Abstract
Macrophage migration inhibitory factor (MIF) is a multi-functional cytokine associated with inflammation and inflammatory bowel disease (IBD). The association between MIF-173G/C polymorphism and IBD risk has been extensively investigated. However, the results were conflicted and inconclusive. Therefore, we performed this meta-analysis. Online electronic databases (PubMed and EMBASE) was searched. All statistical tests were performed with the software STATA version 11.0 (Stata Corporation, College station, TX, USA). A total of nine studies (ten cohorts) with 3436 cases and 2742 controls were included for this meta-analysis. MIF-173G/C polymorphism was associated with a significantly increased risk of IBD when compared with CG and GG genotypes (OR=1.43; 95% CI 1.08-1.90; I2=0%). In the subgroup analysis according to ethnicity, significantly increased IBD risk was observed in Asians (OR=1.74; 95% CI 1.10-2.74; I2=0%) but not in Caucasians (OR=1.27; 95% CI 0.89-1.82; I2=0%). In the subgroup analysis according to IBD type, significantly increased UC risk was observed (OR=1.43; 95% CI 1.04-1.95; I2=0%). In conclusion, this meta-analysis suggested that MIF-173G/C polymorphism was associated with increased IBD risk.
Keywords: Inflammatory bowel disease, macrophage migration inhibitory factor, meta-analysis
Introduction
Inflammatory bowel disease (IBD) includes Crohn’s disease (CD) and ulcerative colitis (UC). Although it is suggested that environmental and immunologic factors are involved in the pathogenesis of IBD, the etiology of IBD is still not completely understood. Some suggest that a family history of IBD may be one of the most important risk factors [1]. A family history of IBD was shown to increase the risk of developing IBD 10- to 15-fold in unaffected first-degree relatives and three-fold among close relatives of IBD patients [A population-based case control study of potential risk factors for IBD.]. Thus, genetic factors are very important for IBD development.
Macrophage migration inhibitory factor (MIF) is a multi-functional cytokine associated with inflammation and tumorigenesis [2]. It promotes the production of inflammatory Th1 cytokines, including TNF-α, IFN-γ, IL-2, and IL-6 [3]. Moreover, MIF inhibits p53 dependent apoptosis [9], and participates in T cell proliferation and activation [4]. de Yong et al. demonstrated that the expression of MIF was increased in a model of experimental colitis induced by the transfer of CD45RB high, and that blockade of MIF with anti-MIF antibody reduced the severity of colitis [5]. Previous study reported that the MIF protein was significantly increased in the sera of patients with Crohn’s disease and those with UC [6].
The association between MIF-173G/C polymorphism and IBD risk has been extensively investigated [7-15]. However, the the results were conflicted and inconclusive. Therefore, we performed this meta-analysis to precisely estimate the association between the MIF-173G/C polymorphism and IBD risk.
Materials and methods
Search for publications
Online electronic databases (PubMed and EMBASE) was searched using the search terms: (Macrophage migration inhibitory factor or MIF) and (“Inflammatory bowel disease” or “IBD”). Additional studies were identifed by a hand search from reference of original studies or review articles on this topic. There was no language restriction.
Inclusion and exclusion criteria
The major inclusion criteria were: (1) case-control studies or cohort studies; (2) investigating the association between MIF-173G/C polymorphism and IBD risk; (3) available genotype distribution information in cases and controls or odds ratios (ORs) with 95% confidence intervals (CIs). The major reasons for exclusion of studies were: (1) reviews and repeated literatures; (2) case-only studies; (3) studies without detail genotype frequencies.
Data extraction
The following data were recorded from each article: first author, year of publication, ethnicity of participants, numbers of cases and controls, Hardy-Weinberg equilibrium (HWE), and genotype frequency in cases and controls. The data were extracted by two of the authors independently. Discrepancies between these two authors were resolved by discussion.
Statistical analysis
The strength of association between the MIF-173G/C polymorphism and IBD risk was assessed by calculating OR with 95% CI. A statistical test for heterogeneity was performed based on the Q statistic. The P>0.10 of the Q-test indicated a lack of heterogeneity among studies. If heterogeneity was observed among the studies, the random-effects model was used to estimate the pooled OR (the DerSimonian and Laird method). Otherwise, the fixed-effects model was adopted (the Mantel-Haenszel method). Stratified analysis was performed by ethnicity and IBD type. Potential publication bias was examined visually in a funnel plot and Egger’s test. All statistical tests were performed with the software STATA version 11.0 (Stata Corporation, College station, TX, USA). A P value <0.05 was considered statistically significant.
Results
Characteristics of studies
A total of nine studies (ten cohorts) with 3436 cases and 2742 controls on the association between MIF-173G/C polymorphism and IBD risk were included for this meta-analysis. There were 4 studies of Asian populations and 6 studies of Caucasian populations. The characteristics of each case-control study and the genotype in each study are presented in Table 1.
Table 1.
Characteristics of the studies included in this meta-analysis
| First author/Year | Ethnicity | IBD type | HWE | No. of Case | No. of Control | Case | Control | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| G | C | G | C | ||||||
| Nohara/2004 | Asian | UC | Yes | 221 | 438 | 346 | 710 | 96 | 166 |
| Oliver 1/2007 | Caucasian | Both | Yes | 623 | 261 | 1024 | 631 | 222 | 91 |
| Oliver 2/2007 | Caucasian | Both | Yes | 672 | 526 | 1133 | 919 | 211 | 133 |
| Dambacher/2007 | Caucasian | CD | Yes | 198 | 159 | 337 | 261 | 59 | 57 |
| Fei/2008 | Asian | Both | Yes | 99 | 142 | 136 | 213 | 62 | 71 |
| Shiroeda/2010 | Asian | UC | Yes | 111 | 209 | 175 | 328 | 47 | 90 |
| Przybylowska/2011 | Caucasian | Both | Yes | 99 | 123 | 163 | 221 | 35 | 25 |
| Sivaram/2012 | Asian | UC | No | 139 | 176 | 224 | 295 | 54 | 57 |
| Falvey/2013 | Caucasian | Both | Yes | 988 | 488 | 2392 | 516 | 799 | 177 |
| Mrowicki/2014 | Caucasian | Both | Yes | 286 | 220 | 481 | 91 | 356 | 84 |
IBM, inflammatory bowel disease; HWE, Hardy-Weinberg equilibrium.
Results of meta-analysis
The CC genotype of MIF-173G/C polymorphism was associated with a significantly increased risk of IBD when compared with CG and GG genotypes (OR=1.43; 95% CI 1.08-1.90; I2=0%; Figure 1). In the subgroup analysis according to ethnicity, significantly increased IBD risk was observed in Asians (OR=1.74; 95% CI 1.10-2.74; I2=0%) but not in Caucasians (OR=1.27; 95% CI 0.89-1.82; I2=0%). In the subgroup analysis according to IBD type, significantly increased UC risk was observed (OR=1.43; 95% CI 1.04-1.95; I2=0%).
Figure 1.
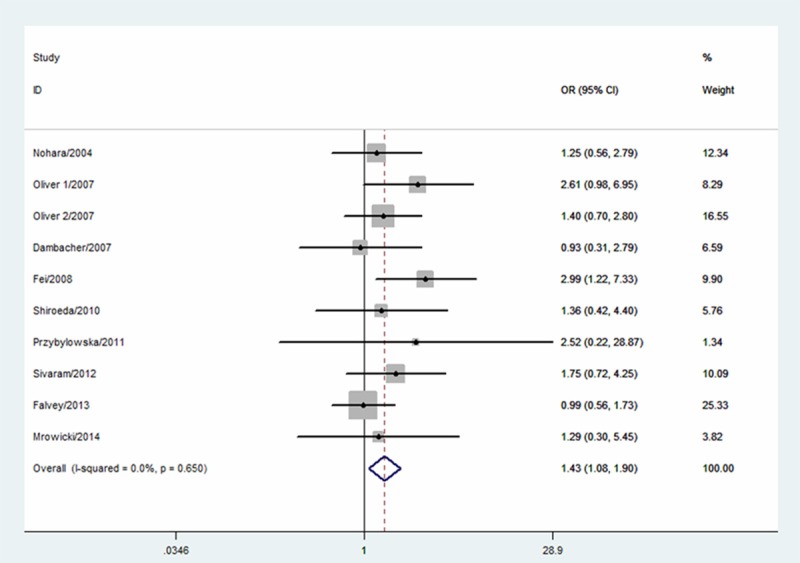
Meta-analysis for the association between MIF-173G/C polymorphism and IBD risk.
The shape of the funnel plot did not reveal any evidence of obvious asymmetry (Figure 2). Egger’s test did not find the evidence of publication bias (P=0.27).
Figure 2.
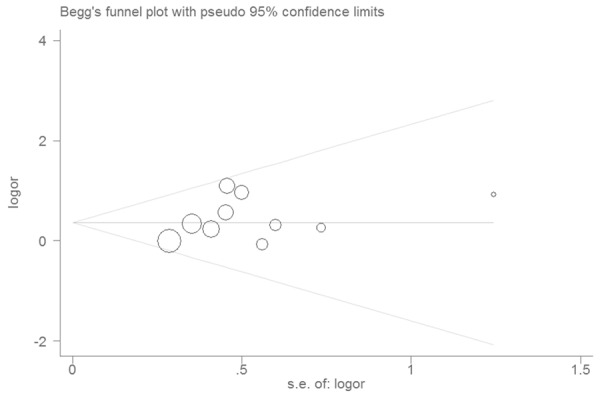
Funnel plot of associations between MIF-173G/C polymorphism and IBD.
Discussion
We performed a systematic search of the literature and combined the available results in this meta-analysis. MIF-173G/C polymorphism has been studied extensively about the relationship with IBD. Previous results of the studies on the relationship between MIF-173G/C polymorphism and IBD risk were contradictory. We thus performed a meta-analysis. We found that MIF-173G/C polymorphism was a risk factor for developing IBD. In the subgroup analysis by ethnicity, we noted that Asians carrying MIF-173G/C polymorphism had an increased IBD risk, while Caucasians carrying MIF-173G/C polymorphism did not have an increased IBD risk. In the subgroup analysis by IBD type, MIF-173G/C polymorphism increased UC risk.
MIF was shown to markedly enhance the production of IL-8 in dendritic cells obtained from patients with UC compared with non-UC patients [16]. As for the G-to-C transition at position -173 of the MIF gene, this site became functionally active in the presence of C through the creation of an activator protein 4 transcription factor binding site [9]. The expression of MIF was increased in colon cancerous lesions [17]. Thus, an anti-MIF strategy for IBD treatment is advantageous not only for suppression of intestinal inflammation, but also for prevention of the colon cancer often seen at the late stage of IBD.
Some limitations should be addressed. First, more studies with large sample sizes are needed to further identify the association among different races. Second, because small negative studies are less likely to published, the possibility of publication bias cannot be ruled out completely. Third, a lack of original data from the eligible studies limited evaluation of the effects of the gene-gene and gene-environment interactions during IBD development.
In conclusion, this meta-analysis suggested that MIF-173G/C polymorphism was associated with increased IBD risk.
Acknowledgements
This work is supported by Zhejiang medical scientific research projects (2013KYB204) and Hangzhou science and technology development plans (20100733Q04).
Disclosure of conflict of interest
None.
References
- 1.Pinsk V, Lemberg DA, Grewal K, Barker CC, Schreiber RA, Jacobson K. Inflammatory bowel disease in the South Asian pediatric population of British Columbia. Am J Gastroenterol. 2007;102:1077–83. doi: 10.1111/j.1572-0241.2007.01124.x. [DOI] [PubMed] [Google Scholar]
- 2.Adamali H, Armstrong ME, McLaughlin AM, Cooke G, McKone E, Costello CM, Gallagher CG, Leng L, Baugh JA, Fingerle-Rowson G, Bucala RJ, McLoughlin P, Donnelly SC. Macrophage migration inhibitory factor enzymatic activity, lung inflammation, and cystic fibrosis. Am J Respir Crit Care Med. 2012;186:162–9. doi: 10.1164/rccm.201110-1864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- 4.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–54. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong YP, Abadia-Molina AC, Satoskar AR, Clarke K, Rietdijk ST, Faubion WA, Mizoguchi E, Metz CN, Alsahli M, ten Hove T, Keates AC, Lubetsky JB, Farrell RJ, Michetti P, van Deventer SJ, Lolis E, David JR, Bhan AK, Terhorst C. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001;2:1061–6. doi: 10.1038/ni720. [DOI] [PubMed] [Google Scholar]
- 6.Murakami H, Akbar SM, Matsui H, Onji M. Macrophage migration inhibitory factor in the sera and at the colonic mucosa in patients with ulcerative colitis: clinical implications and pathogenic significance. Eur J Clin Invest. 2001;31:337–43. doi: 10.1046/j.1365-2362.2001.00796.x. [DOI] [PubMed] [Google Scholar]
- 7.Dambacher J, Staudinger T, Seiderer J, Sisic Z, Schnitzler F, Pfennig S, Hofbauer K, Konrad A, Tillack C, Otte JM, Diebold J, Göke B, Ochsenkühn T, Lohse P, Brand S. Macrophage migration inhibitory factor (MIF) -173G/C promoter polymorphism influences upper gastrointestinal tract involvement and disease activity in patients with Crohn’s disease. Inflamm Bowel Dis. 2007;13:71–82. doi: 10.1002/ibd.20008. [DOI] [PubMed] [Google Scholar]
- 8.Fei BY, Lv HX, Yang JM, Ye ZY. Association of MIF-173 gene polymorphism with inflammatory bowel disease in Chinese Han population. Cytokine. 2008;41:44–7. doi: 10.1016/j.cyto.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Nohara H, Okayama N, Inoue N, Koike Y, Fujimura K, Suehiro Y, Hamanaka Y, Higaki S, Yanai H, Yoshida T, Hibi T, Okita K, Hinoda Y. Association of the -173 G/C polymorphism of the macrophage migration inhibitory factor gene with ulcerative colitis. J Gastroenterol. 2004;39:242–6. doi: 10.1007/s00535-003-1284-7. [DOI] [PubMed] [Google Scholar]
- 10.Przybyłowska K, Mrowicki J, Sygut A, Narbutt P, Dziki Ł, Dziki A, Majsterek I. Contribution of the -173 G/C polymorphism of macrophage migration inhibitory factor gene to the risk of inflammatory bowel diseases. Pol Przegl Chir. 2011;83:76–80. doi: 10.2478/v10035-011-0012-x. [DOI] [PubMed] [Google Scholar]
- 11.Oliver J, Márquez A, Gómez-Garcia M, Martinez A, Mendoza JL, Vilchez JR, López-Nevot MA, Piñero A, de la Concha EG, Nieto A, Urcelay E, Martín J. Association of the macrophage migration inhibitory factor gene polymorphisms with inflammatory bowel disease. Gut. 2007;56:150–1. doi: 10.1136/gut.2006.107649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiroeda H, Tahara T, Nakamura M, Shibata T, Nomura T, Yamada H, Hayashi R, Saito T, Yamada M, Fukuyama T, Otsuka T, Yano H, Ozaki K, Tsuchishima M, Tsutsumi M, Arisawa T. Association between functional promoter polymorphisms of macrophage migration inhibitory factor (MIF) gene and ulcerative colitis in Japan. Cytokine. 2010;51:173–7. doi: 10.1016/j.cyto.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Sivaram G, Tiwari SK, Bardia A, Anjum F, Vishnupriya S, Habeeb A, Khan AA. Macrophage migration inhibitory factor, Toll-like receptor 4, and CD14 polymorphisms with altered expression levels in patients with ulcerative colitis. Hum Immunol. 2012;73:201–5. doi: 10.1016/j.humimm.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Falvey JD, Bentley RW, Merriman TR, Hampton MB, Barclay ML, Gearry RB, Roberts RL. Macrophage migration inhibitory factor gene polymorphisms in inflammatory bowel disease: an association study in New Zealand Caucasians and meta-analysis. World J Gastroenterol. 2013;19:6656–64. doi: 10.3748/wjg.v19.i39.6656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mrowicki J, Przybylowska-Sygut K, Dziki L, Sygut A, Chojnacki J, Dziki A, Majsterek I. The role of polymorphisms of genes CXCL12/CXCR4 and MIF in the risk development IBD the Polish population. Mol Biol Rep. 2014;41:4639–52. doi: 10.1007/s11033-014-3335-y. [DOI] [PubMed] [Google Scholar]
- 16.Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Macrophage migration inhibitory factor activates antigen-presenting dendritic cells and induces inflammatory cytokines in ulcerative colitis. Clin Exp Immunol. 2002;128:504–10. doi: 10.1046/j.1365-2249.2002.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi N, Nishihira J, Sato Y, Kondo M, Ogawa H, Ohshima T, Une Y, Todo S. Involvement of macrophage migration inhibitory factor (MIF) in the mechanism of tumor cell growth. Mol Med. 1998;4:707–14. [PMC free article] [PubMed] [Google Scholar]


