies, only one concerned this issue [26], and thus, this topic has not been assessed in the present analysis.
A total of four studies reported the survival data, and nevertheless, the HR values could be extracted directly from two papers [26,29] and indirectly estimated from the Kaplan-Meier curves in another two papers [28,30]. The pooled HRs for the overall survival failed to show a significant difference between TWIST positive cases and negative cases. However, after the sensitivity analysis, when the study by da Silva et al. [30] contributing to the evident heterogeneity was excluded, the results showed that patients with positive or high TWIST expression had a worse prognosis relative to the ones with negative or low TWIST expression (HR = 3.46, 95% CI = 1.77-6.77). However, since some data were estimated from the Kaplan-Meier curves, systematic errors were inevitable. Thus, any bias might exist and the results should be interpreted with caution.
Several limitations might be included in this study. First, only published data in Chinese and English were involved. Papers written in other languages were missed. Therefore, selection bias might exist. Second, the cut-off definition of TWIST appeared to be different in each study. This might also lead to any bias. Third, the selected studies focused on TWIST expression in tissues, instead of serum. Circulating prognostic markers seemed to be more valuable and convenient for detection throughout the life of patients. Therefore, further well-designed investigations testing circulating biomarkers might be of value and interest for oral cancer research. Furthermore, most included studies in this meta-analysis concerned Chinese population and only one concerned other ethnicities. Thus, the results might only be representative of a proportion of people in the world. Therefore, future primary research on various ethnicities is also required.
Despite the limitations, the data of the present meta-analysis showed a marked association of TWIST over-expression with low differentiation, advanced clinical stages, lymph node and local recurrence, suggesting that TWIST might play critical roles in the development of oral carcinoma. In addition, TWIST over-expression might predict poor survival in patients with oral carcinoma.
Acknowledgements
The present study was supported by Special Foundation of China Postdoctoral Science (2012T50851).
Disclosure of conflict of interest
None.
References
- 1.Furness S, Glenny AM, Worthington HV, Pavitt S, Oliver R, Clarkson JE, Macluskey M, Chan KK, Conway DI. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst Rev. 2011:CD006386. doi: 10.1002/14651858.CD006386.pub3. [DOI] [PubMed] [Google Scholar]
- 2.Zygogianni AG, Kyrgias G, Karakitsos P, Psyrri A, Kouvaris J, Kelekis N, Kouloulias V. Oral squamous cell cancer: early detection and the role of alcohol and smoking. Head Neck Oncol. 2011;3:2. doi: 10.1186/1758-3284-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lambert R, Sauvaget C, de Camargo Cancela M, Sankaranarayanan R. Epidemiology of cancer from the oral cavity and oropharynx. Eur J Gastroenterol Hepatol. 2011;23:633–641. doi: 10.1097/MEG.0b013e3283484795. [DOI] [PubMed] [Google Scholar]
- 4.Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade--an update (2000-2012) Asian Pac J Cancer Prev. 2013;14:5567–5577. doi: 10.7314/apjcp.2013.14.10.5567. [DOI] [PubMed] [Google Scholar]
- 5.Tan M, Myers JN, Agrawal N. Oral cavity and oropharyngeal squamous cell carcinoma genomics. Otolaryngol Clin North Am. 2013;46:545–566. doi: 10.1016/j.otc.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendt MK, Balanis N, Carlin CR, Schiemann WP. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3:e28975. doi: 10.4161/jkst.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macara IG, Guyer R, Richardson G, Huo Y, Ahmed SM. Epithelial Homeostasis. Curr Biol. 2014;24:R815–R825. doi: 10.1016/j.cub.2014.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao XH, Yang XQ, Wang BC, Liu SP, Wang FB. Overexpression of twist and matrix metalloproteinase-9 with metastasis and prognosis in gastric cancer. Asian Pac J Cancer Prev. 2013;14:5055–5060. doi: 10.7314/apjcp.2013.14.9.5055. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Ang L, Liu MQ, Hu HG, Wang J, Zou Q, Zhao Y, Zheng L, Zhao M, Wu ZS. Serum and tissue expression of gelatinase and Twist in breast cancer. Eur Rev Med Pharmacol Sci. 2014;18:2662–2669. [PubMed] [Google Scholar]
- 10.Zhuo X, Chang A, Huang C, Yang L, Xiang Z, Zhou Y. Expression of TWIST, an inducer of epithelial-mesenchymal transition, in nasopharyngeal carcinoma and its clinical significance. Int J Clin Exp Pathol. 2014;7:8862–8868. [PMC free article] [PubMed] [Google Scholar]
- 11.Khan MA, Chen HC, Zhang D, Fu J. Twist: a molecular target in cancer therapeutics. Tumour Biol. 2013;34:2497–2506. doi: 10.1007/s13277-013-1002-x. [DOI] [PubMed] [Google Scholar]
- 12.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry Res. 2004;129:39–44. doi: 10.1016/j.psychres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scanlon CS, Van Tubergen EA, Inglehart RC, D’Silva NJ. Biomarkers of epithelial-mesenchymal transition in squamous cell carcinoma. J Dent Res. 2013;92:114–121. doi: 10.1177/0022034512467352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu KJ, Yang MH. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Biosci Rep. 2011;31:449–455. doi: 10.1042/BSR20100114. [DOI] [PubMed] [Google Scholar]
- 19.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou C, Liu J, Tang Y, Zhu G, Zheng M, Jiang J, Yang J, Liang X. Coexpression of hypoxia-inducible factor-2alpha, TWIST2, and SIP1 may correlate with invasion and metastasis of salivary adenoid cystic carcinoma. J Oral Pathol Med. 2012;41:424–431. doi: 10.1111/j.1600-0714.2011.01114.x. [DOI] [PubMed] [Google Scholar]
- 21.Sakamoto K, Imanishi Y, Tomita T, Shimoda M, Kameyama K, Shibata K, Sakai N, Ozawa H, Shigetomi S, Fujii R, Fujii M, Ogawa K. Overexpression of SIP1 and downregulation of E-cadherin predict delayed neck metastasis in stage I/II oral tongue squamous cell carcinoma after partial glossectomy. Ann Surg Oncol. 2012;19:612–619. doi: 10.1245/s10434-011-2052-1. [DOI] [PubMed] [Google Scholar]
- 22.Silva BS, Yamamoto FP, Pontes FS, Cury SE, Fonseca FP, Pontes HA, Pinto-Junior DD. TWIST and p-Akt immunoexpression in normal oral epithelium, oral dysplasia and in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2012;17:e29–34. doi: 10.4317/medoral.17344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Freitas Silva BS, Yamamoto-Silva FP, Pontes HA, Pinto Junior Ddos S. E-cadherin downregulation and Twist overexpression since early stages of oral carcinogenesis. J Oral Pathol Med. 2014;43:125–131. doi: 10.1111/jop.12096. [DOI] [PubMed] [Google Scholar]
- 24.Jia J, Zhang W, Liu JY, Chen G, Liu H, Zhong HY, Liu B, Cai Y, Zhang JL, Zhao YF. Epithelial mesenchymal transition is required for acquisition of anoikis resistance and metastatic potential in adenoid cystic carcinoma. PLoS One. 2012;7:e51549. doi: 10.1371/journal.pone.0051549. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Wu T, Jia J, Xiong X, He H, Bu L, Zhao Z, Huang C, Zhang W. Increased expression of Lin28B associates with poor prognosis in patients with oral squamous cell carcinoma. PLoS One. 2013;8:e83869. doi: 10.1371/journal.pone.0083869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan CC, Wang TY, Cheng YA, Jiang SS, Cheng CW, Lee AY, Kao TY. Expression of E-cadherin, Twist, and p53 and their prognostic value in patients with oral squamous cell carcinoma. J Cancer Res Clin Oncol. 2013;139:1735–1744. doi: 10.1007/s00432-013-1499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Zhang C, Zhang S, Yue K, Wang X. Epithelial-Mesenchymal Transformation-Mediated Lymph Node Metastasis of Oral Squamous Cell Carcinoma and Its Mechanism. Chin J Clin Oncol. 2012;39:1877–1885. [Google Scholar]
- 28.Liang X, Zheng M, Jiang J, Zhu G, Yang J, Tang Y. Hypoxia-inducible factor-1 alpha, in association with TWIST2 and SNIP1, is a critical prognostic factor in patients with tongue squamous cell carcinoma. Oral Oncol. 2011;47:92–97. doi: 10.1016/j.oraloncology.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 29.Wushou A, Pan HY, Liu W, Tian Z, Wang LZ, Shali S, Zhang ZY. Correlation of increased twist with lymph node metastasis in patients with oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70:1473–1479. doi: 10.1016/j.joms.2011.06.212. [DOI] [PubMed] [Google Scholar]
- 30.da Silva SD, Alaoui-Jamali MA, Soares FA, Carraro DM, Brentani HP, Hier M, Rogatto SR, Kowalski LP. TWIST1 is a molecular marker for a poor prognosis in oral cancer and represents a potential therapeutic target. Cancer. 2014;120:352–362. doi: 10.1002/cncr.28404. [DOI] [PubMed] [Google Scholar]
- 31.Zheng J, Nan X. Expression and significance of TWIST in oral squamous cell carcinoma. Guide of China Med. 2012;10:212–213. [Google Scholar]
- 32.Gong Z, Yan Y. Expression of Twist and E-cadherin in tongue squamous cell carcinoma and its clinical significance. Anhui Med Pharm J. 2012;16:941–943. [Google Scholar]
- 33.Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Techn Bull. 1999;8:15–17. [Google Scholar]
- 34.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11:714–723. doi: 10.4161/cbt.11.8.15274. [DOI] [PubMed] [Google Scholar]
- 35.Steinestel K, Eder S, Schrader AJ, Steinestel J. Clinical significance of epithelial-mesenchymal transition. Clin Transl Med. 2014;3:17. doi: 10.1186/2001-1326-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moyret-Lalle C, Ruiz E, Puisieux A. Epithelial-mesenchymal transition transcription factors and miRNAs: “Plastic surgeons” of breast cancer. World J Clin Oncol. 2014;5:311–322. doi: 10.5306/wjco.v5.i3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–671. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Che N, Zhao XL, Sun T, Zhao XM, Gu Q, Dong XY, Zhao N, Liu YR, Yao Z, Sun BC. The role of Twist1 in hepatocellular carcinoma angiogenesis: a clinical study. Hum Pathol. 2011;42:840–847. doi: 10.1016/j.humpath.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Zhai W, Wang H, Xia X, Zhang C. Benzo(a)pyrene promotes A549 cell migration and invasion through up-regulating Twist. Arch Toxicol. 2015;89:451–458. doi: 10.1007/s00204-014-1269-8. [DOI] [PubMed] [Google Scholar]
- 41.Fondrevelle ME, Kantelip B, Reiter RE, Chopin DK, Thiery JP, Monnien F, Bittard H, Wallerand H. The expression of Twist has an impact on survival in human bladder cancer and is influenced by the smoking status. Urol Oncol. 2009;27:268–276. doi: 10.1016/j.urolonc.2007.12.012. [DOI] [PubMed] [Google Scholar]