Abstract
Introduction/Aims: Hydroxyisoleucine (4-HIL), derived from fenugreek seeds, improves insulin resistance in 3T3-L1 adipocytes and L6 myotubes. However, the effects of 4-HIL on liver glycogen synthesis and hepatic insulin resistance have not been described. The aim of this study was to investigate the effects of 4-HIL on glycogen synthesis in a tumor necrosis factor-α (TNF-α)-induced insulin resistance model using HepG2 cells. Materials and methods: HepG2 cells were divided into eight groups: control; TNF-α; and 5, 10, or 20 μM 4-HIL without or with TNF-α. Glycogen and protein expression were evaluated using a glycogen assay kit and western blotting, respectively. Results: Glycogen levels did not differ between the 4-HIL groups and control (P>0.05), but were decreased significantly in the TNF-α group (P<0.05), indicating the establishment of insulin-resistant HepG2 cells. Adding 20 μM 4-HIL to TNF-α-treated cells increased glycogen levels (P<0.05). Relative to the control group, the P-IRS-1/IRS-1 and P-JNK/JNK ratios were increased (P<0.001) in the TNF-α group, whereas the P-AKT/AKT and P-GSK/GSK ratios were decreased (P<0.001). When 20 μM 4-HIL was added to TNF-α-treated cells, the P-IRS-1/IRS-1 and P-JNK/JNK ratios decreased (P<0.001 and P<0.05, respectively), whereas the P-AKT/AKT and P-GSK/GSK ratios increased (P<0.05 and P<0.001). Conclusions: 4-HIL directly or indirectly reversed TNF-α reduced glycogen levels by inhibiting JNK and IRS-1 (Ser307) phosphorylation and increasing AKT (Ser473) and GSK-3 phosphorylation. These findings demonstrate that 4-HIL modulates hepatic insulin resistance at the molecular level, and suggest that it is a novel potential therapeutic agent for the treatment of insulin resistance in patients with diabetes.
Keywords: Fenugreek, JNK signaling pathway, insulin resistance, 3T3-L1 adipocytes, L6 myotubes
Introduction
Insulin resistance (IR) is the main pathophysiological characteristic of type 2 diabetes. IR can develop in hepatocytes, muscle cells, kidney cells, adipocytes, endothelial cells, and pancreatic β-cells [1]. Because the liver is a site of insulin action, hepatic IR can have significant effects on the body [2]. Increased hepatic glucose production and decreased glycogen synthesis, which are manifestations of hepatic IR, lead to increased blood glucose levels [3]. Many factors contribute to the pathogenesis of IR, including IRS family members [4-6], glucose transporter protein (GLUT) [7,8], the phosphorylation of intracellular glucose, oxidative stress [9], free fatty acids (FFAs) [10-12], and adipokines [13,14].
Studies have indicated that cytokines can also induce IR. Tumor necrosis factor-α (TNF-α) was the first cytokine implicated in the induction of IR [15]. TNF-α inhibits glycogen synthase in the IR state via the JNK signaling pathway: phosphorylation of JNK (P-JNK) and IRS-1 (P-IRS-1) increases, whereas the phosphorylation of AKT (P-AKT) and GSK (P-GSK) decreases, thus reducing glycogen synthesis [16].
Fenugreek (Trigonellafoenum-graecum) is an annual herb that grows primarily in the Mediterranean and countries such as Ukraine, India, and China. Owing to its immunomodulatory and anti-oxidizing effects, it is used as a dietary supplement to treat wounds and several conditions including diabetes, high cholesterol levels, inflammation, and gastrointestinal complaints [17]. 4-Hydroxyisoleucine (4-HIL) is derived from fenugreek seeds, which are composed of 80% free amino acids. 4-HIL is comprised of (2S, 3R, 4S) isomers (~90%) and (2R, 3R, 4S) isomers [18]. Some studies have indicated that (2S, 3R, 4S)-4-HIL enhances islet β-cell insulin secretion and improves IR, although the mechanism behind these effects is unclear [19-23]. Recently, it was reported that 4-HIL ameliorates FFA-induced IR in skeletal muscle cells by inhibiting the production of palmitate-induced reactive oxygen species (ROS) and ROS-associated inflammation [24]. We demonstrated previously that 4-HIL decreases soluble TNF-α expression and IRS-1 (Ser307) phosphorylation and increases GLUT4 expression, leading to enhanced insulin sensitivity in 3T3-L1 adipocytes [25]. However, the effects of 4-HIL on liver glycogen synthesis, its ability to modulate TNF-α-induced hepatic IR, and the underlying mechanisms behind these effects have not been described.
In this study, an insulin-resistant cell model was established by treating HepG2 cells with TNF-α. The cells were then exposed to different concentrations of 4-HIL to determine the effects of 4-HIL on hepatic glycogen synthesis and IR, and investigate the molecular mechanisms underlying these effects.
Materials and methods
Reagents and antibodies
Dulbecco’s modified Eagle’s medium (DMEM), a high-glucose medium purchased from HyClone (Beijing, China), was stored at 4°C. Fetal bovine serum (FBS) was obtained from Gibco (Beijing, China) and stored at -20°C. TNF-α (human) was purchased from ProSpec-Tany (East Brunswick, NJ, USA). Antibiotics (penicillin and streptomycin solution) were purchased from HyClone. 4-HIL was purchased from Sigma (St. Louis, MO, USA). Novolin R was purchased from Novo Nordisk (Bagsvaerd, Denmark). JNK, IRS-1, Akt, GSK, P-JNK, P-Akt (Ser473), P-IRS-1 (Ser307), and P-GSK rabbit anti-mouse antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). β-actin antibody was purchased from Wuhan Antgene Biotechnology Co., Ltd. (China). Goat anti-rabbit IgG (H + L) were purchased from Beijing 4A Biotech Co., Ltd. (China). All antibodies were used at the dilutions recommended by the manufacturer.
Cell culture
HepG2 cells are used commonly as a hepatocyte model for studies of insulin resistance [16,26,27]. Importantly, this cell line was characterized in detail previously, and the authors concluded that HepG2 cells exhibited most of the cellular features of primary hepatocytes, and that they retained the ability to synthesize all cellular proteins, except for C-reactive peptide [28]. HepG2 cells were grown at 37°C with 5% CO2 in DMEM containing 10% FBS, 100 IU/mL penicillin, and 0.1 mg/mL streptomycin.
Induction of IR and analysis of glycogen content
HepG2 cells were divided into eight groups: control, 5 μM 4-HIL, 10 μM 4-HIL, 20 μM 4-HIL, TNF-α, TNF-α + 5 μM 4-HIL, TNF-α + 10 μM 4-HIL, and TNF-α + 20 μM 4-HIL. HepG2 cells were exposed to 10 ng/mL TNF-α without or with different concentrations of 4-HIL for 21 h. Insulin (10 nM; Novo Nordisk) was added thereafter and incubated for another 3 h. The glycogen level in each group was assessed using a glycogen assay kit (Nanjing Jiancheng Bioengineering Institute, China), and the results were normalized to a standard curve and expressed as milligrams of glycogen per milliliter of protein, as per the manufacturer’s instructions.
Western blotting
Cell lysates (20-30 μg of protein) were separated using 8% SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA), which were blocked using either 5% non-fat dried milk (for total proteins) or BSA (for phospho-specific antibodies), and incubated overnight at 4°C in the presence of specific antibodies. The blots were incubated with the antibodies described in Reagents and antibodies above at the dilutions recommended by the manufacturers, and signals were detected using ECL (Beyotime, Shanghai, China). To assess insulin signaling proteins, cells were treated with 10 nM insulin (Novo Nordisk) for 10 min before proteins were collected. Western blots were quantified using ImageJ software (NIH, MD, USA).
Statistical analysis
All values were calculated as the means ± SEMs unless otherwise indicated. Student’s t-test and one-way ANOVA test were used to determine significance. A p-value of <0.05 was considered significant.
Results
4-HIL increases the TNF-α-induced reduction in glycogen levels
Figure 1 shows the glycogen level in the following eight groups of HepG2 cells: control, 5 μM 4-HIL, 10 μM 4-HIL, 20 μM 4-HIL, TNF-α, TNF-α + 5 μM 4-HIL, TNF-α + 10 μM 4-HIL, and TNF-α + 20 μM 4-HIL. The glycogen level was significantly lower (P<0.05) in the TNF-α group compared with the control group. The addition of 4-HIL to TNF-α-treated cells increased the glycogen level (P<0.05, TNF-α + 20 μM 4-HIL group vs. TNF-α group). However, in the absence of TNF-α 4-HIL itself had no effect on the glycogen level compared with the control group.
Figure 1.
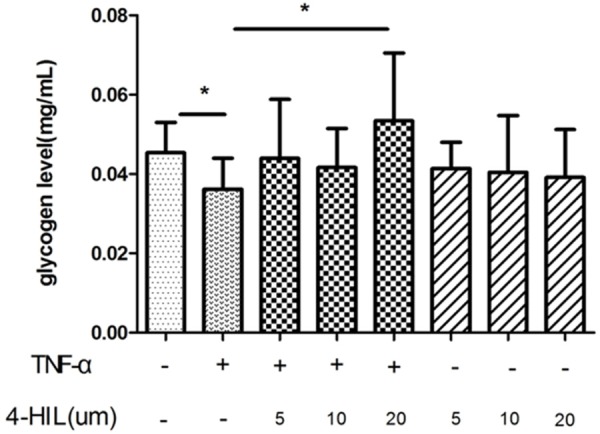
4-HIL increases the TNF-α-induced reduction in glycogen levels. 4-HIL promoted glycogen synthesis in HepG2 cells. HepG2 cells (5×106) were treated with TNF-α or 4-HIL at the indicated concentrations for 24 h. Data are presented as means ± SDs; *P<0.05 vs. TNF-α group.
4-HIL modulates the TNF-α-induced changes in insulin signaling
To assess the expression of insulin signaling proteins using western blotting, HepG2 cells were divided into four groups (control, 4-HIL, TNF-α, and TNF-α + 4-HIL). 4-HIL was used at a concentration of 20 μM because this had the maximal effect on glycogen levels (Figure 1). Figure 2 shows the expression of P-JNK and JNK proteins. As expected, the levels of total JNK were unchanged by treatment with TNF-α and/or 4-HIL. The P-JNK/JNK ratios were similar in the control and 20 μM 4-HIL groups, but the ratio was markedly higher in the TNF-α group (P<0.001). However, the P-JNK/JNK ratio was significantly lower in the TNF-α + 4-HIL group than in the TNF-α group (P<0.05).
Figure 2.
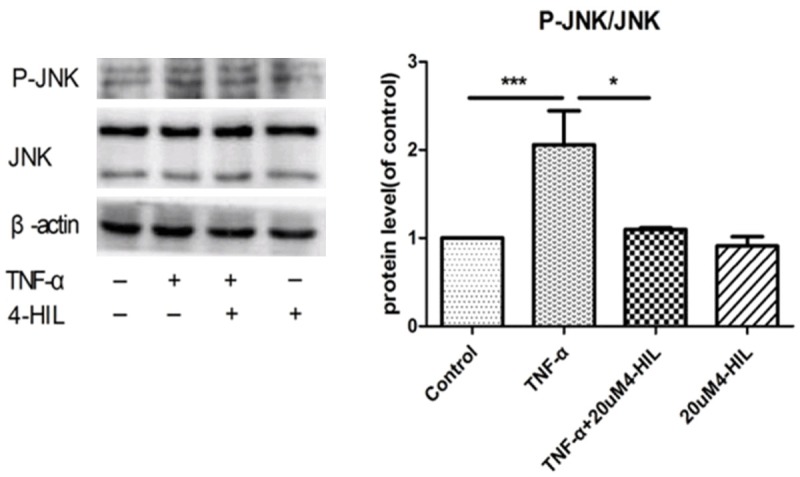
4-HIL reduced phospho-JNK levels. Treatment with 4-HIL led to the reduced expression of phospho-JNK. HepG2 cells (2×106) were incubated with TNF-α alone or TNF-α plus 20 µM 4-HIL for 24 h. Whole-cell extracts were prepared to assess the phosphorylation of JNK. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading (***P<0.001 and *P<0.05 vs. TNF-α group).
Figure 3 shows the expression of P-IRS-1 and IRS-1 proteins. The P-IRS-1 (Ser307)/IRS-1 ratio was higher in the TNF-α group compared with the control group (P<0.001). The addition of 20 μM 4-HIL to TNF-α-treated cells significantly reduced the P-IRS-1 (Ser307)/IRS-1 ratio (P<0.001 vs. the TNF-α group). Moreover, the P-IRS-1 (Ser307)/IRS-1 ratio was significantly lower (P<0.05) in the 20 μM 4-HIL group than in the control group.
Figure 3.
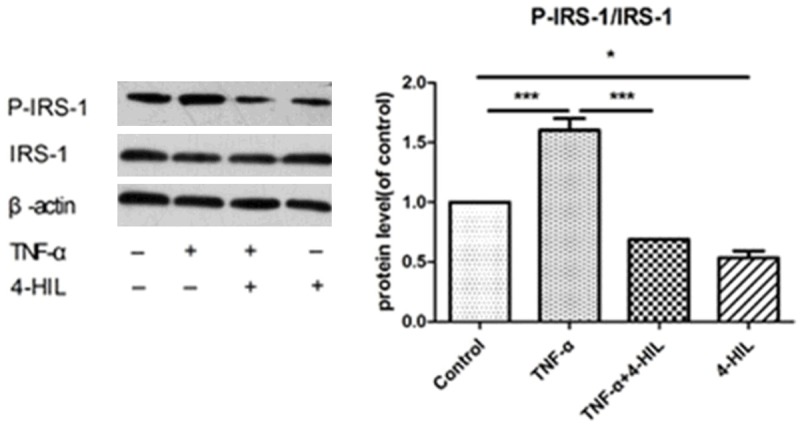
4-HIL reduced phospho-IRS-1 levels. Treatment with 4-HIL led to the reduced expression of phospho-IRS-1 (Ser307). HepG2 cells (2×106) were treated with TNF-α alone or TNF-α plus 20 µM4-HIL for 24 h. Whole cell extracts were prepared to assess the expression of phospho-IRS-1. The same blots were stripped and reprobed with β-actin antibody to show equal protein loading (***P<0.001 and *P<0.05 vs. TNF-α group).
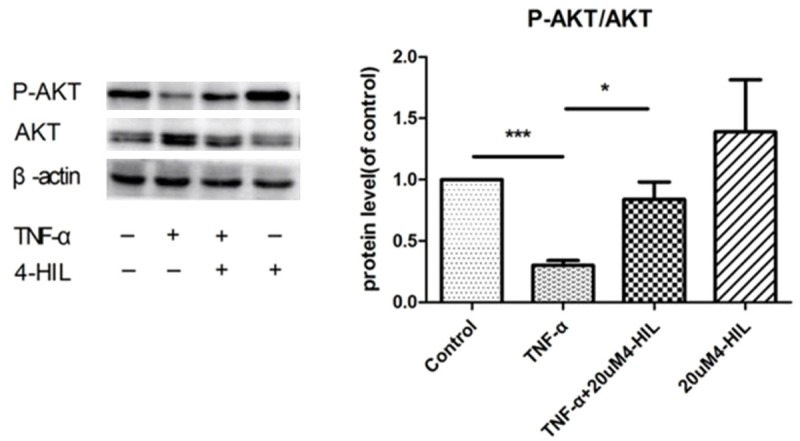
The expression of phospho-AKT (Ser473) and AKT proteins is shown in Figure 4. TNF-α treatment markedly decreased the P-AKT (Ser473)/AKT ratio (P<0.001 vs. the control group). The ratio was significantly higher in the TNF-α + 4-HIL group than in the TNF-α group (P<0.05). In addition, 4-HIL alone slightly increased the P-AKT (Ser473)/AKT ratio, although the difference between the 4-HIL group and the control group was not statistically significant.
Figure 4.
4-HIL reduced phospho-AKT levels. Treatment with 4-HIL led to the reduced expression of phospho-AKT (Ser473). HepG2 cells (2×106) were treated with TNF-α alone or TNF-α plus 20 µM4-HIL for 24 h. Whole cell extracts were prepared to assess the expression of phospho-AKT. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading (***P<0.001 and *P<0.05 vs. TNF-α group).
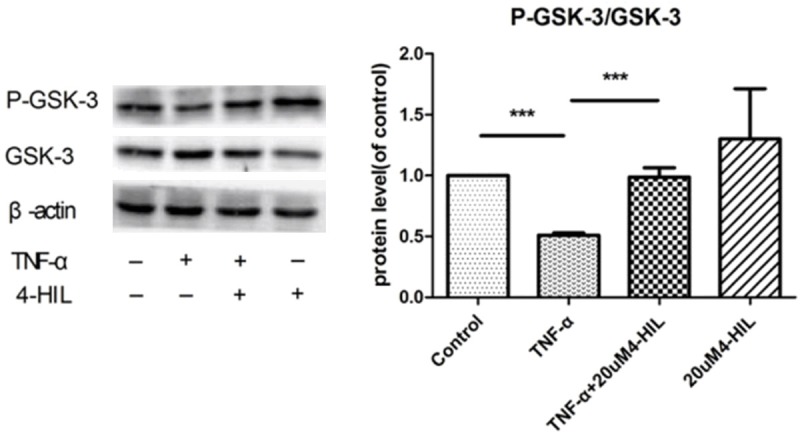
The expression of phospho-GSK-3α/β (Ser21/9) and GSK proteins was also evaluated (Figure 5). TNF-α treatment decreased the P-GSK-3α/β (Ser21/9)/GSK ratio (P<0.001 vs. the control group). The addition of 20 μM 4-HIL to TNF-α-treated cells restored the ratio to control levels (P<0.001 vs. the TNF-α group). In addition, 4-HIL alone slightly increased the P-GSK-3α/β (Ser21/9)/GSK ratio, although the difference between the 4-HIL group and the control group was not statistically significant.
Figure 5.
4-HIL reduced phospho-GSK-3 levels. Treatment with 4-HIL led to the reduced expression of phospho-GSK-3α/β (Ser21/9). HepG2 cells (2×106) were treated with TNF-α alone or TNF-α plus 20 µM 4-HIL for 24 h. Whole-cell extracts were prepared to assess the expression of phospho-GSK-3α/β. The same blots were stripped and reprobed with β-actin antibody to verify equal protein loading (***P<0.001 vs. TNF-α group).
Discussion
TNF-α, a major inflammatory cytokine secreted by macrophages, is involved in cell proliferation, differentiation, survival, and apoptosis [29,30]. Abnormal increases in TNF-α stimulate the production of cytokines, which promote the activation of various inflammatory signaling pathways [31]. TNF-α is involved in the development of obesity-related insulin resistance, and TNF-α levels are closely related to the severity of IR [32,33]. Studies have demonstrated that TNF-α increases the expression of phosphorylated JNK in 3T3-L1 adipocytes and worsens FFA-induced IR. However, inhibiting JNK phosphorylation might improve IR [34]. A reduction in hepatic glycogen synthesis is an important manifestation of IR. Li et al reported that TNF-α acts on JNK, which further affects the expression of IRS-1, AKT, and GSK, which subsequently reduces hepatic glycogen synthesis [16]. The mechanism by which TNF-α decreases hepatic glycogen synthesis is shown in Figure 6. The current study demonstrated that 4-HIL successfully inhibited the effects of TNF-α on inhibiting glycogen synthesis by preventing JNK and IRS-1 phosphorylation and increasing Akt and GSK-3 phosphorylation, and thereby improving hepatic IR. Therefore, these results suggest that 4-HIL might protect against inflammation-induced IR.
Figure 6.
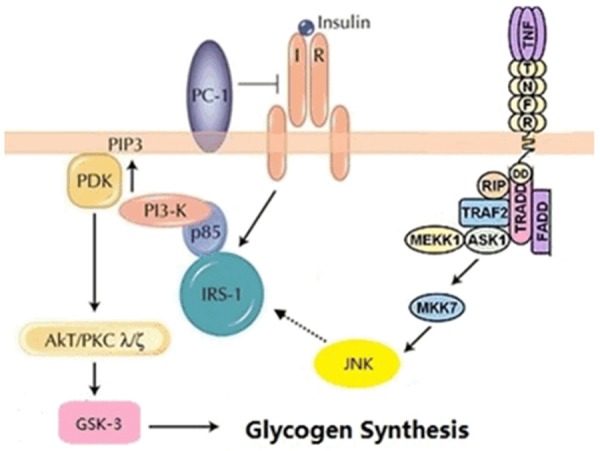
The mechanism by which TNF-α inhibits glycogen synthesis.
4-HIL promotes insulin secretion in a glucose-dependent manner in vitro. Furthermore, 4-HIL might improve IR within the liver, skeletal muscle, and adipose tissue [19-23]. Recently, Maurya et al reported that 4-HIL ameliorates FFA-induced IR in L6 myotubes [24]. In their study, 4-HIL enhanced insulin sensitivity by inhibiting the ability of palmitate to decrease the insulin-induced activation of the insulin receptor pathway. In addition, 4-HIL suppressed the production of ROS and ROS-associated inflammation induced by palmitate [24]. In an additional study, Rawat et al demonstrated that 4-HIL ameliorated IR in streptozotocin-treated rats and L6 myotubes via an AMPK- and AKT-dependent pathway in the liver [35]. Therefore, 4-HIL elicits multiple effects on insulin metabolism in a variety of tissues. The current study expands on these previous observations by highlighting at least some of the mechanisms of action of 4-HIL in hepatocytes. Further studies will expand on these observations and assess the ability of 4-HIL to reduce or prevent TNF-α-induced IR in vivo.
We demonstrated previously that 4-HIL downregulates TNF-α expression by inhibiting TNF-α-converting enzyme and promoting tissue inhibitor of metalloproteinase, thus suppressing IRS-1 (Ser307) phosphorylation and increasing GLUT4 expression [25]. These findings confirmed that 4-HIL could improve insulin resistance, but it remained unclear whether 4-HIL affected hepatic glycogen synthesis. Glycogen synthesis in the liver is highly complex, and involves multiple biological pathways and processes. In this study, we found that 4-HIL reversed TNF-α-induced reduction of hepatic glycogen levels, thus improving hepatic IR. These findings expand our knowledge of the effects of 4-HIL on hepatic IR, and also reveal at least some of the underlying mechanisms behind the pharmacological effects of 4-HIL in improving hepatic IR. As mentioned above, 4-HIL also modulates IR in other tissues such as adipose tissue and skeletal muscle. Therefore, future studies will focus on these tissues to increase our understanding of the mechanism of action of 4-HIL in other settings that mimic the in vivo environment, as well as on the hepatic IR induced by other factors including oxidative stress, free fatty acids, and other cytokines.
Conclusions
This study assessed the effects of 4-HIL on hepatic glycogen levels and IR, and also investigated the molecular mechanisms underlying these effects. We found that 4-HIL reversed the TNF-α-induced reduction in glycogen levels in HepG2 cells via the JNK signaling pathway. These results suggest a novel role for 4-HIL in alleviating insulin resistance at the molecular level, and suggest that 4-HIL warrants attention in future studies in vivo as a potential antidiabetogenic agent.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (registered no: 81001670).
Disclosure of conflict of interest
None.
References
- 1.Goldstein BJ. Insulin resistance as the core defect in type 2 diabetes mellitus. Am J Cardiol. 2002;90:3G–10G. doi: 10.1016/s0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Ding L, Hassan W, Abdelkader D, Shang J. Adipokines and hepatic insulin resistance. J Diabetes Res. 2013;2013:170532. doi: 10.1155/2013/170532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol. 2007;47:142–156. doi: 10.1016/j.jhep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Valverde AM, Kahn CR, Benito M. Insulin signaling in insulin receptor substrate (IRS)-1-deficient brown adipocytes: requirement of IRS-1 for lipid synthesis. Diabetes. 1999;48:2122–2131. doi: 10.2337/diabetes.48.11.2122. [DOI] [PubMed] [Google Scholar]
- 5.Bloomgarden ZT. American Diabetes Association Annual Meeting, 1999: insulin action and the development of type 2 diabetes. Diabetes Care. 2000;23:248–252. doi: 10.2337/diacare.23.2.248. [DOI] [PubMed] [Google Scholar]
- 6.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 7.Bonadonna RC, Del Prato S, Bonora E, Saccomani MP, Gulli G, Natali A, Frascerra S, Pecori N, Ferrannini E, Bier D, Cobelli C, DeFronzo RA. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes. 1996;45:915–925. doi: 10.2337/diab.45.7.915. [DOI] [PubMed] [Google Scholar]
- 8.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 10.Capaldo B, Napoli R, Di Marino L, Picardi A, Riccardi G, Sacca L. Quantitation of forearm glucose and free fatty acid (FFA) disposal in normal subjects and type II diabetic patients: evidence against an essential role for FFA in the pathogenesis of insulin resistance. J Clin Endocrinol Metab. 1988;67:893–898. doi: 10.1210/jcem-67-5-893. [DOI] [PubMed] [Google Scholar]
- 11.Groop LC, Saloranta C, Shank M, Bonadonna RC, Ferrannini E, DeFronzo RA. The role of free fatty acid metabolism in the pathogenesis of insulin resistance in obesity and noninsulindependent diabetes mellitus. J Clin Endocrinol Metab. 1991;72:96–107. doi: 10.1210/jcem-72-1-96. [DOI] [PubMed] [Google Scholar]
- 12.Boden G. Free fatty acids-the link between obesity and insulin resistance. Endocr Pract. 2001;7:44–51. doi: 10.4158/EP.7.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Zou C, Shao J. Role of adipocytokines in obesity- associated insulin resistance. J Nutr Biochem. 2008;19:277–286. doi: 10.1016/j.jnutbio.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang XF, Zhao MM, Weng CL, Sun NL. Adipocytokines: a bridge connecting obesity and insulin resistance. Med Hypotheses. 2009;73:981–985. doi: 10.1016/j.mehy.2009.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 16.Li L, He Q, Huang X, Man Y, Zhou Y, Wang S, Wang J, Li J. NOX3-derived reactive oxygen species promote TNF-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 2010;584:995–1000. doi: 10.1016/j.febslet.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 17.Upaganlawar A, Badole S, Bodhankar S. Antidiabetic potential of trigonelline and 4-hydroxyisoleucine in fenugreek. San Diego: Academic Press; 2013. [Google Scholar]
- 18.Broca C, Manteghetti M, Gross R, Baissac Y, Jacob M, Petit P, Sauvaire Y, Ribes G. 4-Hydroxyisoleucine: effects of synthetic and natural analogues on insulin secretion. Eur J Pharmacol. 2000;390:339–345. doi: 10.1016/s0014-2999(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 19.Sauvaire Y, Petit P, Broca C, Manteghetti M, Baissac Y, Fernandez-Alvarez J, Gross R, Roye M, Leconte A, Gomis R, Ribes G. 4-Hydroxyisoleucine: a novel amino acid potentiator of insulin secretion. Diabetes. 1998;47:206–210. doi: 10.2337/diab.47.2.206. [DOI] [PubMed] [Google Scholar]
- 20.Broca C, Gross R, Petit P, Sauvaire Y, Manteghetti M, Tournier M, Masiello P, Gomis R, Ribes G. 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am J Physiol. 1999;277:E617–E623. doi: 10.1152/ajpendo.1999.277.4.E617. [DOI] [PubMed] [Google Scholar]
- 21.Broca C, Breil V, Cruciani-Guglielmacci C, Manteghetti M, Rouault C, Derouet M, Rizkalla S, Pau B, Petit P, Ribes G, Ktorza A, Gross R, Reach G, Taouis M. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am J Physiol Endocrinol Metab. 2004;287:E463–E471. doi: 10.1152/ajpendo.00163.2003. [DOI] [PubMed] [Google Scholar]
- 22.Jetté L, Harvey L, Eugeni K, Levens N. 4-Hydroxyisoleucine: a plant-derived treatment for metabolic syndrome. Curr Opin Investig Drugs. 2009;10:353–358. [PubMed] [Google Scholar]
- 23.Ogawa J, Kodera T, Smirnov SV, Hibi M, Samsonova NN, Koyama R, Yamanaka H, Mano J, Kawashima T, Yokozeki K, Shimizu S. A novel L-isoleucine metabolism in Bacillus thuringiensis generating (2S,3R,4S)-4-hydroxyisoleucine, a potential insulinotropic and anti-obesity amino acid. Appl Microbiol Biotechnol. 2011;89:1929–1938. doi: 10.1007/s00253-010-2983-7. [DOI] [PubMed] [Google Scholar]
- 24.Maurya CK, Singh R, Jaiswal N, Venkateswarlu K, Narender T, Tamrakar AK. 4-Hydroxyisoleucine ameliorates fatty acid-induced insulin resistance and inflammatory response in skeletal muscle cells. Mol Cell Endocrinol. 2014;395:51–60. doi: 10.1016/j.mce.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Wu M, Lu FR, Xie J, Zheng N, Qin Y, Gao F, Du W, Jian LM. [Effect of Trigonella foenumgraecum 4-hydroxyisoleucine on high-glucose induced insulin resistance in 3T3-L1 adipocytes of mice] . Zhongguo Zhong Xi Yi Jie He Za Zhi. 2013;33:1394–1399. [PubMed] [Google Scholar]
- 26.Ishikawa M, Yoshida K, Okamura H, Ochiai K, Takamura H, Fujiwara N, Ozaki K. Oral Porphyromonas gingivalis translocates to the liver and regulates hepatic glycogen synthesis through the Akt/GSK-3β signaling pathway. Biochim Biophys Acta. 2013;1832:2035–2043. doi: 10.1016/j.bbadis.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y, Man Y, Wang S, Yang J, Li J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem. 2010;285:29965–29973. doi: 10.1074/jbc.M110.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouma ME, Rogier E, Verthier N, Labarre C, Feldmann G. Further cellular investigation of the human hepatoblastoma-derived cell line HepG2: morphology and immunocytochemical studies of hepatic-secreted proteins. In Vitro Cell Dev Biol. 1989;25:267–275. doi: 10.1007/BF02628465. [DOI] [PubMed] [Google Scholar]
- 29.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramírez Alvarado MM, Sánchez Roitz C. [Tumor necrosis factor-α, insulin resistance, the lipoprotein metabolism and obesity in humans] . Nutr Hosp. 2012;27:1751–1757. doi: 10.3305/nh.2012.27.6.6004. [DOI] [PubMed] [Google Scholar]
- 33.Kong P, Chi R, Zhang L, Wang N, Lu Y. Effects of paeoniflorin on tumor necrosis factor-α-induced insulin resistance and changes of adipokines in 3T3-L1 adipocytes. Fitoterapia. 2013;91:44–50. doi: 10.1016/j.fitote.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen MTA, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 35.Rawat AK, Korthikunta V, Gautam S, Pal S, Tadigoppula N, Tamrakar AK, Srivastava AK. 4-Hydroxyisoleucine improves insulin resistance by promoting mitochondrial biogenesis and act through AMPK and Akt dependent pathway. Fitoterapia. 2014;99:307–317. doi: 10.1016/j.fitote.2014.10.006. [DOI] [PubMed] [Google Scholar]


