Abstract
The impact of pre-existing diabetes on the development of deep vein thrombosis (DVT) remains unclear. We performed a meta-analysis of cohort studies to evaluate the risk of DVT in patients with and without pre-existing diabetes. We searched Pubmed, Embase, and Cochrane databases from the inception to December 2014 for cohort studies assessing the effect of diabetes on the incidence of DVT. Pooled odds ratio (OR) with 95% confidence interval (CI) was calculated using random- or fixed-effect models. Six cohort studies were included in this meta-analysis. A fixed-effects model meta-analysis showed that pre-existing diabetes was associated with an increased risk of DVT (OR 1.36; 95% CI 1.07-1.72; P=0.01), with moderate heterogeneity among the studies (I2=46.2; P=0.10). When patients were divided into two subgroups based on the method of screening DVT, there was no significant heterogeneity in each subgroup. Our meta-analysis suggested that pre-existing diabetes was associated with an increased risk of DVT after total knee replacement. However, the result should be interpreted with caution because of the potential bias and confounding in the included studies.
Keywords: Arthroplasty, knee replacement, diabetes mellitus, deep venous thrombosis, meta-analysis
Introduction
Deep vein thrombosis (DVT), as one kind of venous thromboembolisms, is commonly seen after hip and knee replacement surgery. Many factors that may increase the incidence of DVT have been investigated, such as smoking, obesity, cancer, immobilization, congestive heart failure, and so on [1-3]. In clinical practice, DVT is frequently seen in patients with diabetes after total knee replacement. It is possible that diabetes is another risk factor for DVT. However, relevant studies on this topic have not draw a definite conclusion, and some of the results were even controversial. For example, Zhao et al. [4] considered that patients with diabetes have a higher risk of DVT, but in the studies conducted by Meding et al. [5], there is a converse conclusion. Besides, some of these studies are limited by the small sample size [6]. Thus, we undertook this systematic review and meta-analysis of cohort studies to assess the effect of diabetes on DVT after total knee replacement.
Material and methods
Literature search and inclusion criteria
This meta-analysis was undertaken in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement. A literature search was carried out using Pubmed, Embase, and Cochrane databases from inception to December 2014. The search was limited to human subjects and English language. We used the following search terms and boolean operators: (“diabetes” or “diabetic” or “diabetics”) and (“knee” or “joint”) and (“replacement” or “arthroplasty”) and “thrombosis”. In addition, we reviewed the reference lists of retrieved papers and recent reviews to identify other potentially eligible studies that we had not captured with our primary search.
The following inclusive selection criteria were applied: (a) study design: cohort study; (b) study population: adult patients with total knee replacement; (c) comparison intervention: with and without pre-existing diabetes; and (d) outcome measure: the development of DVT. In the case of duplicate data publication (several studies with overlapping samples), we only included the most informative article or complete study to avoid duplication of information.
Data extraction and outcome measures
The following data was extracted from each included article: first author, publication year, number of patients with and without diabetes, method of screening DVT, and odds ratios (ORs) with the corresponding 95% confidence intervals (CIs). The supplementary files were also examined for data extraction. When it is necessary, we contacted authors of included studies for additional information.
Assessment of methodological quality
The quality of the included studies was evaluated using the Newcastle-Ottawa Scale (NOS) [7] based on three main items: the selection of the study groups (0-4 points), the comparability of the groups (0-2 points) and the determination of either the exposure or the outcome of interest (0-3 points), with a perfect score of 9.
Two investigators independently conducted the study selection, data extraction and quality assessment. Any disagreements were resolved by discussion and consensus.
Statistical analysis
From each study, we extracted the adjusted OR with 95% CI. When the adjusted OR was not available, a crude OR was calculated. We pooled across studies to assess the associations between diabetes and the risk of DVT with P<0.05 indicating a significant difference. Heterogeneity among the studies was tested by Q-test statistics with the significance set at P<0.10. The I2 statistics was used as a second measure of heterogeneity, with I2 more than 50% indicating inconsistency. A random effects model was used to calculate pooled ORs in the case of significant heterogeneity (P<0.10 or I2>50%), otherwise, a fixed-effects model was used.
Furthermore, we conducted sensitivity analyses by omitting one study in each turn to investigate the influence of a single study on the overall pooled estimate. To explore the possible source of heterogeneity and to examine the influence of various clinical factors on the overall risk estimate, we further carried out subgroup analysis according to sample size (≤1000 and >1000), pooled ORs (crude and adjusted ORs), and method of screening DVT (routine and non-routine sonography).
Potential publication bias was detected by Begg’s funnel plots, and P<0.05 was judged as statistically significant. All statistical analyses were performed using STATA, version 11.0 (StataCorp, College Station, TX).
Results
Literature search results
The initial search yielded 84 relevant publications, of which 78 were excluded for duplicate studies and various reasons (reviews, letters, or not relevant to our analysis) on the basis of the title/abstract and full text (Figure 1). The remaining 6 cohort studies were included in the final analysis [4,5,8-11].
Figure 1.
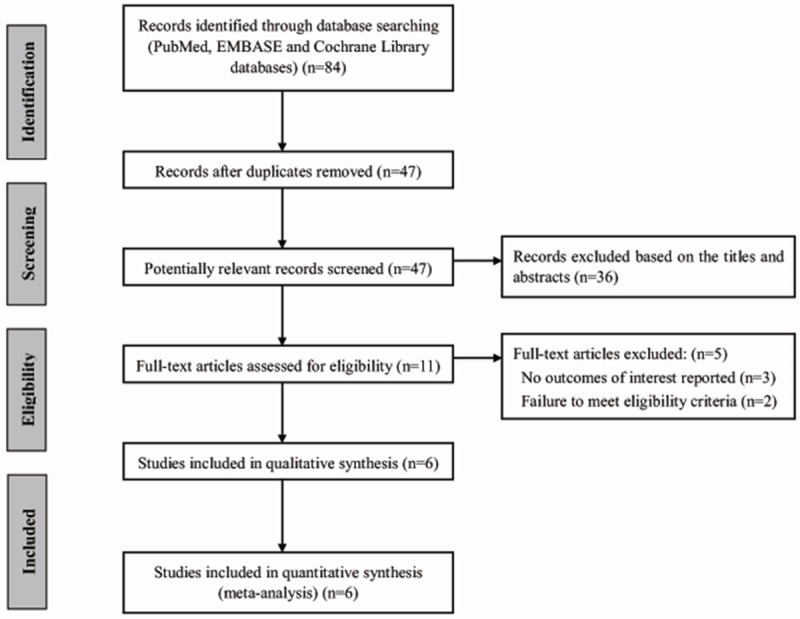
Flow diagram showing the process of selection for meta-analysis.
Study characteristics and methodological quality assessment
The main characteristics of the six included cohort studies are shown in Table 1. These studies were published between 2003 and 2014. The average NOS score of the studies included was 7 (range from 6 to 8). One study [8] scored 8, 4 studies [4,5,9,11] scored 7, and 1 study [10] scored 6. Overall, a total of 503941 patients were included in this analysis. Among them, 54196 patients had diabetes and 449745 patients had DVT.
Table 1.
Main characteristics of cohort studies included in the meta-analysis
| First author | Publication year | Country | NOS score | Total patients | Patients with diabetes | Patients without diabetes | Methods of screening DVT |
|---|---|---|---|---|---|---|---|
| Meding [5] | 2003 | USA | 7 | 3519 | 291 | 3228 | Clinically screened and confirmed with Doppler ultrasonography |
| Bolognesi [9] | 200 | USA | 7 | 458986 | 46044 | 412942 | Not reported |
| Moon [10] | 2008 | Korea | 6 | 342 | 171 | 171 | Clinically screened and confirmed with Doppler ultrasonography |
| Adams [8] | 2013 | USA | 8 | 40491 | 7567 | 32924 | Clinically diagnosis and imaging study |
| Wang [11] | 2013 | China | 7 | 245 | 53 | 192 | Routine Duplex sonography examination |
| Zhao [4] | 2014 | China | 7 | 358 | 70 | 288 | Routine Duplex sonography examination |
NOS, Newcastle-Ottawa Scale; DVT, deep vein thrombosis.
Diabetes and the risk of DVT
Figure 2 shows the pooled results from the fixed-effects model combing the ORs for DVT. Pre-existing diabetes was associated with an increased risk of DVT in patients underwent total knee replacement (OR 1.36; 95% CI 1.07-1.72; P=0.01), with moderate heterogeneity among the studies (I2=46.2; P=0.10). Further exclusion of some single studies altered the overall combined OR, with a range from 1.23 (95% CI, 0.95-1.58) to 1.64 (95% CI, 0.94-2.88). Table 2 shows the results of subgroup analyses for DVT. When patients were divided into two subgroups based on the method of screening DVT, there was no significant heterogeneity in each subgroup (Figure 3).
Figure 2.
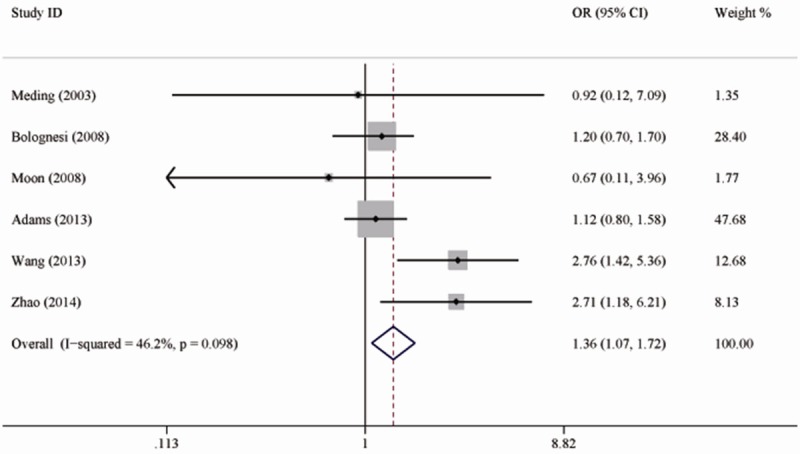
Forest plots showing the risk of deep vein thrombosis in patients with diabetes.
Table 2.
Subgroup analyses for DVT
| Subgroup | No. of studies | OR (95% CI) | P value | Q-test (P) | I2 (%) |
|---|---|---|---|---|---|
| Total [4,5,8-11] | 6 | 1.36 (1.07-1.72) | 0.01 | 0.10 | 46.2 |
| Sample size | |||||
| ≤1000 [4,10,11] | 3 | 2.45 (1.49-4.04) | <0.01 | 0.33 | 10.1 |
| >1000 [5,8,9] | 3 | 1.15 (0.88-1.50) | 0.32 | 0.95 | 0 |
| Pooled ORs | |||||
| Crude ORs [5,8,10] | 3 | 1.10 (0.79-1.53) | 0.59 | 0.85 | 0 |
| Adjusted ORs [4,9,11] | 3 | 1.95 (1.06-3.60) | 0.03 | 0.06 | 64.5 |
| Method of screening DVT | |||||
| Routine sonography [4,11] | 2 | 2.74 (1.63-4.60) | <0.01 | 0.97 | 0 |
| Non-routine sonography [5,8-10] | 4 | 1.13 (0.87-1.48) | 0.36 | 0.93 | 0 |
DVT, deep vein thrombosis.
Figure 3.
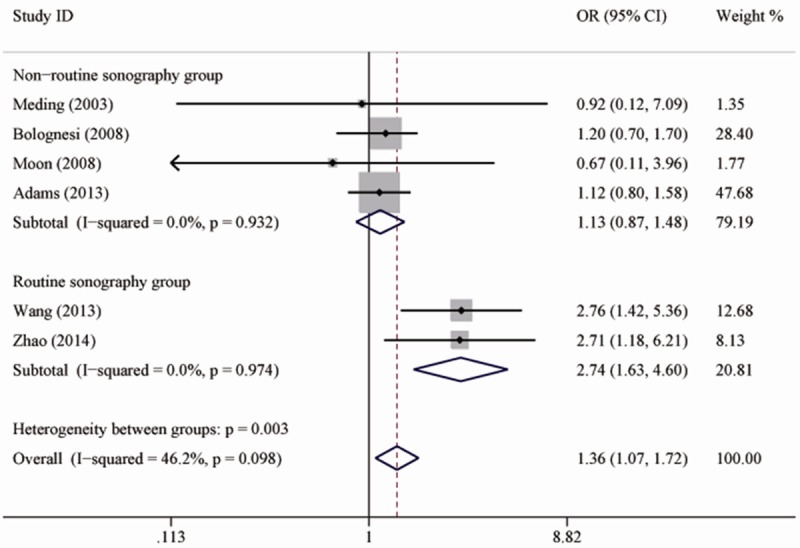
Forest plots showing the risk of deep vein thrombosis in patients with diabetes after subgroup analysis according to the method of screening deep vein thrombosis.
Publication bias
Assessment of publication bias using Begg’s test showed that no potential publication bias existed among the included studies (P=0.71, Figure 4).
Figure 4.
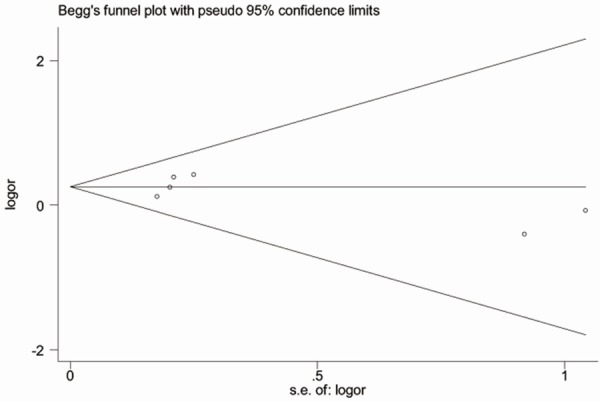
Begg’s funnel plot for the risk of deep vein thrombosis in patients with diabetes.
Discussion
Diabetes has been proven to be a risk factor for poor functional outcome after total knee replacement [12-14]. However, to the best of our knowledge, whether pre-existing diabetes will predispose patients to the development of DVT remains unclear. The current meta-analysis of cohort studies suggests that pre-existing diabetes was associated with an increased incidence of DVT after total knee replacement.
In this meta-analysis, we included only six cohort studies in an attempt to produce robust results. However, there was moderate heterogeneity among the studies, and the combined ORs changed essentially when some single studies were excluded. Thus, we further conducted subgroup analyses to seek the possible source of heterogeneity. When studies were divided into two groups by the methods of screening DVT, heterogeneity among the studies in each subgroup decreased significantly (I2=0), which means that the methods of screening DVT might contribute to the significant heterogeneity, and affected the final result.
As we know, venography is the gold standard for detecting DVT [15]. However, it is an invasive examination procedure. Doppler ultrasonography, on the other hand, is more commonly used in clinical practice. As an examination with good sensitivity, Doppler ultrasonography is able to image thrombosis in most patients [16,17]. In the studies conducted by Zhao and Wang et al. [4,11], the color Duplex sonography examination was routinely performed on the third and fourteenth day postoperatively. Thrombosis appearing either proximal or distal to the trifurcation of the popliteal vein were regarded as the incidence of DVT. In this way, the sensitivity of color Doppler examination in DVT would be significantly higher, and the results in the two studies might be more reliable. The pooled result in this subgroup showed that patients with diabetes have a higher chance to suffer DVT after total knee replacement. In the other subgroup, some studies assessed only proximal DVT, excluding patients with calf muscular venous thrombosis; some studies screened DVT by clinical symptoms and confirmed it with Doppler ultrasonography, in which occasion, DVT might not be detected if patients were asymptomatic. As a result, there was a significant lower incidence of DVT in these studies. However, in this subgroup, there was still a trend that diabetes increases the incidence of DVT (OR=1.13). Larger sample sizes in these studies may make the result statistically significant.
Although pre-existing diabetes may increase the risk of DVT, the mechanism remains unclear. Virchow proposed that venous thrombosis may be precipitated by three factors: venous stasis, increased coagulability of the blood, and damage to the vessel wall. Diabetes mellitus has been proven to be associated with endothelial damage, increased blood coagulability, and decrease fibrinolysis function [18]. Raised concentrations of fibrinogen, von Willebrand factor and other endothelium-derived mediators increase blood viscosity and promote platelet adhesion. Besides, fibrinolysis is impaired by raised concentrations of plasminogen activator inhibitor-1 and depressed concentrations of plasminogen activator. It is reported that diabetes could contribute to an increase of venous thromboembolisms [19] and cardiovascular events [20]. The increased risk of DVT in diabetic patients might be associated with this mechanism too.
Our findings are of clinical significance to some extent. Surgeons caring for total knee replacement patients can not accurately determine which patient will develop DVT, because no markers have been found to predict it precisely so far. However, surgeons could roughly predict who was more likely to develop DVT according to the risk factors in individuals. Several risk factors have been found to be associated with the development of DVT, such as old age, obesity, and smoking. In this study, diabetes is proven to be another risk factor. This requires surgeons to pay more attention to this population at risk. Strategies to decrease the risk of DVT should be provided to these patients shortly after total knee replacement.
This study has several limitations. First, our analysis was based on only six cohort studies and some of them have a modest sample size. Compared to a review with a large sample size, our study is more likely to overestimate or underestimate the true results. Besides, there was considerable heterogeneity among the included studies. The country, lengths of follow-up, and quality of study were varied among these studies. These factors might have an impact on our results. Third, not all the extracted ORs are adjusted. Crude ORs calculated from univariate statistics could result in some bias.
Despite the limitations mentioned above, this study is clinically valuable to some extent. In summary, this meta-analysis of six cohort studies suggests that diabetes increase the risk of DVT in patients with total knee replacement. Surgeons should pay more attention to these patients in order to prevent the incidence of DVT as early as possible.
Disclosure of conflict of interest
None.
References
- 1.Abdollahi M, Cushman M, Rosendaal FR. Obesity: risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Haemost. 2003;3:493–498. [PubMed] [Google Scholar]
- 2.Howell MD, Geraci JM, Knowlton AA. Congestive heart failure and outpatient risk of venous thromboembolism: a retrospective, case-control study. J Clin Epidemiol. 2001;8:810–816. doi: 10.1016/s0895-4356(00)00373-5. [DOI] [PubMed] [Google Scholar]
- 3.Umesh Y, Mahemuti A, Zhou XH. Smoking is a risk factor for venous thromboembolism. Chin Med J (Engl) 2013;16:3177–3180. [PubMed] [Google Scholar]
- 4.Zhao Z, Wang S, Ma W, Kong G, Zhang S, Tang Y, Zhao Y. Diabetes mellitus increases the incidence of deep vein thrombosis after total knee arthroplasty. Arch Orthop Trauma Surg. 2014;1:79–83. doi: 10.1007/s00402-013-1894-3. [DOI] [PubMed] [Google Scholar]
- 5.Meding JB, Reddleman K, Keating ME, Klay A, Ritter MA, Faris PM, Berend ME. Total knee replacement in patients with diabetes mellitus. Clin Orthop Relat Res. 2003;416:208–216. doi: 10.1097/01.blo.0000093002.90435.56. [DOI] [PubMed] [Google Scholar]
- 6.Papagelopoulos PJ, Idusuyi OB, Wallrichs SL, Morrey BF. Long term outcome and survivorship analysis of primary total knee arthroplasty in patients with diabetes mellitus. Clin Orthop Relat Res. 1996;330:124–132. doi: 10.1097/00003086-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;9:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 8.Adams AL, Paxton EW, Wang JQ, Johnson ES, Bayliss EA, Ferrara A, Nakasato C, Bini SA, Namba RS. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am. 2013;6:481–487. doi: 10.2106/JBJS.L.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolognesi MP, Marchant MH Jr, Viens NA, Cook C, Pietrobon R, Vail TP. The impact of diabetes on perioperative patient outcomes after total hip and total knee arthroplasty in the United States. J Arthroplasty. 2008;23(Suppl 1):92–98. doi: 10.1016/j.arth.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Moon HK, Han CD, Yang IH, Cha BS. Factors affecting outcome after total knee arthroplasty in patients with diabetes mellitus. Yonsei Med J. 2008;1:129–137. doi: 10.3349/ymj.2008.49.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Zhao Y. Diabetes mellitus and the incidence of deep vein thrombosis after total knee arthroplasty: a retrospective study. J Arthroplasty. 2013;4:595–597. doi: 10.1016/j.arth.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Singh JA, Lewallen DG. Diabetes: a risk factor for poor functional outcome after total knee arthroplasty. PLoS One. 2013;11:e78991. doi: 10.1371/journal.pone.0078991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amusat N, Beaupre L, Jhangri GS, Pohar SL, Simpson S, Warren S, Jones CA. Diabetes that impacts on routine activities predicts slower recovery after total knee arthroplasty: an observational study. J Physiother. 2014;4:217–223. doi: 10.1016/j.jphys.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Robertson F, Geddes J, Ridley D, McLeod G, Cheng K. Patients with Type 2 diabetes mellitus have a worse functional outcome post knee arthroplasty: a matched cohort study. Knee. 2012;4:286–289. doi: 10.1016/j.knee.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Schellong SM, Beyer J, Kakkar AK, Halbritter K, Eriksson BI, Turpie AG, Misselwitz F, Kälebo P. Ultrasound screening for asymptomatic deep vein thrombosis after major orthopaedic surgery: the VENUS study. J Thromb Haemost. 2007;7:1431–1437. doi: 10.1111/j.1538-7836.2007.02570.x. [DOI] [PubMed] [Google Scholar]
- 16.Zierler BK. Ultrasonography and diagnosis of venous thromboembolism. Circulation. 2004;109(Suppl 1):I9–14. doi: 10.1161/01.CIR.0000122870.22669.4a. [DOI] [PubMed] [Google Scholar]
- 17.Rose SC, Zwiebel WJ, Nelson BD, Priest DL, Knighton RA, Brown JW, Lawrence PF, Stults BM, Reading JC, Miller FJ. Symptomatic lower extremity deep venous thrombosis: accuracy, limitations, and role of color duplex flow imaging in diagnosis. Radiology. 1990;3:639–644. doi: 10.1148/radiology.175.3.2188293. [DOI] [PubMed] [Google Scholar]
- 18.Jones E, Mitchell J. Venous thrombosis in diabetes mellitus. Diabetologia. 1983;6:502–505. doi: 10.1007/BF00284459. [DOI] [PubMed] [Google Scholar]
- 19.Petrauskiene V, Falk M, Waernbaum I, Norberg M, Eriksson JW. The risk of venous thromboembolism is markedly elevated in patients with diabetes. Diabetologia. 2005;5:1017–1021. doi: 10.1007/s00125-005-1715-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang PY. Cardiovascular disease in diabetes. Eur Rev Med Pharmacol Sci. 2014;15:2205–2214. [PubMed] [Google Scholar]


