Abstract
The study was designed to investigate the effect of various concentrations of cannabidiol (CBD) in rats with chronic epilepsy. The chronic epilepsy rat model was prepared by intraperitoneally injecting pentylenetetrazole to the rats pre-treated with CBD (10, 20 and 50 mg/kg) for 28 consecutive days. Behavioral measurements of convulsion following pentylenetetrazole treatment and morphological changes of the hippocampal neurons with hematoxylin and eosin staining were used to observe the epileptic behaviour. Immunohistochemistry was used to detect the expression levels of glial fibrillary acidic protein and inducible nitric oxide synthase (iNOS) in the hippocampus. The mRNA expression of N-methyl-D-aspartic acid (NMDA) receptor subunits (NR1 and NR2B) was detected by reverse transcription polymerase chain reaction. The results revealed a significant decrease in the daily average grade of epileptic seizures on treatment with CBD (50 mg/kg). The neuronal loss and astrocyte hyperplasia in the hippocampal area were also decreased. CBD treatment did not affect the expression of iNOS in the hippocampus; however, the expression of NR1 was decreased significantly. Thus, CBD administration inhibited the effect of pentylenetetrazole in rats, decreased the astrocytic hyperplasia, decreased neuronal damage in the hippocampus caused by seizures and selectively reduced the expression of the NR1 subunit of NMDA. Therefore, CBD exhibits an anticonvulsive effect in the rats with chronic epilepsy.
Keywords: Chronic epilepsy, anticonvulsive, astrocyte hyperplasia, hippocampus, pentylenetetrazole
Introduction
Epilepsy, a common disease of the nervous system has an incidence rate of 0.4-0.7% as per the epidemiology survey. Epilepsy is one of the most common diseases of the brain, affecting at least 50 million people globally [1]. There are some of the neurological disorders like cerebral tumors, arteriovenous malformation, cavernous hemangioma, craniocerebral injury and cerebral cortical dysplasia which are frequently complicated with epilepsy. It is reported that there is formation of lesions which damage the brain structure and impair the nerve conduction function [2]. About 30-40% of the supratentorial brain gliomas are complicated with epilepsy and the epilepsy incidence rate of low-grade gliomas is estimated to be 70% [2-5]. Despite development of a number of new antiepileptic drugs, epilepsy could not be significantly reduced and is a challenge to the clinicians [6,7]. For the discovery of novel antiepileptic drugs (AEDs) efficacy and safety is established in animal models [8]. For any given seizure type the more predictive the animal model, the greater is the likelihood of efficacy in human clinical trials [9].
Many plants, known for their anticonvulsant activity are subjected to phytochemical and pharmacological studies [10,11]. This process resulted in the identification of some potent anticonvulsant candidates. Cannabidiol (CBD, Figure 1) a constituent of the hemp seed exhibits potent anticonvulsant activity. CBD is produced and stored in the glandular structures of the plant and its concentration is higher in most fiber and oil varieties of hemp. The CBD possess anticonvulsive, anti-epileptic, and antimicrobial properties [12,13]. Although the specific physiological actions of CBD are yet to be determined, reports have confirmed that CBD inhibits maximal electroshock and pentylenetetrazole induced seizures in rat models [14-18]. It is believed that selective reduction of NMDA receptor [15,16] and the inhibition of nitric oxide synthase [14,18] may be responsible for the seizure inhibition. The present study was performed to examine the anticonvulsive effects of CBD in pentylenetetrazole-induced chronic epilepsy rat models.
Figure 1.
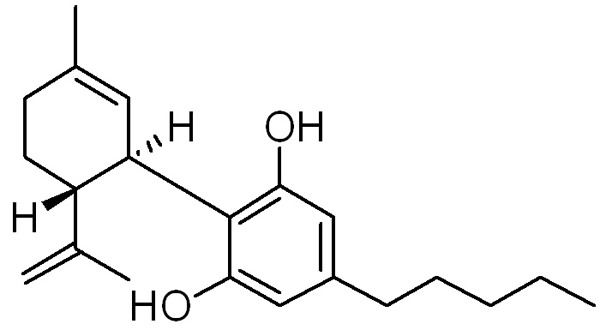
Structure of cannabidiol (CBD).
Materials and methods
Cannabidiol (CBD), pentylenetetrazole were purchased from Sigma (Sigma, St. Louis, MO, USA).
Animals
Male Sprague-Dawley rats were obtained from Chengdu Dashuo Biological Technology Co., Ltd., Chengdu, China. The animals weighed 170 ± 10 g and were acclimatized to the housing facilities before 7 days of use in the experiments. Rats were maintained on a 12 h light-dark cycle and had free access to standard diet and water. The use of experimental animals was performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The use was approved by the Local Committee on Animal Use and Protection.
Treatment strategy
For inducing convulsion the rats in CBD and model control groups were intraperitoneally administered pentylenetetrazole (35 mg/kg) daily for 28 consecutive days. The rats were randomly assigned to five groups with 8 each: i) normal control group, injected only saline; ii) model control group, received saline 1 h before intraperitoneal injection of pentylenetetrazole; iii) 10 mg/kg CBD group, received pentylenetetrazole + 10 mg/kg CBD; iv) 20 mg/kg CBD group, given pentylenetetrazole + 20 mg/kg CBD, and v) 50 mg/kg CBD group, given pentylenetetrazole + 50 mg/kg CBD.
Behavioral observations of convulsion
After pentylenetetrazole injection the behavior of each animal was observed for 1 h on five-point scale. The seizure activity was scored as: no response, stage 0; ear and facial twitching, stage 1; convulsive wave throughout the body, stage 2; myoclonic jerks, stage 3; turn onto their side, stage 4; and turn over onto their back, stage 5. For each of the animal the grade of convulsion was recorded daily. The rats with stable epileptic behavior for 5 days were considered as kindling rats and for such animals kindling rate was calculated.
Sample preparation
On completion of the treatment the animals were sacrificed to dissect out the entire brain under cold condition. The half of the hippocampus was stored at -80°C for reverse transcription polymerase chain reaction (RT-PCR) experiments. The other half was fixed in paraformaldehyde, embedded in paraffin and then cut into 2-μm thick sections using a microtome.
Hematoxylin and eosin staining
The paraffin embedded slices were deparaffinized and then stained with hematoxylin and eosin. The slices were examined under light microscope (BX51M; Olympus, Tokyo, Japan; magnification, ×10). In each slice, hippocampal CA3, CA1 and DG regions were examined to observe the changes in morphology of the hippocampal neurons.
Immunohistochemistry
Polink-2 Plus® HRP Polymer Detection System (PV-9001; GBI Labs, Mukilteo, WA, USA) was used for the immunostaining of the brain slices. The sections were dehydrated in graded ethanol series and incubated with rabbit anti-mouse glial fibrillary acidic protein (GFAP) antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The sections were washed with phosphate-buffered saline (Shanghai Generay Biotech Co., Ltd.), incubated with poly horseradish peroxidase anti-rabbit secondary antibody (PV-9001). The avidin-biotin complex and diaminobenzidine were used to obtain a visible reaction product. As a negative control, the specimens in the control experiments were processed without primary or secondary antibodies. The immunostaining of inducible nitric oxide synthase (iNOS) was performed in a similar manner, however, the primary antibodies were substituted with rabbit anti-mouse iNOS anti-body (Santa Cruz Biotechnology, Inc.). A Leica microscope equipped with a digital camera was used for the examination and imaging of the sections (Leica, Solms, Germany).
Image analysis
The sections were focussed under the microscope (magnification, ×20) and five high power field areas were randomly selected. The average number of positive cells in the hippocampal CA1 and CA3 areas were calculated for the quantification of GFAP expression. Prism 3.0 software (GraphPad Software, Inc., San Diego, CA, USA) was used to plot the data obtained. For the quantification of iNOS expression we used ImagePro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA) software. The iNOS immunohistochemical images were analysed to calculate the average light density values (IOD/area) for each section of five randomly selected high power fields in the hippocampal CA1 and CA3 areas (magnification, ×40).
RT-PCR
Total RNA was prepared using Sepasol (Nacalai Tesque), according to the manufacturer’s protocols. Using M-MLV reverse transcriptase (Life Technologies Japan Ltd.), total RNA was reverse-transcribed using a thermal cycler (Takara PCR Thermal Cycler SP: Takara Bio Inc., Shiga, Japan). To determine mRNA expression levels, real-time quantitative RT-PCR analysis was performed with a Light Cycler System (Roche Diagnostics) using SYBR green fluorescence signals. The following specific primers were used: NR1, forward 5’-‘GCTGCACGCCTTTATCTG-3’ and reverse 5’-TCCTACGGGCATCCTTGT-3’; NR2b, forward 5’-CACGGTGCCTTCAGAGTT-3’ and reverse 5’-CCTCCTCCAAGGTGACAA-3’. The PCR products were separated using electrophoresis on a 2.0% agarose gel. The intensity of the bands was analyzed using BioSense SC-810 Gel Documentation System (Shanghai Bio-Tech Co., Ltd., Shanghai, China) and Gel-Pro 3.1 software (Media Cybernetics, Inc., Bethesda, MD, USA).
Statistical analysis
Each assay was performed in triplicates. The data were expressed as the mean ± standard deviation (S.D.). SPSS 16 software was used for all statistical analyses. One-way analysis of variance (ANOVA) was used to determine the significant differences in two comparisons. Statistical significance was set at P < 0.05.
Results
CBD treatment reduces the severity of pentylenetetrazole-induced chronic seizures
The effect of CBD on pentylenetetrazole induced chronic seizures was examined by measuring convulsions using a five-point scale. It was observed that the rats reached a completely kindled condition after 30 days of the treatment. In two CBD treatment groups with higher CBD dosage (20 and 50 mg/kg), the daily average seizure grades were significantly lower compared with those of the model control group (P < 0.001; Figure 2A). However, the CBD treatment groups with lower CBD dosage (10 and 20 mg/kg) as well as model control groups showed the same kindling rate (Table 1).
Figure 2.
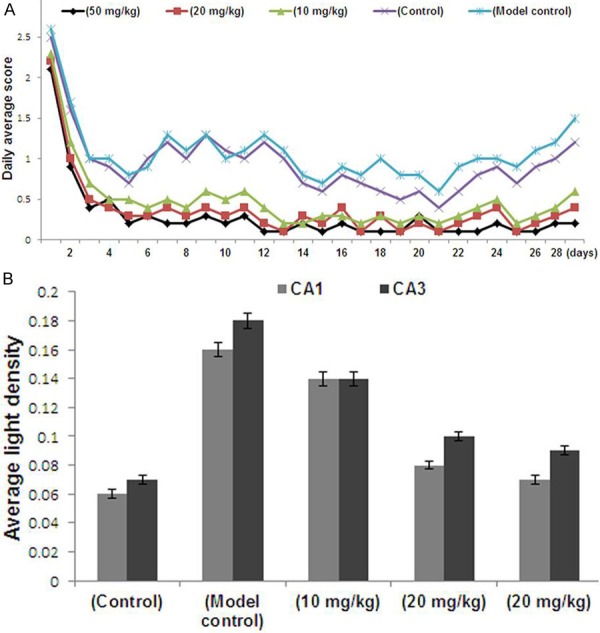
A. Behavioral scores of the animals for each group. B. Average light density (IOD/Area) values of inducible nitric oxide synthase expression in the CA1 and CA3 regions of the hippocampus. CBD, cannabidiol.
Table 1.
Severity and the kindling rate of rats in each treatment group
| Seizure grade | |||||
|---|---|---|---|---|---|
|
|
|||||
| Group | Sample (n) | Survival rate | Moderate ( ≤ III) | Severe ( ≥ III) | Kindling rate |
| Normal control | 8 | - | - | - | - |
| Model control | 8 | 8/8 | 2/8 | 6/8 | 8/8 |
| CBD 10 mg/kg | 8 | 8/8 | 4/8 | 4/8 | 8/8 |
| CBD 20 mg/kg | 8 | 8/8 | 6/8 | 2/8 | 7/8 |
| CBD 50 mg/kg | 8 | 8/8 | 7/8 | 1/8 | 4/8 |
A marked decrease in the rate of severe grade seizures was observed in CBD treatment groups compared with the model control group. The CBD treatment group (50 mg/kg) also showed significant difference in the kindling rates.
CBD has no effect on the expression of iNOS
We examined the effect of CBD on the expression of iNOS in the CA1 and CA3 areas of the hippocampus. The average light density (IOD/Area) values of iNOS-positive regional expression were obtained. The results showed a markedly higher IOD/Area values for the pentylenetetrazole group compared with the normal group (P < 0.05). However, treatment with CBD caused a significant decrease in the rats of CBD group compared to control group.
This suggested that iNOS activity may be increased in chronic epileptic seizures, however, the long-term usage of CBD does not significantly inhibit iNOS expression (Figure 2B).
CBD treatment decreases cell injury in the hippocampal area of pentylenetetrazole-treated rats
The microscopic examination of the hippocampal pyramidal cells revealed irregular distribution, abnormal structures, wider interspaces and even loss of cells in the model control rat group compared to normal group. However, the animals in CBD group showed reduced neuronal loss in the hippocampal area (Figure 3). Thus CBD treatment decreased the cell injury in the hippocampal area of pentylenetetrazole-treated rats.
Figure 3.
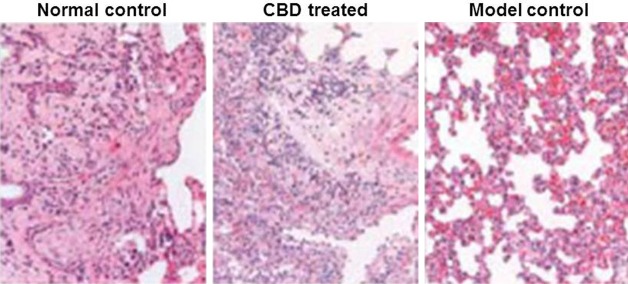
Decreased neuronal injury in the hippocampal region of CBD-treated rats. The hippocampus region of rats was sectioned and stained using hematoxylin and eosin to compare the severity of neuron loss between the (A) normal control, (B) model control and (C) the CBD groups (magnification, × 4).
Treatment with CBD suppresses astrocytic hyperplasia
The hematoxylin and eosin staining and image analysis of the CA1 and CA3 hippocampal regions of normal rats revealed the presence of few scattered, light brown-yellow GFAP-positive cells in the normal rats. GFAP-positive cells were markedly higher in number with more intense staining, as well as thicker and extended neuritis in the model control group. However, CBD treatment resulted in a significant increase in GFAP expression but the number of GFAP-positive cells and the level of staining were less compared with the model control group. The neurites were relatively thinner and shorter. The CBD treatment group showed significant differences compared to the model control group (Figure 4). Thus, suggesting that CBD suppressed astrocytic hyperplasia.
Figure 4.
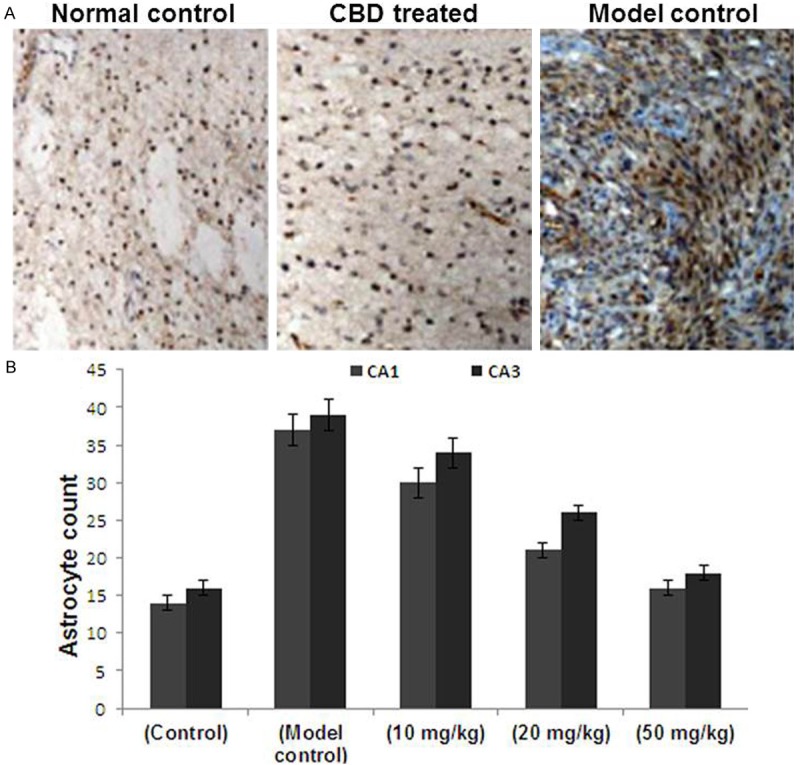
A. Hyperplasia of astrocytes in the hippocampus of CBD-treated rats was reduced. Immunostaining of GFAP was performed on the sections to detect increased astrocyte expression. The expression of astrocytes in the normal control group and the CBD group was decreased compared with the model control group (magnification, × 4; boxed area, magnification, × 20). B. The number of GFAP positive cells was counted from five randomly selected microscopic fields (magnification, × 20) and plotted. Data are presented as the mean ± the standard deviation. CBD, cannabidiol; GFAP, glial fibrillary acidic protein.
CBD treatment decreases the expression of the NMDA receptor
The effect of CBD treatment on changes in the expression of NMDA receptor was studied by analysing NR1 and NR2b mRNA expression in the rat hippocampus (Figure 5A). The results revealed a marked decrease in the quantity of NR1 mRNA in the CBD treatment groups (40 and 80 mg/kg) compared to the model control group (Figure 5B). The suppression of hippocampal NR1 was significant in the rats treated with 80 mg/kg compared with animals treated with 20 mg/kg. Thus, CBD treatment suppresses the actions of the hippocampal NR1 (Figure 5B). However, CBD treatment did not affect the expression of NR2b mRNA (data not shown).
Figure 5.
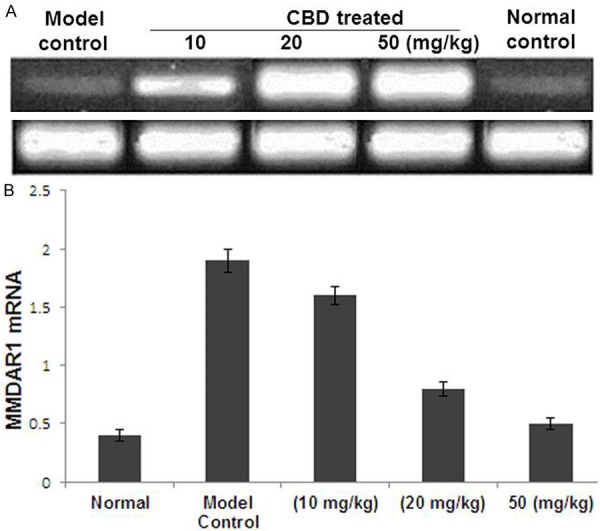
A. Detection of NMDAR1 mRNA expression in the rat hippocampus using reverse transcription polymerase chain reaction. A, normal control group; B, model control group; C1, C2 and C3, Cannabidiol groups (10, 20 and 50 mg/kg, respectively). B. Quantification of NMDAR1 mRNA expression of the five groups. The Y axis indicates the ratio of optical density (OD) of the samples to the corresponding internal standard (β-actin). Data are expressed as the mean ± standard error of the mean (n = 10). *P < 0.05, **P < 0.01, compared with the normal control group. ΔP < 0.01, for comparisons between the cannabidiol group and the model control group. NMDAR1, N-methyl-D-aspartic acid receptor; CBD, cannabidiol; PTZ, pentylenetetrazole.
Discussion
Our study clearly demonstrates that CBD administration protects against pentylenetetrazole-induced chronic seizures in rats. In addition, the CBD treated rats exhibited significantly lower astrocytic hyperplasia and neurological defects in the hippocampal area compared with rats in the model control group. The expression of the NMDA1 receptor was selectively suppressed in CBD-treated rats. The rats treated with a high dose of CBD (20 and 50 mg/kg/d) showed a clear inhibitory effects. This is believed to be due to the rapid metabolism of CBD in the peripheral tissues. The blood-brain barrier may also restrict the penetration of CBD into the brain [19]. Therefore, an adequate peripheral dose is required to produce apparent protective effects.
Astrocytes comprise an important class of glial cells in the brain. It is reported that epilepsy leads to an increase in the number of astrocytes and changes in their morphology and function. Astrocytes are known to play a crucial role in the mechanisms underlying epilepsy [20]. It reported that astrocytes are involved in the maintenance of the inflammatory state during epilepsy by releasing inflammatory cytokines. These cytokines directly alter the excitability of the neurons and promote mossy fiber budding of the dentate gyrus to form an excitability loop, which may induce seizures [21]. Our results from GFAP immunohistochemistry demonstrated that CBD was able to significantly reduce hippocampal astrocytic cell proliferation in a dose-dependent manner following pentylenetetrazole-induced seizures. This may contribute to the inhibitory effect of CBD on seizures.
Development of seizures is induced by the activation of NMDA receptors and it is observed that in convulsant animal models binding sites of NMDA receptors are upregulated. Administration of NMDA receptor antagonists has been shown to inhibit convulsion in the animal models [22]. The results from our study revealed a significant inhibition of NR1 mRNA expression on treatment with CBD (20 and 50 mg/kg). However, CBD did not affect the expression of NMDA R2b mRNA receptor.
It is reported that loss of cells increases the potential for seizure activity which in turn result in neuronal death. This process is believed to be involved in the chronic course of epilepsy [23]. Earlier pentylenetetrazole was showed to decrease the number of hippocampal neurons in seizures. Our results revealed a significant decrease in the cell loss in rat hippocampus on treatment with CBD. It also suppresses the progression of seizures and protects neuronal damage.
The results from our study revealed enhanced iNOS expression in the hippocampus on administration of pentylenetetrazole. However, CBD was not found to significantly inhibit iNOS expression.
Conclusions
The present study demonstrates that CBD protects against pentylenetetrazole-induced chronic seizures, decreases astrocytic hyperplasia, decreases neuronal cell loss and selectively suppresses NMDA1 receptor in the hippocampus.
Disclosure of conflict of interest
None.
References
- 1.Beaumont A, Whittle IR. The pathogenesis of tumor associated epilepsy. Acta Neurochir (Wien) 2000;142:1–15. doi: 10.1007/s007010050001. [DOI] [PubMed] [Google Scholar]
- 2.Govori V, Humolli I, Cepreganov M, Dedushaj I, Gjikolli B. Brain tumors and epilepsy. Acta Clin Croat. 2010;49:133–8. [PubMed] [Google Scholar]
- 3.Rosati A, Tomassini A, Pollo B, Ambrosi C, Schwarz A, Padovani A, Bonetti B. Epilepsy in cerebral glioma: timing of appearance and histological correlations. J Neurooncol. 2009;93:395–400. doi: 10.1007/s11060-009-9796-5. [DOI] [PubMed] [Google Scholar]
- 4.Roux FX, Nataf F. Cerebral oligodendrogliomas in adults and children. Current data and perspectives. Neurochirurgie. 2005;51:410–4. [PubMed] [Google Scholar]
- 5.Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- 6.Mackay MT, Bicknell-Royle J, Nation J, Humphrey M, Harvey AS. The ketogenic diet in refractory childhood epilepsy. J Paediatr Child Health. 2005;41:353–7. doi: 10.1111/j.1440-1754.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 7.Scheuer ML, Pedley TA. The evaluation and treatment of seizures. New Engl J Med. 1990;323:1468–74. doi: 10.1056/NEJM199011223232107. [DOI] [PubMed] [Google Scholar]
- 8.Löscher W, Schmidt D. New horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res. 2006;69:183–272. doi: 10.1016/j.eplepsyres.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith M, Wilcox KS, White HS. Discovery of antiepileptic drugs. Neurotherapeutics. 2007;4:12–7. doi: 10.1016/j.nurt.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan AK, Dobhal MP, Joshi BC. A review of medicinal plants showing anticonvulsant activity. J Ethnopharmacol. 1988;22:11–23. doi: 10.1016/0378-8741(88)90226-7. [DOI] [PubMed] [Google Scholar]
- 11.Nsour WN, Lau CB, Wong IC. Review on phytotherapy in epilepsy. Seizure. 2000;9:96–107. doi: 10.1053/seiz.1999.0378. [DOI] [PubMed] [Google Scholar]
- 12.Karler R, Cely W, Turkanis SA. The anticonvulsant activity of cannabidiol and cannabinol. Life Sciences. 1973;13:1527–31. doi: 10.1016/0024-3205(73)90141-0. [DOI] [PubMed] [Google Scholar]
- 13.Ferenczy L, Gracza L, Jakobey I. An antibacterial prepartum from hemp (Cannabis sativa L) Naturwissenschaften. 1958;45:188. [Google Scholar]
- 14.Demehri S, Homayoun H, Honar H, Riazi K, Vafaie K, Roushanzamir F, Dehpour AR. Agmatine exerts anticonvulsant effect in mice: modulation by α2-adrenoceptors and nitric oxide. Neuropharmacology. 2003;45:534–42. doi: 10.1016/s0028-3908(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 15.Su RB, Wei XL, Zheng JQ, Liu Y, Lu XQ, Li J. Anticonvulsive effect of agmatine in mice. Pharmacol Biochem Behav. 2004;77:345–9. doi: 10.1016/j.pbb.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, LeBlanc MH, Regunathan S. Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: a potential mechanism for the anticonvulsive effects. Neurosci Lett. 2005;390:129–33. doi: 10.1016/j.neulet.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luszczki JJ, Czernecki R, Wojtal K, Borowicz KK, Czuczwar SJ. Agmatine enhances the anticonvulsant action of Phenobarbital and valproate in the mouse maximal electroshock seizure model. J Neural Transm. 2008;115:1485–94. doi: 10.1007/s00702-008-0046-3. [DOI] [PubMed] [Google Scholar]
- 18.Bahremand A, Ziai P, Khodadad TK, Payandemehr B, Rahimian R, Ghasemi A, Ghasemi M, Hedayat T, Dehpour AR. Agmatine enhances the anticonvulsant effect of lithium chloride on pentylenetetrazole-induced seizures in mice: Involvement of L-arginine/nitricoxide pathway. Epilepsy Behav. 2010;18:186–92. doi: 10.1016/j.yebeh.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Stringer JL. Repeated seizures increase GFAP and vimentin in the hippocampus. Brain Res. 1996;717:147–53. doi: 10.1016/0006-8993(96)00059-5. [DOI] [PubMed] [Google Scholar]
- 20.Ravizza T, Gagliardi B, Noé F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–60. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Ekonomou A, Angelatou F. Upregulation of NMDA receptors in hippocampus and cortex in the pentylenetetrazol-induced ‘kindling’ model of epilepsy. Neurochem Res. 1999;24:1515–22. doi: 10.1023/a:1021143813935. [DOI] [PubMed] [Google Scholar]
- 22.Bengzon J, Kokaia Z, Elmér E, Nanobashvili A, Kokaia M, Lindvall O. Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A. 1997;94:10432–7. doi: 10.1073/pnas.94.19.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oses JP, Leke R, Portela LV, Lara DR, Schmidt AP, Casali EA, Wofchuk S, Souza DO, Sarkis JJ. Biochemical brain markers and purinergic parameters in rat CSF after seizure induced by pentylenetetrazol. Brain Res Bull. 2004;64:237–42. doi: 10.1016/j.brainresbull.2004.07.006. [DOI] [PubMed] [Google Scholar]


