Abstract
Chicken meat is a valuable source of protein and consumption of it continues to rise day to day. The aim of the present study was to evaluate the genetic homogeneity of Listeria monocytogenes (L. monocytogenes) isolates obtained from chicken carcasses and human. Random Amplification of Polymorphic DNA (RAPD) PCR with three different primers were used to analyze the 31 L. monocytogenes isolates recovered from human and chicken carcasses. Primers were D8635, HLWL74, and OPM01. Scanned images of RAPD-PCR products were analyzed using Photocap software. The data were analyzed by SPSS software using Jaccard distance matrix and Ward’s hierarchical cluster technique, isolates were clustered and displayed in dendrogram form. Molecular serotyping of the isolate was done. Most of the isolates were grouped into two serogroup IIb and IIa. However some of them were serotyped as IVb serogroup. In the RAPD assay, all of the primers gave amplified bands. Among these three primers, OPM01 had the most discriminatory power due to producing polymorph bands. Totally, 75 different bands with sizes ranging from 150 bp to 3300 bp, were produced. The dendrogram for Listeria monocytogenes isolates from chicken and human showed five different clusters (designed as A to E). In this study, there wasn’t any association between food and human isolates of L. monocytogenes. RAPD has more discriminatory power than serotyping. On the other hand, there were different RAPD profiles among isolates of the same serotype and also, similar RAPD profile among different serotypes were observed.
Keywords: RAPD PCR, Listeria monocytogenes, serotyping
Introduction
Listeria monocytogenes is a gram positive bacillus, which is widely distributed in nature. Listeria is able to infect wide variety of animals, however ruminants and rarely pigs develop disease. Birds are generally subclinical carriers of the organism. Direct transmission of Listeria monocytogenes from infected animals, especially during calving or lambing [1] are very rare. Zoonotic transmission of the disease to humans is not clear, and contamination of food-processing environment is of greater public health importance [2].
Listeria contaminate different types of food products and can survive in the environment for a long time. However, due to its multiplication in refrigerator temperature, readily transfer to other foods especially ready to eat ones. Because of L. monocytogenes pathogenicity in neonates, elderly and especially pregnant women, became a significant hazard to public health [3].
For a long time, food-borne listeriosis was considered as an invasive disease that affected only susceptible population groups, which rarely associated with gastrointestinal symptoms [3]. However, later noninvasive form of listeriosis was observed in persons with no predisposing conditions. These findings increase the public health significance of L. monocytogenes [4-8].
Although the morbidity of listeriosis is relatively low, the mortality of the systemic/encephalitic disease can be very high, with values of 20-30%. In Europe, the hospitalization rate is estimated at more than 95% [3].
In order to characterization of food and clinical Listeria monocytogenes strains different phenotypic and genotypic methods exist. Phenotypic methods as like as serotyping due to existence of untypable strains have low discriminating power. Therefore, genotyping method such as pulsed-field gel electrophoresis (PFGE) [9] and random amplified polymorphic DNA (RAPD) [10] with higher discriminatory power are needed.
A rapid and highly promising tool for discrimination of L. monocytogenes strains is RAPD [11]. RAPD has been widely used in epidemiological studies [12-14]. Although, investigations on the association of isolates from foods and clinical incidences showed the diversity of L. monocytogenes was unable to cluster the causative agent to the source [13,15].
However, the RAPD-PCR is a suitable technique for monitoring strains on a wide scale and for comparison of whole genome diversity [16].
In this paper, different strains of L. monocytogenes recovered from chicken carcasses and human patients were used in RAPD test, with three different primer in order to investigate their genetic homogeneity.
Materials and methods
Isolation of L. monocytogenes
A total of 31 isolates of L. monocytogenes were recovered from chicken carcasses (26 isolates) and Human (5 isolates from CSF samples). Those isolates were detected by biochemical method and were confirmed by molecular method. Serotyping were carried out according to Kérouanton et al. [17]. The human isolates had been recovered from different area and further analysis was done in the laboratory (unpublished data).
DNA isolation
DNA templates were prepared using freshly grown Listeria monocytogenes on Oxford Agar, a loop full of bacteria were added to 250 μL sterile distilled water and boiled in a water bath at 100°C for 10 min [18]. Template DNA was stored at -20°C until used for PCR reactions.
Random amplified polymorphic DNA analysis
The 31 isolates were analyzed using RAPD PCR with three different primers, including D8635 [19], HLWL74 [20] and OPM01 [21], which had been previously published. Table 1 showed the sequence of these primers. These three primers were synthetized by Macrogen, Korea. In each reaction 10 picomol of primer and 20 nanogram of extracted DNA was used. In fact, all reaction mixtures were carried out in a final volume of 25 µl and amplified by a thermal cycler (Techne TC-512, UK).
Table 1.
Sequence of RAPD primers
The amplification reaction for D8635 primer was followed by a 35-cycles program: Denaturation at 94°C for 4 min; Annealing at 39°C for 45 s; and extension at 72°C for 1 min. For HLWL74, the thermal condition was as following: an initial denaturation step of 95°C for 4 min, followed by 45 amplification cycles of 1 min at 95°C, 2 min at 35°C, and 1 min at 72°C and followed by a final extension of 72°C for 10 min. In the case of OPM01 primer, the reaction mixture was cycled through the following temperature profile, 44 cycles: 94°C for 1 min, 2 min at 30°C, and 72°C for 2 min. This was followed by a final cycle at 94°C for 1 min, 30°C for 2 min, and 72°C for 10 min.
Four microliter of PCR products were separated on a 1% agarose gel which was pre-stained by ethidium bromide and visualized under UV illumination. All isolates were analyzed at least twice and controls were included in all the reactions to ensure reproducibility.
Interpretation of PCR fingerprint images and statistical analysis
Scanned images were analyzed using Photocap software. Bands were assigned on a presence-absence basis. The software estimated band sizes for RAPD PCR data.
The data were analyzed using SPSS software, ver.16. Because the data were binary, in order to classify the isolates Jaccard distance matrix and Ward’s hierarchical cluster technique were used. Isolates were clustered and displayed in dendrogram form.
Results
Serotyping
From the 31 isolates of Listeria monocytogenes, 19 (18 of chicken carcasses and 1 from human patients) of them were classified as IIb serogroup, which is mostly containing 1.2b and 3b serovars of L. monocytogenes. The next common serogroup was IIa (L. monocytogenes serovars 1/2a, 1/2c, 3a, and 3c) which contained 9 isolates (7 of chicken carcasses and 2 from human patients). At the last was IVb serogroup (L. monocytogenes serovars 4b, 4d, and 4e) which consisted of 3 isolates (1 of chicken carcasses and 2 from human patients).
Analysis of the amplified DNA polymorphisms
All of the primers gave amplified bands. Among these three primers, OPM01 had the most discriminatory power due to producing polymorph bands. Totally, 75 different bands with sizes ranging from 150 bp to 3300 bp, were produced. Fifteen bands were polymorph. According to this, low polymorphism (20%) were detected among these isolates. Otherwise, genetic diversity was low among 31 isolates.
In our study, 60 monomorph bands were detected among 31 isolates of L. monocytogenes. No unique band was detected among these isolates.
Similarity matrix was constructed based on the presence or absence of a band for each isolate which was scored as 1 and 0, respectively.
The dendrogram for L. monocytogenes isolates from chicken and human (Showed Five different clusters (designed as A to E) (Figure 1).
Figure 1.
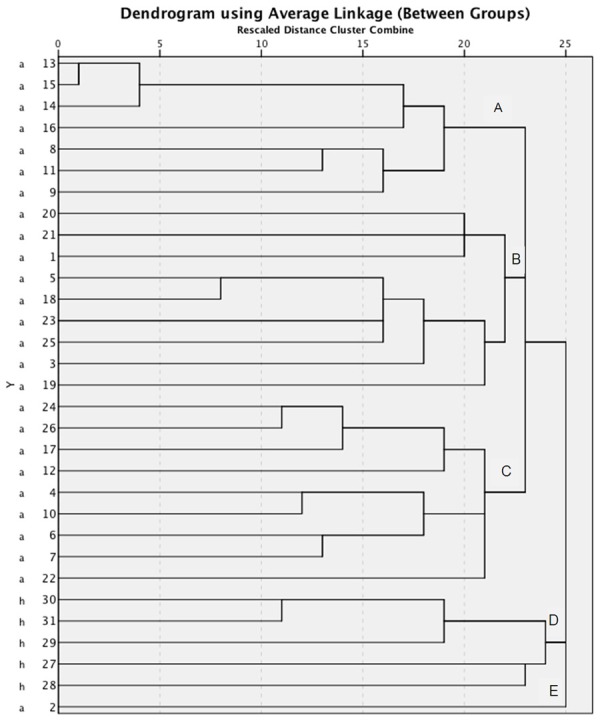
Dendrogram of chicken (a) and human (h) Listeria monocytogenes isolates using three random primers. A to E showed different clusters.
All human isolates were grouped in one cluster, cluster D (27 h, 28 h, 29 h, 30 h, 31 h). 12 (38.7%) isolates displayed a single RAPD profile (cluster C) which all of them were isolated from chicken carcasses (1a, 4a, 6a, 7a, 10a, 12a, 17a, 20a, 21a, 22a, 24a, and 26a).
The second most common RAPD profile, cluster A, accounted for 22.6% of isolates (7 of 31 isolates). Also, this profile consisted of chicken carcass isolates. However, type A appeared to be more prevalent in two points of sampling area. The most genetic similarity (86.7%) was seen between 13a and 15a isolates. 14a and 15a had about 80% genetic similarity.
The remaining of L. monocytogenes isolates (38.7%) distributed in three profiles. RAPD profile, type B, consisted of 6 isolates (3a, 5a, 18a, 19a, 23a and 25a) from chicken carcasses, but type E consisted of only one L. monocytogenes isolate from chicken carcass (2a).
Type D, RAPD profile, contained all human isolates.
RAPD showed a reproducibility level of more than 95%. Control assays, which contained no cell lysate, yielded no detectable amplified product.
Discussion
Molecular methods such as RAPD-PCR technique and pulsed field gel electrophoresis (PFGE) are the two main methods used by different authors for bacterial strain characterization. Typing of L. monocytogenes is very important for identifying the sources of contamination and investigation of foodborne outbreaks [22].
Unlike developed countries, organized studies on the association of pathogenic L. monocytogenes with human listeriosis are lacked in Iran. L. monocytogenes has been isolated from different sources, including foods of animal origin and clinical cases in animals and humans [23-25].
Medrala et al. was used RAPD-PCR to establish the persistence of L. monocytogenes strains isolated from products in a Polish fish-processing plant. Moreover, molecular epidemiology of L. monocytogenes strains in cheese [16], poultry, and pork [27] plants were investigated. In these papers different levels of differentiation were obtained.
World Health Organization suggested more studies to make RAPD test as a standard method for wide scale typing of L. monocytogenes [22].
In this study, a comparison of Chicken and human L. monocytogenes isolates was performed by RAPD PCR using three different primers. RAPD analysis of 26 isolates of L. monocytogenes from chicken carcasses and 5 isolates recovered from human patients revealed five major clusters designated as A to E.
Clusters A, B, C and E belonged to isolates of chicken carcasses, but cluster D belonged to human isolates.
Cluster A was constituted of two serogroup, IIa (8a, 9a, 13a and 15a) and IIb (11a, 14a and 16a) (unpublished data). Also, cluster B contained 2 different serogroups. Isolates no. 5a, 18a, 23a and 25a belonged to serogroup IIb and were associated to cluster B. Also, isolate 3a belonged to these cluster and the IIa serogroup. But, cluster C include 3 serogroups, IIa (4a and 10a), IIb and IVb (6a). However, identical RAPD profiles were also observed for some isolates distinguishable by serotyping. Similar results were obtained by other researchers [13,21,28].
Whereas the isolates from clusters A to C produced mixed profile pertaining to their serogroups, but cluster E only consists of one isolate (2a) belonged to serogroup IIb (1.2b, 3b) (unpublished data). Similar findings were also reported by earlier studies [19].
At a genetic similarity of more than 70%, 3 different RAPD types (13a, 14a and 15a) were detected and all of them were isolated from the same area of sampling. Two of them (13a and 15a) belonged to the same serovar group i.e. 1.2a, 3a, 1.2c and 3c. Also, they have more than 80% similarity. Strains of lactobacillus, which had common origin in terms of their area had identical RAPD profiles has been reported by Corroler et al. [29].
In Current study, RAPD test offered greater discrimination of isolates rather than serotyping. Isolates no. 14a, 5a and 20a had the same serogroup but classified in three different RAPD type A, B and C respectively. This technique allowed discrimination among isolates of the same serogroup and also among isolates recovered from the same sampling areas or from those isolated from different areas [28]. This would suggest that the regions of amplification of DNA in the PCR are not serotype specific [21].
Isolates belonged to IIa serogroup distributed in different clusters of chicken isolates. It may be related to heterogeneous nature of this serogroup.
In addition, five isolates from chicken (isolate no. 20a, 21a, 22a, 24a and 26a), had similar RAPD profile (cluster C), belonged to the same serovar group.
On the other hand, three isolates from chicken carcasses (isolate no. 1a, 6a and 4a), had identical RAPD profiles (cluster C), belonged to different serovar group (1/2b, 3b and 4b, 4d, 4e and 1/2a, 3a) and were isolated from different areas. Similar findings were reported in other studies [13,14,20,21,30].
The three isolates (5a, 11a and 21a) recovered from chicken, belonged to the same serovar group (1.2b, 3b), but, RAPD analysis of these isolates revealed three different profile A, B, and C. Amount of 12 kinds of RAPD profiles were observed for serogroup 4 [21]. In addition, the observation of most of the L. monocytogenes isolates of potential serotype 1.2b is of public health concern, as serotype 1.2b has been one of the serotype mainly associated with human listeriosis.
In conclusion, RAPD has certain advantages over more-traditional typing techniques such as serotyping, because RAPD offers greater discrimination of isolates. So, combination of serotyping and RAPD offer a higher level of differentiation rather than each method used alone. Although, the number of human isolates was low, however, in this study, there was no association between food and human isolates of L. monocytogenes. On the other hand, there were different RAPD profiles among isolates of the same serotype and also, similar RAPD profile among different serotypes were observed. Also, OPM01 had the most discriminatory power among the three used primers.
This study also demonstrates the rationale for further research into the safety of final food products sold at restaurants or caterings. This information may also be useful to local and state regulatory officials responsible for food safety.
Acknowledgements
The authors would like to sincere their special thanks for veterinary medicine College of Ferdowsi University of Mashhad for financial support of this study (Grant No. 3/28199) and also, Mrs. Khajehnasiri for her technical assistance. The authors also declared that there is no conflict of interest in the findings of this study.
Disclosure of conflict of interest
None.
References
- 1.Wesley GN. Listeriosis in Animals. In: Ryser E, Marth E, editors. Listeria, Listeriosis, and Food Safety. New York: Marcel Dekker; 1999. pp. 39–73. [Google Scholar]
- 2.Roberts AJ, Wiedemann M. Pathogen, host and environmental factors contributing to the pathogenesis of listeriosis. Cell Mol Life Sci. 2003;60:904–918. doi: 10.1007/s00018-003-2225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slutsker L, Schuchat A. Ryser E, Marth E. Listeria, Listeriosis, and Food Safety. New York: Marcel Dekker; 1999. Listeriosis in Humans; pp. 75–95. [Google Scholar]
- 4.Aureli P, Fiorucci GC, Caroli D, Marchiaro G, Novara O, Leone L, Salmaso S. An outbreak of febrile gastroenteritis associated with corn contaminated by Listeria monocytogenes . N Engl J Med. 2000;342:1236–1241. doi: 10.1056/NEJM200004273421702. [DOI] [PubMed] [Google Scholar]
- 5.Dalton C, Austin C, Sobel J, Hayes PS, Bibb WF, Graves LM, Swaminathan B, Proctor ME, Griffin PM. An outbreak of gastroenteritis and fever due to Listeria monocytogenes in milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 6.Heitmann M, Gerner-Smidt P, Heltberg O. Gastroenteritis caused by Listeria monocytogenes in a private day-care facility. Pediatr Infect Dis J. 1997;16:827–882. doi: 10.1097/00006454-199708000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen MK, Siitonen A, Heiskanen P, Haajanen H, Bjfrkroth KJ, Korkeala HJ. Molecular epidemiology of an outbreak of febrile gastroenteritis caused by Listeria monocytogenes in cold-smoked rainbow trout. J Clin Microbiol. 1999;37:2358–2360. doi: 10.1128/jcm.37.7.2358-2360.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salamina G, Dalle Donne E, Niccolini A, Poda G, Cesaroni D, Bucci M, Fini R, Maldini M, Schuchat A, Swaminathan B, Bibb W, Rocourt J, Binkin N, Salmaso S. A foodborne outbreak of gastroenteritis involving Listeria monocytogenes . Epidemiol Infect. 1996;117:429–436. doi: 10.1017/s0950268800059082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerouanton A, Brisabois A, Denoyer E, Dilasser F, Grout J, Salvat G, Picard B. Comparison of five typing methods for the epidemiological study of Listeria monocytogenes . Int J Food Microbiol. 1998;43:61–71. doi: 10.1016/s0168-1605(98)00098-1. [DOI] [PubMed] [Google Scholar]
- 10.Vogel BF, Jorgensen LV, Ojeniyi B, Huss HH, Gram L. Diversity of Listeria monocytogenes isolates from cold-smoked salmon produced in different smokehouses as assessed by random amplified polymorphic DNA analyses. Int J Food Microbiol. 2001;65:283–292. doi: 10.1016/s0168-1605(00)00503-1. [DOI] [PubMed] [Google Scholar]
- 11.Boerlin P, Bannerman E, Ischer F, Rocourt J, Bille J. Typing Listeria monocytogenes: a comparison of random amplification on polymorphic DNA with 5 other methods. Res Microbiol. 1995;146:35–49. doi: 10.1016/0923-2508(96)80269-5. [DOI] [PubMed] [Google Scholar]
- 12.Giovannacci I, Ragimbeau C, Queguiner S, Salvat G, Vendeuvre JL, Carlier V, Ermel G. Listeria monocytogenes in pork slaughtering and cutting plants: use of RAPD, PFGE and PCR-REA for tracing and molecular epidemiology. Int J Food Microbiol. 1999;53:127–140. doi: 10.1016/s0168-1605(99)00141-5. [DOI] [PubMed] [Google Scholar]
- 13.Aurora R, Prakash A, Prakash S. Genotypic characterization of Listeria monocytogenes isolated from milk and ready-to-eat indigenous milk products. Food Control. 2009;20:835–839. [Google Scholar]
- 14.Atil E, Ertas HB, Ozbey G. Isolation and molecular characterization of Listeria spp. from animals, food and environmental samples. Vet Med. 2011;56:386–394. [Google Scholar]
- 15.Martinez I, Rorvik LM, Brox V, Lassen J, Seppola M, Gram L, Fonnesbech-Vogel B. Genetic variability among isolates of Listeria monocytogenes from food products, clinical samples and processing environments, estimated by RAPD typing. Int J Food Microbiol. 2003;84:285–297. doi: 10.1016/s0168-1605(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 16.Wagner M, Maderner A, Brandl E. Random amplification of polymorphic DNA for tracing and molecular epidemiology of Listeria contamination in a cheese plant. J Food Protect. 1996;59:384–389. doi: 10.4315/0362-028X-59.4.384. [DOI] [PubMed] [Google Scholar]
- 17.Kérouanton A, Marault M, Petit L, Grout J, Dao TT, Brisabois A. Evaluation of a multiplex PCR assay as an alternative method for Listeria monocytogenes serotyping. J Microbiol Meth. 2010;80:134–137. doi: 10.1016/j.mimet.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Adzitey F, Rusul G, Huda N, Cogan T, Corry J. Prevalence, antibiotic resistance and RAPD typing of Campylobacter species isolated from ducks, duck rearing and processing environments in Penang, Malaysia. Int J Food Microbiol. 2012;154:197–205. doi: 10.1016/j.ijfoodmicro.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Cocolin L, Stella S, Nappi R, Bozzetta E, Cantoni C, Comi G. Analysis of PCR-based methods for characterization of Listeria monocytogenes strains isolated from different sources. Int J Food Microbiol. 2005;103:167–178. doi: 10.1016/j.ijfoodmicro.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 20.Park S, Jung J, Choi S, Oh Y, Lee J, Chae H, Ryu S, Jung H, Park G, Choi S, Kim B, Kim J, Chae YZ, Jung B, Lee M, Kim H. Molecular Characterization of Listeria monocytogenes Based on the PFGE and RAPD in Korea. Adv Microbiol. 2012;2:605–616. [Google Scholar]
- 21.Lawrence LM, Harvey J, Gilmour A. Development of a random amplification of polymorphic DNA typing method for Listeria monocytogenes . Appl Environ Microbiol. 1993;59:3117–3119. doi: 10.1128/aem.59.9.3117-3119.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wernars K, Boerlin P, Audurier A, Russell EG, Curtis GD, Herman L, van der Mee-Marquet N. The WHO multicenter study on Listeria monocytogenes subtyping: random amplification of polymorphic DNA (RAPD) Int J Food Microbiol. 1996;32:325–41. doi: 10.1016/s0168-1605(96)01146-4. [DOI] [PubMed] [Google Scholar]
- 23.Fallah AA, Saei-Dehkordi SS, Rahnama M, Tahmasby H, Mahzounieh MR. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control. 2012;28:327–332. [Google Scholar]
- 24.Safarpoor Dehkordi F, Barati S, Momtaz H, HosseiniAhari SN, Nejat Dehkordi S. Comparison of Shedding, and Antibiotic Resistance Properties of Listeria monocytogenes Isolated From Milk, Feces, Urine, and Vaginal Secretion of Bovine, Ovine, Caprine, Buffalo, and Camel Species in Iran. Jundishapur J Microbiol. 2013;6:284–294. [Google Scholar]
- 25.Eslami G, Samadi R, Taherpanah R, Taherpor A, Baseri N. Detection of actA and InlB genes in Listeria monocytogenes Isolated from women with Spontaneous abortions. Novel Biomed. 2014;2:18–21. [Google Scholar]
- 26.Medrala D, Dabrowski W, Czekajlo-Kolodziej U, Daczkowska-Kozon E, Koronkiewicz A, Augustynowicz E, Manzano M. Persistence of Listeria monocytogenes strains isolated from products in a Polish fish-processing plant of a 1-year period. Food Microbiol. 2003;20:715–724. doi: 10.1078/1438-4639-00243. [DOI] [PubMed] [Google Scholar]
- 27.Chasseignaux E, Toquin MT, Ragimbeau C, Salvat G, Colin P, Ermel G. Molecular epidemiology of Listeria monocytogenes isolates collected from the environment, raw meat and raw products in two poultry- and pork-processing plants. J Appl Microbiol. 2001;91:888–899. doi: 10.1046/j.1365-2672.2001.01445.x. [DOI] [PubMed] [Google Scholar]
- 28.Mazurier SI, Wernars K. Typing of Listeria strains by random amplification of polymorphic DNA. Res Microbiol. 1992;143:499–505. doi: 10.1016/0923-2508(92)90096-7. [DOI] [PubMed] [Google Scholar]
- 29.Corroler D, Mangin I, Desmasures N, Gueguen M. An ecological study of lactococci isolated from raw milk in the camembert cheese registered designation of origin area. Appl Environ Microbiol. 1998;64:4729–4735. doi: 10.1128/aem.64.12.4729-4735.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lawrence L, Gilmour A. Characterization of Listeria monocytogenes isolated from poultry products and from the poultry-processing environment by random amplification of polymorphic DNA and multilocus enzyme electrophoresis. Appl Environ Microbiol. 1995;61:2139–2144. doi: 10.1128/aem.61.6.2139-2144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


