Abstract
Objective: To observe the clinical effects and adverse reactions, and analyze the clinical significance of L-asparaginase (L-ASP) containing multidrug chemotherapy regimen in incipient peripheral T-cell lymphoma (PTCL). Methods: A retrospective analysis was conducted of 102 patients with incipient PTCL who received L-ASP containing multidrug chemotherapy regimens or not in our hospital from January 2010 to December 2013. Complete remission (CR) rate, partial remission (PR) rate, overall remission (OR) rate, progression free survival (PFS), overall survival (OS) and adverse reactions were compared. Results: Patients who received L-ASP containing multidrug chemotherapy (L-ASP group) had higher OR rate than those who received L-ASP-free ones (non L-ASP group) (83.3% vs 61.7%, P=0.016), particularly those at phase III/IV (82.4% vs 54.0%, P=0.007) and with an international prognostic index (IPI) score of ≥2 (82.1% vs 50.0%, P=0.006). The median survival time (OS) was 10.5 months (range, 1-47months) in L-ASP group, while 13 months (range, 0.3-68 months) in non L-ASP group, and they had no statistically significance (P=0.754). Similarly, the progression free survival time(PFS)was 10 months (range, 1-47 months) in L-ASP group,while 11 months (range, 0.3-68 months) in non L-ASP group, also had no statistically significance (P=0.414). The 3-year OS rate of L-ASP group and non L-ASP group were 48.9% and 65.0% , respectively (P=0.974) and the 3-year PFS rate of L-ASP group and non L-ASP group were 40.8% and 61.0%, respectively (P=0.479). They all had no statistically significance. The L-ASP group had more adverse reactions than the non L-ASP group, though most of them were mild and could be improved by symptomatic and supportive care. Conclusion: L-ASP containing multidrug chemotherapy regimen in incipient PTCL showed a better short-term effect and controllable adverse reactions. A large prospective clinical trial of use L-ASP in first-line treatment of PTCL is worthy of further research and investigation.
Keywords: Lymphoma, peripheral t-cell, l-asparaginase, effect, adverse reaction, safety
Introduction
Peripheral T-cell lymphomas (PTCLs) are a series of malignant lymphoproliferative disoders with high heterogeneity. Except for prolonged natural course of T-large granular lymphocytic leukemia (T-LGLL) and cutaneous T-cell lymphomas (CTCL) and better chemotherapy efficacy of ALK-positive anaplastic large cell lymphoma (ALCL), most of PTCLs have poor outcomes, with an overall 5-year survival rate of less than 30% [1]. So far, treatment of PTCLs is principally based on the CHOP (cyclophosphamide, adriamycin, vincristine and prednisone) chemotherapy. Research results from the International PTCL Clinical and Pathologic Review Project [2] showed that, for peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) at least, anthracyclines-based therapetic regimens had poor outcomes and were notideal for PTCLs, and other more intensive chemotherapy regimens, i.e., Hyper-CVAD (high-dose cyclophosphamide, vincristine, adriamycin and dexamethasone) and higher-dose EPOCH, had not shown even better outcomes [3]. A prospective study concluded that patients who received autologous stem cell transplantation (ASCT) had better outcomes than those who received conventional chemotherapies, but 1/3 to 2/3 of the patients threw away a chance to receive ASCT due to progression of disease. This suggests the significance of pre-ASCT chemotherapy and a need for development of new therapies and drugs which can improve pretransplant remission rate, creating the conditions for ASCT. We retrospectively analyzed 102 patients with incipient PTCLs who received L-asparaginase (L-ASP) containing multidrug chemotherapy regimens or not in our hospital from January 2010 to December 2013, and short-term effects and safety of L-ASP as well.
Materials and methods
Cases
Subjects were recruited from 102 patients with incipient PTCL who received L-ASP containing multidrug chemotherapy regimens or not from January 2010 to December 2013. All subjects were diagnosed pathologically, with complete clinical and follow-up data. The follow-up was completed on March 31, 2014. Pathological classification referred to WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 2008 [4], while clinical stages to Ann Arbor Staging System [5].
Ethics statement
This study complied fully with all provisions of the Declaration of Helsinki. All patients gave written informed consent to participate. This study was proved by the medical ethics committee of the first Affiliated Hospital of Medical School of Zhejiang University.
Therapy
The therapeutic principle was determined by pathological types and clinical stages. The therapeutic principles of predominantly nodal PTCLs including PTCL-NOS, Angioimmunoblastic T cell lymphoma (AITL), anaplastic large cell lymphoma (ALCL) were as follows: patients with clinically limited-stage disease were mainly treated with CHOP or CHOP-like chemotherapy regimens, if necessary, in conjunction with radiotherapy for involved or extended field. Those with advanced diseases mainly received not only similar chemotherapy regimens to the former, but additional radiotherapy for masses or local lesions. The therapeutic principles of the extranodal NK/T-cell lymphoma were as follows: stage I patients received additional radiotherapy followed by consolidation chemotherapy; stage II patients received multidrug chemotherapy and then radiotherapy; advanced stage patients received chemotherapy mainly, if necessary, in conjunction with radiotherapy for nasal cavity. The L-ASP group were given CHOP or CHOP-like chemotherapy regimens with additional intravenous drip of L-ASP at the dose of 6 000 U/m2, once a day, for 7 days (which required a negative skin test and before medication, with specific dose and frequency as regulated by patients’ condition), and at least two L-ASP containing multidrug chemotherapy regimens should be employed.
Major multidrug chemotherapy regimens and usage: CHOP regimen: cyclophosphamide 750 mg/m2, Day 1, vindesine 4 mg, Day 1, epirubicin 80 mg/m2 or adriamycin 50 mg/m2, Day 1, prednisone 100 mg, at draught, Day 1 to 5, for a 21-day cycle; CHOPE regimen: CHOP regimen plus etoposide 100 mg, Day 1 to 3 or 5, for a 21-day cycle. Adverse reactions of the chemotherapy were evaluated by NCI Common Terminology Criteria for Adverse Events Version 3.0 (NCI.CTCAE V3.0).
Therapeutic evaluation and statistical treatment
Therapeutic effects were classified as complete remission (CR), partial remission (PR), stable disease (SD), relapse or progressive disease (PD) by IWG criteria, and CR+PR referred to overall remission (OR). OS were calculated beginning from the date of diagnosis to death or the last follow-up date. PFS were calculated beginning from the date of diagnosis to progression or death or the last follow-up date. Enumeration data of independent samples were compared with χ2 test. Kaplan-Meier method was used to estimate OS and PFS, and the differences were compared by Log-rank test. COX regression model was used for multivariate analysis. P<0.05 was considered statistically significant. SPSS 20.0 software was used for statistical analysis.
Results
General information
Of 102 patients with incipient PTCL, 42/102 patients received L-ASP containing multidrug chemotherapy regimens (L-ASP group), PTCL-NOS developed in 17, extranodal NK/T-cell lymphoma (NK/TCL) in 15, AITL in 6, ALCL (ALK-negative) in 2, and subcutaneous panniculitis-like T-cell lymphoma (SCPTCL) in one. 60/102 received L-ASP-free ones (non L-ASP group), PTCL-NOS developed in 33, extranodal NK/T-cell lymphoma (NK/TCL) in 9, AITL in 7, ALCL (ALK-negative) in 10, enteropathy-associated T-cell lymphoma (EATL) in one, and ALCL (ALK-positive) in one. 6/102 got lost to follow-up, 96/102 had complete follow-up data. Patients were randomized, but their clinical features were similar (Table 1). t test was used for measurement data, while χ 2 test for enumeration data.
Table 1.
Clinical features of patients in L-ASP group and control group [number of cases (%)]
| Group | L-ASP Group | Control Group |
|---|---|---|
| Number of cases (n) | 42 | 60 |
| Gender | ||
| Male | 35 (83.3) | 40 (66.7) |
| Female | 7 (16.7) | 20 (33.3) |
| Median age (year) | 51.5 (18-80) | 49.0 (16-78) |
| Clinical stage | ||
| I/II | 8 (19.0) | 10 (16.7) |
| III/IV | 34 (81.0) | 50 (83.3) |
| IPI score | ||
| Low-risk | 14 (33.3) | 18 (30.0) |
| low-intermediate risk | 25 (59.5) | 35 (58.3) |
| high-intermediate risk | 3 (7.2) | 7 (11.7) |
| median number of chemotherapies (n) | 6 (1-18) | 4.5 (1-12) |
Note: 1. Comparison of all parameters between ASP group and control group, P>0.05; 2: International poor prognostic factors: age > 60 years, stage III/IV, elevated serum LDH, number of extranodal lesions ≥2, ECOG score ≥2. There was no or only one adverse factor in low-risk group, 2 in low-intermediate risk group, 3 in high-intermediate group and 4 or 5 in high-risk group.
Short-term effects
CR and PR rates were 45.2% and 38.1% in the L-ASP group and 51.7% and 10% in the non L-ASP group, respectively; OR rate in the L-ASP group was superior to that in the non L-ASP group (83.3% vs 61.7%, P=0.016). According to the clinical stages, patients were divided into early-stage (I/II) group and advanced-stage (III/IV) group. It was found that, in patients from the advanced-stage group, CR and PR rates were 35.3% and 47.1% in the L-ASP group and 42% and 12% in the non L-ASP group, respectively, and that OR rate in the L-ASP group was superior to that in the non L-ASP group (82.4% vs 54.0%, P=0.007). According to the IPI scores, the risk stratification was divided into low (0 to 1 point), intermediate (2 to 3 points) and high (4 to 5 points) risk groups. It was found that, overall, in patients from intermediate and high risk groups, CR and PR rates were 35.7% and 46.4% in the L-ASP group and 38.1% and 11.9% in the non L-ASP group, respectively, and that OR rate in the L-ASP group was superior to that in the non L-ASP group (82.1% vs 50%, P=0.006); especially, among intermediate-risk patients, CR and PR rates were 36% and 48% in the L-ASP group and 40% and 18.6% in the non L-ASP group, respectively, OR rate in the L-ASP group was superior to that in the non L-ASP group (84.0% vs 48.6%, P=0.005); while among high-risk ones, no significant difference was noted in OR rate between L-ASP group and non L-ASP group (66.7% vs 57.1%, P=1) (Tables 2, 3 and 4).
Table 2.
Comparison of short-term effects between L-ASP group and control group [number of cases (%)]
| Group | Number of cases (n) | CR | Short-ter PR | effect SD | PD | OR |
|---|---|---|---|---|---|---|
| L-ASP group | 42 | 19 (45.2) | 16 (38.1) | 3 (7.1) | 4 (9.5) | 35 (83.3%) |
| Control group | 60 | 31 (51.7) | 6 (10.0) | 6 (10.0) | 17 (28.3) | 37 (61.7%) |
| P value | 0.052 | 0.016 |
Table 3.
Comparison of short-term effects between L-ASP group and control group according to the clinical stages [number of cases (%)]
| Stage | Number of cases | L-ASP group | Number of cases | Control group | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| CR | PR | OR | CR | PR | OR | CR | OR | |||
| l+ll | 8 | 7 (87.5) | 0 (0) | 7(87.5) | 10 | 10 (100) | 0 (0) | 10 (100) | 0.444 | 0.444 |
| lll+lV | 34 | 12 (35.3) | 16 (47.1) | 28 (82.4) | 50 | 21 (42.0) | 6 (12.0) | 27 (54.0) | 0.537 | 0.007 |
Table 4.
Comparison of short-term effects between L-ASP group and control group according to the IPI scores [number of cases (%)]
| IPI score | Number of cases | L-ASP group | Number of cases | Control group | P value | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| CR | PR | OR | CR | PR | OR | CR | OR | |||
| 0-1 | 14 | 9 (64.3) | 3 (21.4) | 12 (85.7) | 18 | 15 (83.3) | 1 (5.5) | 16 (88.8) | 0.252 | 1.000 |
| 2-3 | 25 | 9 (36.0) | 12 (48.0) | 21 (84.0) | 35 | 14 (40.0) | 3 (18.6) | 17 (48.6) | 0.753 | 0.005 |
| 4-5 | 3 | 1 (33.3) | 1 (33.3) | 2 (66.7) | 7 | 2 (28.6) | 2 (28.6) | 4 (57.1) | 1.000 | 1.000 |
| 2-5 | 28 | 10 (35.7) | 13 (46.4) | 23 (82.1) | 42 | 16 (38.1) | 5 (11.9) | 1 (50.0) | 0.840 | 0.006 |
Long-term effects
The median overall survival time(OS)was 10.5 months (range, 1-47 months) in L-ASP group, while 13 months (range, 0.3-68 months) in non L-ASP group, and they had no statistically significance (P=0.754). Similarly, the progression free survival time (PFS) was 10 months (range, 1-47 months) in L-ASP group, while 11 months (range, 0.3-68 months) in non L-ASP group, also had no statistically significance (P=0.414). The 3-year OS rate of L-ASP group and non L-ASP group were 48.9% and 65.0% , respectively (P=0.974) (Figure 1) and the 3-year PFS rate of L-ASP group and non L-ASP group were 40.8% and 61.0%, respectively (P=0.479) (Figure 2). They all had no statistically significance.
Figure 1.
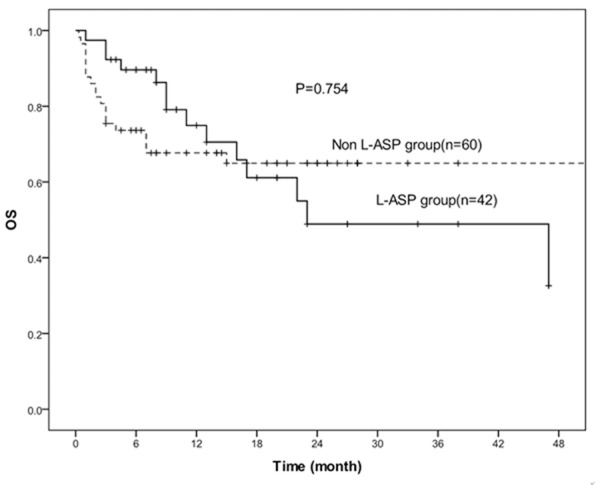
Kaplan-Meier estimates for the OS rates of 42 patients treated with L-asparaninase and 60 patients without L-asparaginase. There was no statistically significant difference between the two groups (P=0.754).
Figure 2.
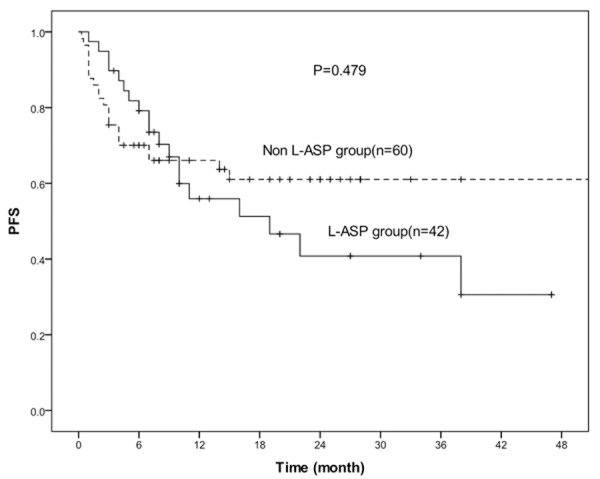
Kaplan-Meier estimates for the PFS rates of 42 patients treated with L-asparaninase and 60 patients without L-asparaginase. There was no statistically significant difference between the two groups (P=0.479).
Adverse reactions
Common adverse reactions of both groups included hypocytosis, elevated transaminase, decreased albumin and fever. Hypocytosis was manifested with anemia, neutropenia and thrombocytopenia. In grade I/II hypocytosis, the incidence of anemia was higher in the L-ASP group than in the non L-ASP group (P=0.005), but it could be improved by transfusion and supportive treatment; in grade III/IV, the incidence of neutropenia was higher in the L-ASP group than in the non L-ASP group (P=0.016), which probably led to higher incidence of fever in the L-ASP group than in the non L-ASP group, but fever could be controlled after active anti-infective treatment while no significant difference was noted in incidence of severe infection (grade III/IV) between the two groups (P=0.777). No significant difference was noted in the incidence of elevated transaminase between both groups (P=0.194); incidence of decreased albumin was higher in the L-ASP group than in the non L-ASP group (P=0.047). Incidence of coagulation disorders was higher in the L-ASP group than in the non L-ASP group (P=0.008), but no significant difference was noted in prolongation of PT and APTT between both groups, and coagulation function was recovered from supplementation of fibrinogen and plasma. Only one patient died of encephalorrhagia, which was manly attributed to his weak compliance and lack of close monitoring of the coagulation function. In the L-ASP group, allergic reaction occurred in 3 patients, being mainly manifested with rash, which was improved by drug withdrawal and anti-allergic therapy. One patient with aggressive NK/T cell lymphoma developed hyperamylasemia and acute pancreatitis, and the latter was improved after treatment, however, he failed to tolerate subsequent chemotherapy and died early due to his poor general condition. Two patients developed incomplete intestinal obstruction and were improved after treatment. One had elevated serum creatinine (grade II) and was improved after treatment (Table 5).
Table 5.
Comparison of incidence of adverse reactions between L-ASP group and control group [number of cases (%)]
| Adverse reaction | L-ASP (n=42) | Control (n=60) | P value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| l~ll | lll~lV | l~ll | lll~lV | l~ll | lll~lV | |
| Hematological toxicity | ||||||
| Anemia | 21 (50.0) | 16 (38.1) | 14 (23.3) | 26 (43.3) | 0.005 | 0.596 |
| Neutropenia | 3 (7.1) | 35 (83.3) | 8 (13.3) | 37 (61.7) | 0.310 | 0.016 |
| Thrombocytopenia | 10 (23.8 | 18 (42.9) | 11 (18.3) | 26 (43.3) | 0.503 | 0.962 |
| Elevated transaminase | 12 (28.6) | 4 (9.5) | 12 (20.0) | 2 (3.3) | 0.318 | 0.194 |
| Fever | 32 (76.2) | 1 (2.4) | 22 (36.7) | 2 (3.3) | 0.000 | 0.777 |
| Decreased albumin | 20 (47.6) | 17 (28.3) | 0.047 | |||
| Decreased FIB | 13 (31.0) | 6 (10.0) | 0.008 | |||
| Prolonged PT | 3 (7.1) | 3 (5.0) | 0.653 | |||
| Prolonged APTT | 6 (14.3) | 8 (13.3) | 0.891 | |||
| Allergic reaction | 3 (7.1) | 1 (1.7) | 0.161 | |||
| Hyperamylasemia | 1 (2.4) | 0 | ||||
| Acute pancreatitis | 1 (2.4) | 0 | ||||
| Incomplete intestinal obstruction | 2 (4.8) | 0 | ||||
| Elevated creatinine | 1 (2.4) | 0 | ||||
Note: FIB: fibrinogen; PT: prothrombin time; APTT: activated partial thromboplastin time.
Prognostic analysis
A univariate analysis was conducted of factors reflecting states of the first visit in patients, including gender, age, clinical stage, serum LDH level, ECOG score, absence or presence of B symptoms, number of extranodal invasions and serum ferritin (SF) level. Results showed that high clinical stage (P=0.001), elevated serum LDH level (P<0.001), high ECOG score (P<0.001), presence of B symptoms (P=0.016), elevated serum SF level (P=0.021) were influencing factors for poor prognosis, the others showed no statistical significance (P>0.05) (Table 6). Five significant factors in abovementioned univariate analysis entered into the COX regression model, showing that ECOG score was an independent risk factor influencing patients’ survival in individual group (RR=1.585, P=0.036) while clinical stages tended to be of independent prognostic significance (RR=1.992, P=0.063).
Table 6.
Univariate analysis of influence of prognosis of patients with incipient PTCL
| Factor | Number of cases | 1-year survival rate (%) | 2-year survival rate (%) | P value |
|---|---|---|---|---|
| Gender | 0.734 | |||
| Male | 70 | 70.2 | 56.0 | |
| Female | 26 | 73.1 | 68.2 | |
| Age (year) | 0.596 | |||
| ≤60 | 73 | 71.4 | 56.5 | |
| >60 | 23 | 69.7 | 69.7 | |
| Stage | 0.001 | |||
| l | 11 | 90.9 | 90.9 | |
| ll | 7 | 85.7 | 85.7 | |
| lll | 20 | 93.3 | 84.8 | |
| lV | 58 | 57.9 | 42.1 | |
| LDH (U/L) | <0.001 | |||
| ≤245 | 41 | 86.1 | 77.3 | |
| >245 | 52 | 57.4 | 41.7 | |
| ECOG score | <0.001 | |||
| 0 | 25 | 90.4 | 75.8 | |
| 1 | 51 | 74.2 | 66.2 | |
| 2 | 12 | 41.7 | 27.8 | |
| 3 | 8 | 37.5 | 0 | |
| Symptom B | 0.016 | |||
| Presence | 58 | 62.2 | 52.0 | |
| Absence | 38 | 84.5 | 71.1 | |
| Number of extranodal invasions | 0.929 | |||
| ≥2 | 23 | 68.6 | 60.1 | |
| <2 | 73 | 71.9 | 59.8 | |
| Ferritin (ng/ml) | 0.021 | |||
| >328 | 50 | 60.5 | 42.4 | |
| ≤328 | 35 | 77.5 | 68.9 |
Discussion
PTCLs are a series of malignant lymphoproliferative disorders with heterogeneity, derived from postthymic mature T-cells or natural killer cells. PTCLs account for 10% of non-Hodgkin’s lymphomas (NHLs) [6], but its morbidity varies in regions and races significantly, i.e., incidence of PTCLs is higher in East Asia, accounting for 20% to 30% of NHLs [7]. Of all patients with PTCLs, around 68%, 45%, 26% and 37% of them are manifested with diffuse disease process, systemic symptoms, bone marrow involvement and extranodal diseases, respectively, and 23% of them are complicated with hemophagocytic syndrome [8]. ALK-positive ALCL has a better prognosis, with a 5-year survival rate of 70%. However, survival rates of ALK-negative ALCL, PTCL-NOS and AITL are only 49%, 32% and 14%, respectively [9]. Conventional chemotherapy of PTCL usually has a poor outcome, prompting many studies to explore autologous stem cell transplantation (ASCT) as the first-line consolidation therapy. Several retrospective studies had revealed improved prognosis of PTCL patients. The 3-year OS rate was 53% to 58%, and the 3-year PFS rate was 44% to 50% [10]. The 2013 NCCN Guidelines recommended that all patients, except of ALK-positive ALCL with low IPI scores, should conduct ASCT as the first-line consolidation therapy. However, one recent study had indicated that ALK-positive ALCL patients with higher IPI scores still needed an ASCT therapy. Pre-transplant status was closely related to the prognosis. A French research team conducted a retrospective analysis of 77 patients who received allo-HSCT, showing that patients received transplantation after achieving CR or PR had a higher 5-year OS than those who were not (69% vs 29%); multivariate analysis showed that diseases being not at least in PR state before allo-SCT contributed to be an independent predictive factor for poor prognosis [11]. Hence, it is very important for patients to be at least in PR state through pre-transplant chemotherapy. CHOP and CHOP-like chemotherapies are the most commonly used conventional standard first-line treatment for PTCL patients. However, compared with diffuse large B cell lymphoma (DLBCL), except for ALK-positive ALCL, the outcomes of PTCLs are disappointing. Anthracyclines show poor efficacy for invasive T cell lymphoma, and the mechanism is related to the P-gp-induced multidrug resistance pathway [12]. L-ASP has a different mechanism of action from other P-gp-related chemotherapeutics, independent of high expression of P-gP. L-ASP can hydrolyse plasma L-asparagine into L-aspartic acid and ammonia; if L-asparagine, as an essential amino acid with which cells make proteins, if exhausting, it may lead to protein synthesis disorder and cell death [13]. Normal cells contain asparagine synthetase (AS) which synthesizes the asparagine itself, whereas lymphoma cells cannot. NHL cells are apt to cause cell death because they usually contain only a small number of AS and do not upregulate the AS gene [14]. Use of L-ASP-based chemotherapy regimens in acute lymphoblastic leukemia (ALL) and adult malignant lymphomas has a history of more than 30 years, and has attracted considerable attention in recent years. These regimens signifcantly improve the short-tserm effect and long-term survival for patients, particularly for NK/T cell lymphomas and prosomal T-immunoblastic lymphomas [15]. L-ASP-containing chemotherapy regimens can lead to an objective remission rate (ORR) of 79%, a CR rate of 63% and a 5-year OS rate of 66.9% in patients with refractory or relapsing extranodal NK/T-cell lymphoma [16-18]. In a phase I study, a new chemotherapy regimen of L-asparaginase in conjunction with ifosfamide, etoposide, dexamethasone and methotrexate (SMILE) allowed relapsing patients to achieve an overall response rate of 67% [19]. A further prospective phase II study had also investigated the efficacy of the SMILE regimen in patients with newly diagnosed stage IV, relapsed, or refractory NK/T-cell lymphoma [20], with an ORR of 74% and a CR rate of 38%. Tsutomu Takahashi et al. [21] reported an L-ASP-induced complete response in a relapsed patient with Epstein-Barr virus and cytotoxic peripheral T-cell lymphoma not otherwise specified, followed by allogeneic bone marrow transplantation (allo- BMT), and had continuous remission for a 2-year follow-up period since the treatment with L-ASP. Obama K et al. [22] reported an L-ASP-induced complete response in a patient with Epstein-Barr virus and multidrug resistant cutaneous T-cell lymphoma. In 2010, a phase II clinical study of the SMILE regimen in PTCLs (NKTSG-03) was launched in Japan, South Korea and China. Results of this study may reveal an effective type of PTCL to L-ASP containing chemotherapy. In view of few reports in respect to L-ASP in treating PTCLs at home and abroad so far and limitation to studies of NK/T-cell lymphoma, as well as only case reports in respect to other types, and results of NKTSG-03 have not been reported yet, we conducted this retrospective study in which untreated patients were enrolled. Statistical results found that the L-ASP group had a higher OR rate than the non L-ASP group, with a statistically significant difference. There was a particularly significant difference in stage III/IV patients with an IPI score of ≥2; among them, patients scored 2 to 3 points had significantly difference while those scored 4 to 5 had not, which we believe to be related to too small sample size and should expand the sample size to further verify. As for long-term effects, PFS and OS both were not improved, corresponding to multiple research institutions at home and abroad. This suggests, for PTCL patients, only transplantation can improve patients’ survival time. Using L-ASP containing chemotherapy regimens as the first-line treatment, especially in high-risk patients with advanced stages and higher IPI scores, can achieve higher short-term remission rate to offer opportunities for more patients to receive transplants, thereby improve PFS and long-term survival.
Multivariate analysis revealed that ECOG score was an important risk factor affecting survival of patients in the individual group. Whereas the multivariate analysis result of the clinical stage was (RR=1.992, P=0.063), which was likely to be an independent prognostic factor if the sample size was expanded to study further. These two results were consistent with the IPI scores we used so far, but others, such as age >60 years, elevation of LDH level, number of extranodal invasions ≥2, showed no independent prognostic significance in this study. IPI had a better prognostic value in B-NHL, and studies showed that IPI was also fit for prognosis of PTCL-NOS and ALCL, but further studies are necessary to investigate whether it is fit for other types of PTCL. Besides clinical prognostic factors, scholars are also positively studying biological and molecular prognostic factors, such as chemokine receptors, gene expression profile and others, i.e., Ki-67, cytotoxic molecules in azurophilic granules of T-lymphocytes and p53 [23,24], also, some scholars established new prognostic scoring systems based on clinical and molecular factors. However, prospective clinical studies are necessary to verify the superiority of new prognostic factors and scoring systems.
L-asparaginase leads to some adverse reactions, it was reported that some serious ones may cause allergic shock and severe acute pancreatitis. In this study, adverse reactions significantly increased by adding L-ASP included grade I/II anemia, grade III/IV neutropenia, grade I/II fever, decreased albumin and fibrinogen. In the case of medication after negative skin test, only three patients developed mild allergy, and there were also one with renal dysfunction and two with incomplete intestinal obstruction; mild adverse reactions were improved by symptomatic and supportive treatment. The L-ASP group had a higher incidence of grade III/IV neutropenia than the non L-ASP group, but there was no difference in consequent severe infection between both groups. Furthermore, other severe adverse reactions, i.e., encephalorrhagia and acute pancreatitis, occurred in one patient, respectively. Causes of death of the two patients were related to their poor compliance and progression of diseases. Hence, adverse reactions can be controlled when using L-ASP under the close observation of medical staff.
L-ASP has a significant effect on NK/T cell lymphomas, and there are also case reports regarding the successful treatment of other types of PTCLs. Furthermore, our retrospective analysis also confirmed significant short-term effects of L-ASP containing chemotherapy regimens and controllable adverse reactions, providing more opportunities for patients to receive transplantation and advantages in long-term survival after transplantation. A large prospective clinical trial of use of L-ASP in first-line consolidation treatment of PTCL is worthy of further research and investigation.
Acknowledgements
This work was supported in part by the Research Plan from the National Natural Science Foundation of China (No. 81372256), the Research Plan of Education Administration of Zhejiang Province, China (No. Y201327033) and the Research Plan of Science Technology Department of Zhejiang Province, China (No. 2013C33125).
Disclosure of conflict of interest
None.
References
- 1.Savage KJ. Aggressive peripheral T-cell lymphomas (specified and unspecified types) Hematology Am Soc Hematol Educ Program. 2005:267–277. doi: 10.1182/asheducation-2005.1.267. [DOI] [PubMed] [Google Scholar]
- 2.Weisenburger DD, Savage KJ, Harris NL, Muller-Hermelink K, Rudiger T, Coiffier B, Gascoyne RD, Berger F, Tobinai K, Au WY, Liang R, Montserrat E, Hochberg EP, Pileri S, Federico M, Nathwani B, Armitage JO, Weisenburger DD. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 3.Escalon MP, Liu NS, Yang Y, Hess M, Walker PL, Smith TL, Dang NH. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer. 2005;103:2091–2098. doi: 10.1002/cncr.20999. [DOI] [PubMed] [Google Scholar]
- 4.Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102:83–87. [PubMed] [Google Scholar]
- 5.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 6.Savage KJ. Peripheral T-cell lymphomas. Blood Rev. 2007;21:201–216. doi: 10.1016/j.blre.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Kojima H, Hasegawa Y, Suzukawa K, Mukai HY, Kaneko S, Kobayashi T, Kamoshita M, Shinagawa A, Komeno T, Komatsu T, Mitsuhashi S, Kawachi Y, Yamashita Y, Mori N, Nagasawa T. Clinicopathological features and prognostic factors of Japanese patients with “peripheral T-cell lymphoma, unspecified” diagnosed according to the WHO classification. Leuk Res. 2004;28:1287–1292. doi: 10.1016/j.leukres.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Xie W, Hu K, Xu F, Zhou D, He J, Shi J, Luo Y, Zhu J, Zhang J, Lin M, Ye X, Huang H, Cai Z. Clinical analysis and prognostic significance of lymphoma-associated hemophagocytosis in peripheral T cell lymphoma. Ann Hematol. 2013;92:481–486. doi: 10.1007/s00277-012-1644-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmitz N, Trumper L, Ziepert M, Nickelsen M, Ho AD, Metzner B, Peter N, Loeffler M, Rosenwald A, Pfreundschuh M. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116:3418–3425. doi: 10.1182/blood-2010-02-270785. [DOI] [PubMed] [Google Scholar]
- 10.Liu T. [Progress of diagnosis and treatment in peripheral T cell lymphoma] . Zhonghua Xue Ye Xue Za Zhi. 2014;35:361–366. doi: 10.3760/cma.j.issn.0253-2727.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Le Gouill S, Milpied N, Buzyn A, De Latour RP, Vernant JP, Mohty M, Moles MP, Bouabdallah K, Bulabois CE, Dupuis J, Rio B, Gratecos N, Yakoub-Agha I, Attal M, Tournilhac O, Decaudin D, Bourhis JH, Blaise D, Volteau C, Michallet M Société Française de Greffe de Moëlle et de Thérapie Cellulaire. Graft-versus-lymphoma effect for aggressive T-cell lymphomas in adults: a study by the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. J. Clin. Oncol. 2008;26:2264–2271. doi: 10.1200/JCO.2007.14.1366. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P-glycoprotein in previously untreated extranodal NK/T-cell lymphoma, nasal type. Am J Hematol. 2008;83:795–799. doi: 10.1002/ajh.21256. [DOI] [PubMed] [Google Scholar]
- 13.Cooney DA, Handschumacher RE. L-asparaginase and L-asparagine metabolism. Annu Rev Pharmacol. 1970;10:421–440. doi: 10.1146/annurev.pa.10.040170.002225. [DOI] [PubMed] [Google Scholar]
- 14.Haskell CM, Canellos GP. l-asparaginase resistance in human leukemia--asparagine synthetase. Biochem Pharmacol. 1969;18:2578–2580. doi: 10.1016/0006-2952(69)90375-x. [DOI] [PubMed] [Google Scholar]
- 15.Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, Katz J, Yu A, Laver J, Ravindranath Y, Kurtzberg J, Desai S, Camitta B, Murphy SB. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 16.Jaccard A, Gachard N, Marin B, Rogez S, Audrain M, Suarez F, Tilly H, Morschhauser F, Thieblemont C, Ysebaert L, Devidas A, Petit B, de Leval L, Gaulard P, Feuillard J, Bordessoule D, Hermine O GELA and GOELAMS Intergroup. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011;117:1834–1839. doi: 10.1182/blood-2010-09-307454. [DOI] [PubMed] [Google Scholar]
- 17.Yong W, Zheng W, Zhang Y, Zhu J, Wei Y, Zhu D, Li J. L-asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol. 2003;78:163–167. doi: 10.1007/BF02983387. [DOI] [PubMed] [Google Scholar]
- 18.Yong W, Zheng W, Zhu J, Zhang Y, Wang X, Xie Y, Lin N, Xu B, Lu A, Li J. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009;88:647–652. doi: 10.1007/s00277-008-0669-3. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi M, Suzuki R, Kwong YL, Kim WS, Hasegawa Y, Izutsu K, Suzumiya J, Okamura T, Nakamura S, Kawa K, Oshimi K. Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci. 2008;99:1016–1020. doi: 10.1111/j.1349-7006.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi M, Kwong YL, Kim WS, Maeda Y, Hashimoto C, Suh C, Izutsu K, Ishida F, Isobe Y, Sueoka E, Suzumiya J, Kodama T, Kimura H, Hyo R, Nakamura S, Oshimi K, Suzuki R. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J. Clin. Oncol. 2011;29:4410–4416. doi: 10.1200/JCO.2011.35.6287. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Ikejiri F, Onishi C, Kawakami K, Inoue M, Miyake T, Tanaka J, Araki A, Maruyama R, Ohshima K, Suzumiya J. L-asparaginase-induced complete response in a relapsed patient with Epstein-Barr virus and cytotoxic peripheral T-cell lymphoma not otherwise specified. Intern Med. 2010;49:2505–2508. doi: 10.2169/internalmedicine.49.4083. [DOI] [PubMed] [Google Scholar]
- 22.Obama K, Tara M, Niina K. L-asparaginase induced complete remission in Epstein-Barr virus positive, multidrug resistant, cutaneous T-cell lymphoma. Int J Hematol. 1999;69:260–262. [PubMed] [Google Scholar]
- 23.Tsuchiya T, Ohshima K, Karube K, Yamaguchi T, Suefuji H, Hamasaki M, Kawasaki C, Suzumiya J, Tomonaga M, Kikuchi M. Th1, Th2, and activated T-cell marker and clinical prognosis in peripheral T-cell lymphoma, unspecified: comparison with AILD, ALCL, lymphoblastic lymphoma, and ATLL. Blood. 2004;103:236–241. doi: 10.1182/blood-2002-05-1352. [DOI] [PubMed] [Google Scholar]
- 24.Ballester B, Ramuz O, Gisselbrecht C, Doucet G, Loï L, Loriod B, Bertucci F, Bouabdallah R, Devilard E, Carbuccia N, Mozziconacci MJ, Birnbaum D, Brousset P, Berger F, Salles G, Briere J, Houlgatte R, Gaulard P, Xerri L. Gene expression profiling identifies molecular subgroups among nodal peripheral T-cell lymphomas. Oncogene. 2006;25:1560–1570. doi: 10.1038/sj.onc.1209178. [DOI] [PubMed] [Google Scholar]


