Abstract
Background: Recent studies have suggested soluble tumor necrotizing factor-like weak inducer of apoptosis (sTWEAK) and sCD163 may be a potential cardiovascular biomarker. We aimed to evaluate sTWEAK and sCD163 levels and predictive values in patients with chronic coronary artery disease (CAD) and acute coronary syndrome (ACS). Methods: Two hundred fourteen angiography-made patients were enrolled in the study and divided into 3 groups: 30 controls with normal angiograms, 99 patients with ACS, 85 patients with chronic CAD. sTWEAK, sCD163 and CRP levels were measured. Receivers operating characteristic (ROC) curve analysis were performed to determine the predictive values of sTWEAK and sCD163 levels and the sCD163/sTWEAK ratio. Gensini scores were used to assess severity of CAD. Results: sTWEAK levels in chronic CAD and ACS patients were lower compared to the control group (P<0.0001). sCD163 levels (P<0.0001) and the sCD163/sTWEAK ratio (P<0.0001) were higher in the ACS patients compared to the control and chronic CAD patients. ROC analysis revealed low sTWEAK level and high sCD163/sTWEAK ratio predicted chronic CAD, and low sTWEAK, high sCD163, CRP levels and sCD163/sTWEAK ratio predicted ACS. According to ROC analyses, significance of sTWEAK levels for chronic CAD was more marked compared to ACS (P<0.0001 vs P=0.001) and significance of sCD163/sTWEAK ratio was greater than sTWEAK for ACS (P<0.0001 vs P=0.001). These parameters didn’t correlate with severity of disease, obtained gensini scoring, in chronic CAD. Conclusions: It was concluded thatsTWEAK level may be a diagnostic marker of especially chronic CAD, sCD163 level of ACS, and the sCD163/sTWEAK ratio of both chronic CAD and ACS.
Keywords: sTWEAK, sCD163, coronary artery disease, acute coronary syndrome, gensini scoring
Introduction
Atherosclerosis related cardiovascular diseases are the most important cause of death worldwide. Various biomarkers have been investigated in order to identify patients at high risk for atherosclerosis. The most widely investigated molecule for this purpose, C-reactive protein (CRP), has only a moderate predictive value and is not widely used in clinical practice [1,2]. CD40L, monocyte chemoattractant protein-1 (MCP-1), adhesion molecules, myeloperoxidase and various interleukins have been investigated for that purpose in addition to CRP in recent years, but no significant additional predictive value has been determined [3].
Recent studies have suggested that tumor necrotizing factor-like weak inducer of apoptosis (TWEAK) may be a potential cardiovascular biomarker [4,5]. TWEAK was first described in 1997 and is a cytokine from the tumor-necrotizing factor (TNF) super family. It is initially expressed as a transmembrane protein and is released into the extracellular space in the form of a shorter bioactive fragment known as soluble TWEAK (sTWEAK) [5]. TWEAK is involved in cell proliferation, differentiation, migration, growth, apoptosis and angiogenesis, depending on the type of cell involved, and stimulates expression of several proinflammatory proteins [5-8].
Two receptors have been described for TWEAK, Fn14 (human fibroblast growth factor-inducible 14) [9-11] and CD163 [11]. Fn14 is the first receptor described. It has been suggested that TWEAK exhibits proatherogenic effects by binding to this receptor [8]. CD163, a scavenger described receptor in recent years, neutralizes TWEAK by binding to it [12]. Soluble CD163 (sCD163) results from the proteolytic shedding of CD163 on the cell surface, and its levels in plasma can be measured [11].
The main characteristic of CD163 is that its acts as a scavenger receptor for Hp-Hb complexes in intralesional hemorrhages [13]. Its atheroprotective effect is the result of the removal of pro-oxidant Hb from the vessel and induction of anti-inflammatory pathways by IL-10 and hemeoxygenase [14]. Expression and levels of CD163 have been shown to rise in acute and chronic inflammatory conditions associated with macrophage activation [15]. sCD163 is thought to be a potential biomarker in inflammatory conditions (such as the autoimmune diseases and atherosclerosis) [16,17]. TWEAK possesses a similar structure of the Hb-Hp complex binding site, and competes with that complex to bind to CD163. TWEAK neutralization in conditions of oxidative stress and inflammation is inhibited by these complexes, and this cytokine becomes suitable for the Fn14 receptor in cells [12].
To our knowledge, there is no report investigating levels of sTWEAK and sCD163 together in patients with angiographically diagnosed chronic coronary artery disease (CAD) and acute coronary syndrome (ACS). The purpose of this study was to evaluate sTWEAK and sCD163 levels in patients with chronic CAD and ACS, the relation between them and the predictive significance of these parameters.
Material and methods
Study population
Two hundred fourteen patients who referred coronary care unit and performed coronary angiography between October 2011 and December 2012 were included in the study. Patients who fulfilled inclusion criteria recruited consecutively, and divided into 3 groups on the basis of their clinical status, 30 controls with normal angiograms, 99 patients with ACS, 85 patients with chronic CAD.
Detailed clinical information was obtained from all patients, and physical examinations were performed. Patients’ height, weight and waist were measured. All patients were investigated in terms of atherosclerotic risk factors such as age, gender, family history, diabetes, hyperlipidemia, hypertension and cigarette use. Patients with heart failure, prior stroke, periphery artery disease, immunodeficiency or HIV-positive status, malignant neoplasm, severe renal/hepatic disease, endocrine disorder or systemic infection, with a recent history of surgery, pregnant women and subjects aged under 18 were excluded. Local ethical committee approval was obtained for the study.
Evaluation of biochemical parameters
Blood samples were obtained after a 12 hour overnight fasting. After the subjects rested for 15 min, blood samples were collected into tubes with and without ethylenediamine tetraacetic acid anticoagulant to obtain serum and plasma. Blood samples of patients presenting with ACS were drawn within the first 24 h after admission to hospital. Samples were obtained by low-speed centrifugation at 1500 × g for 15 min at 4°C. To reduce inter-assay variation, samples were stored at-80°C and analyzed at end of the study. sTWEAK (Human TWEAK instant enzyme-linked immunosorbent assay kit, eBioscience, catalog no: BMS2006INST) and sCD163 (Human CD163 kit, R& D systems, catalog no: DC1630) levels were determined by using commercialELISA kits. Specimens were examined in accordance with the kit procedures. Specimen concentrations were calculated using the formula obtained from the standard figures using kit standards.
Serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and glucose levels were determined by enzymatic methods using ROCHE autoanalyzer (Modular system, Gmbh, Mannheim, Germany). Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated using Friedewald’s equation. CRP, well-known inflammatory marker, was assessed using the latex enhanced immunonephelometric method (DABE BEHRING, BN II, Gmbh, Marburg, Germany).
Coronary angiography
Selective coronary angiography was performed with a right femoral approach, using a Siemens Axiom Artis coronary angiography device and the standard Judkins technique. Coronary arteries were visualized in the right and left oblique positions using cranial and caudal angulation. Coronary angiography analyses were performed by specialist cardiologists. In the epicardial arteries (including side branches), coronary angiography was regarded as normal in the absence of plaque, marginal irregularities, ectasia and slow flow. Cases with at least one of these were assessed as patients having CAD. Gensini scores were used to assess severity of CAD [18]. On the basis of that system, angiographic narrowing of 0-25% was given 1 point, 25-50% 2 points, 50-75% 4 points, 75-90% 8 points, 90-99% 16 points and 100% complete obstruction was given 32 points. These were then multiplied by a coefficient defined for each main coronary artery and each segment (5 points for left main coronary lesion, 2.5 points for proximal left anterior descending branch and left circumflex artery, 1.5 points for middle left descending artery lesion, 1 point for the diagonal branch and obtuse marginal branches and 0.5 points for the second diagonal and left circumflex artery posterolateral branch). The results were then added to give a Gensini score for each patient group. Total Gensini score <20 was regarded as mild CAD and >20 as severe CAD [19].
Statistical analysis
Variables were expressed as mean±standard deviation or percentage. The distribution of variables was assessed by Kolmogorov-Smirnov test. Patient characteristics and risk factors were compared using the chi-square test. Normally distributed continuous variables were compared using ANOVA with post-hoc analysis using Bonferroni test and Student’s t test. Not-normally distributed data was compared using Kruskal Wallis test and Mann Whitney U test. Skewed variables (sTWEAK, sCD163, CD163/sTWEAK and CRP) were logarithmically transformed prior to statistical analyses to approximate a normal distribution. Relations between normally and non-normally distributed continuous variables were assessed using Pearson’s or Spearman’s correlation, respectively. Receiver operating characteristic (ROC) curve analysis was performed to determine the predictive values of sTWEAK and sCD163 levels and the sCD163/sTWEAK ratio. All statistical analysis were performed on SPSS (13.0, SPSS Inc, Chicago, Illinois). A p value <0.05 was considered to indicate statistical significance; all tests were two-sided.
Results
Patient characteristics
Two hundred fourteen patients, 57 women (26.6%) and 157 men (73.4%) were included in the study. Mean age was 62.4±12.1 years. Ninety nine patients were enrolled in the ACS subgroup, 24 women (24.2%) and 75 men (75.8%). Mean age of the ACS patients was 63.8±11.4 years. Thirty one of the ACS patients had unstable angina, 36 had myocardial infarction (MI) with non-ST elevation and 32 had MI with ST elevation. The baseline characteristics and several biochemical parameters of the patients and the controls were shown in Table 1.
Table 1.
Baseline group characteristics and laboratory of the study population
| Control N=30 | ACS N=99 | Chronic CAD N=85 | P-value | |
|---|---|---|---|---|
| Age, years | 62.9±11.5 | 63.8±11.4 | 62.9±11.5 | 0.09 |
| Female, % | 53.3 | 24.2* | 20* | 0.001 |
| BMI, kg/m2 | 28±4.26 | 28.6±3.8 | 29.2±4 | 0.39 |
| Waist, cm | 97.3±11.2 | 99.8±11.2 | 101.4±10 | 0.18 |
| Hypertension, % | 40 | 59.6 | 49.4 | 0.29 |
| Diabetes mellitus, % | 3.3 | 21.2 | 23.5 | 0.12 |
| Smoking, % | 3.3 | 31.3* | 20* | 0.004 |
| Family history, % | 37.9 | 34.3 | 45.7 | 0.29 |
| Hyperlipidemia, % | 20 | 29.3 | 50.6*,** | 0.001 |
| SBP, mmHg | 125±13 | 125±16 | 125±13 | 0.99 |
| DBP, mmHg | 73±12 | 71±11 | 74±10** | 0.01 |
| Heart rate, beats/min | 73.1±8.2 | 73.5±11.3 | 72.2±12.6 | 0.72 |
| History of MI, % | - | 20.2 | 22.4 | 0.72 |
| History of CABG, % | - | 10.1 | 10.6 | 0.18 |
| ASA, % | 16.7 | 39.4* | 47.1* | 0.01 |
| Clopidogrel, % | - | 17.2 | 14.1 | 0.05 |
| BB, % | 13.3 | 29.3* | 42.4* | 0.01 |
| ACE inhibitor, % | 16.7 | 23.2* | 35.6* | 0.04 |
| Statin, % | 3.3 | 25.3 | 32.9 | 0.06 |
| Spironolactone, % | - | 3 | 4.7 | 0.45 |
| TC, mg/dL | 170±34 | 172±41 | 164±42 | 0.38 |
| TG, mg/dL | 131±71 | 137±70 | 136±60 | 0.66 |
| LDL-C, mg/dL | 120±30 | 126±35 | 118±38 | 0.31 |
| HDL-C, mg/dL | 41.5±10.2 | 36.5±7.5* | 36.2±6.7** | 0.004 |
| sTWEAK, pg/mL | 799±208 | 756±624* | 728±652* | <0.0001 |
| sCD163, ng/mL | 593±212 | 866±282* | 643±28** | <0.0001 |
| sCD163/sTWEAK | 0.8±0.3 | 1.6±0.9* | 1.2±0.6** | <0.0001 |
| CRP, mg/dL | 0.9±1.6 | 1.5±2.6* | 1.2±3.6** | 0.007 |
Data expressed as mean±SD or percentage. Statistical significance was set at p-value <0.05. Bold values indicate significant values (P<0.05). ACE: angiotensin converting enzyme; ASA: acetyl salicylic acid; BB: beta blocker; BMI: body mass index; CRP: C-reactive protein; CABG: coronary artery bypass graft; DB, diastolic blood pressure; HDL-C: high-density lipoprotein-cholesterol; LDL-C: low-density lipoprotein-cholesterol; MI: myocardial infarction; SBP: systolic blood pressure; sCD163: soluble CD163; sTWEAK: soluble tumor necrotizing factor-like weak inducer of apoptosis; TC: total cholesterol; TG: total glyceride.
Significantly different compared to the control group;
Significantly different compared to the ACS group.
Comparison of sTWEAK, sCD163 and CRP levels and the sCD163/sTWEAK ratio Comparison of sTWEAK, sCD163 and CRP levels and the sCD163/sTWEAK ratio in all 3 groups were shown in Table 1. sTWEAK levels in chronic CAD and ACS patients were significantly lower compared to the control group. sCD163 levels and the sCD163/sTWEAK ratio were highest in the ACS group, these differences were statistically significant when compared to the control and chronic CAD group (Table 1).
Correlation analysis
Correlations among sTWEAK, sCD163 and CRP levels and the sCD163/sTWEAK ratio were investigated in all three groups. In the control group, while sCD163 level was positively correlated with the sCD163/sTWEAK ratio (r=0.71, P<0.0001), it were no observed significant correlations among other parameters. Correlation analysis in the ACS group revealed that sTWEAK levels were negatively correlated with sCD163 levels and the sCD163/sTWEAK ratio (r=-0.31, P=0.001 and r=-0.84, P<0.0001, respectively), and that sCD163 levels were positively correlated with the sCD163/sTWEAK ratio (r=0.73, P<0.0001). In the ch=ronic CAD group, sTWEAK levels were negatively correlated with the sCD163/sTWEAK ratio (r=-0.7, P<0.0001), sCD163 levels were positively correlated with sCD163/sTWEAK ratio (r=0.49, P<0.0001) and CRP levels were positively correlated with the sCD163/sTWEAK ratio (r=0.26, P=0.01). Consequently, sTWEAK levels were significantly correlated with sCD163 levels in only the ACS group.
ROC analysis
Receiver operating characteristic curve analysis was performed separately between the control-chronic CAD groups and the control-ACS groups in order to determine the diagnostic predictive value for chronic CAD and ACS of sTWEAK, sCD163, CRP levels, and the sCD163/sTWEAK ratio. The predictive value of sTWEAK and sCD163 was assessed together with CRP as a known inflammatory marker. Logarithms were obtained of non-normally distributed parameters.
According to ROC analysis between the control-chronic CAD groups, it was observed an increased sTWEAK level (Figure 1A) and sCD163/sTWEAK ratio (Figure 1B) significantly predicted chronic CAD. According to ROC analysis between the control-ACS groups, it was observed all parameters significantly predicted ACS (Figure 2A, 2B).
Figure 1.
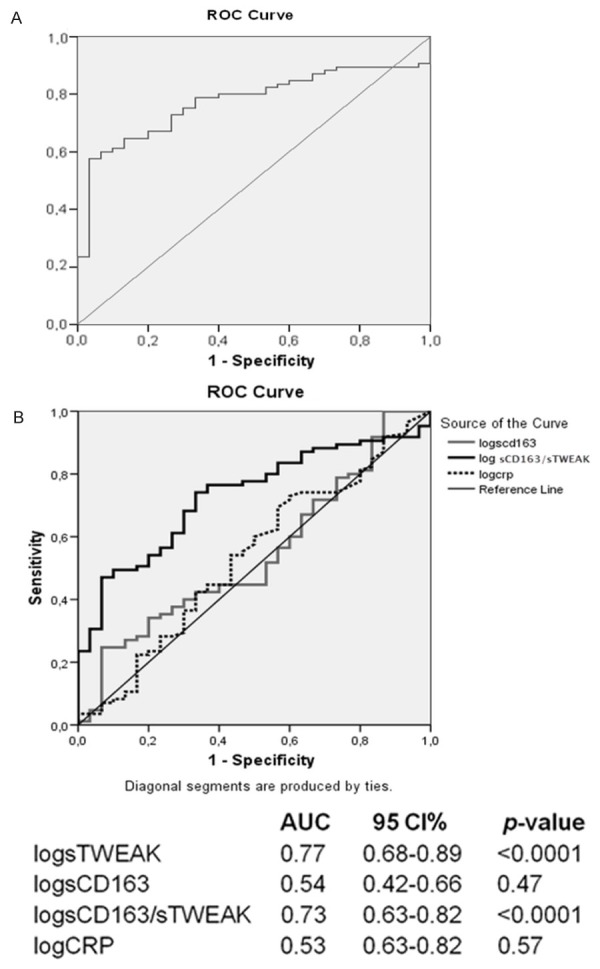
ROC analysis between the control-chronic CAD groups. ROC curves for sTWEAK (A), CD163, CRP and sCD163/STWEAK (B) AUC: area under curve; CAD: coronary artery disease; CI: confidence interval; CRP: C-reactive protein; ROC: receiver operating characteristic; sTWEAK:soluble TWEAK.
Figure 2.
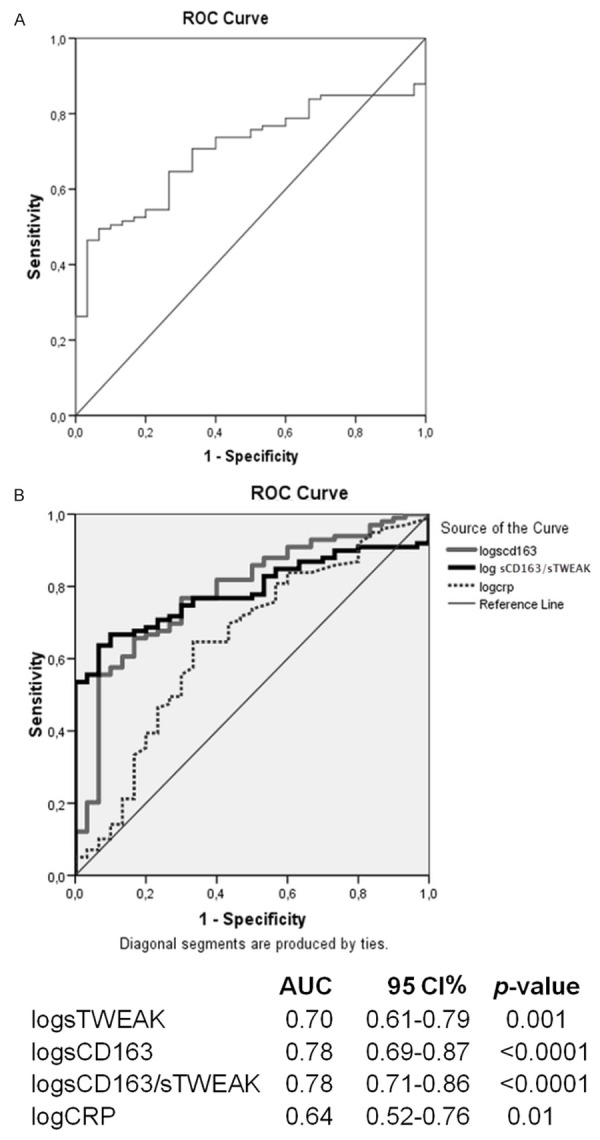
ROC analysis between the control-ACS groups. ROC curves for sTWEAK (A), sCD163, CRP and sCD163/sTWEAK (B). AUC: area under curve; CAD: coronary artery disease; CI: confidence interval; CRP: C-reactive protein; ROC: receiver operating characteristic; sTWEAK: soluble TWEAK.
The relation between sTWEAK, sCD163, CRP levels, sCD163/sTWEAK ratio and severity of CAD
Gensini scoring was performed in chronic CAD in order to investigate relation betweensTWEAK, sCD163, CRP levels and sCD163/STWEAK ratio and severity of CAD. Since these parameters can behave as an acute phase reactant, ACS patients were excluded from this analysis. The mild CAD group (Group 1) contained 50 patients and the severe CAD group (Group 2) contained 35 patients. Comparison of the two groups revealed no significant difference in these parameters [sTWEAK (793.3±793.2 vs 635.4±358.3, P=0.63), sCD163 (607.8±194.9 vs 693±262, P=0.18), sCD163/sTWEAK (1±0.52 vs 1.74±4.5, P=0.11) and CRP [(0.89±2.69 vs 1.74±4.56, P=0.06)].
Discussion
To our knowledge, this is the first study evaluating both sTWEAK and sCD163 levels in patients with both chronic CAD and ACS. sTWEAK levels were significantly lower in patients with chronic CAD and ACS compared to the control group. A number of studies have shown that sTWEAK levels decreased in CAD and various inflammatory diseases [20-22]. Although the exact cause for this decrease in sTWEAK levels in inflammatory diseases has not revealed, it has been explained by several mechanisms. Various studies have suggested that the decrease in sTWEAK in inflammatory diseases may be due to it being brought into the arterial wall by the Fn14 receptor or being removed by the scavenger receptor sCD163 [10,12,23]. Another study showed that the expression of TWEAK mRNA that permits the synthesis of sTWEAK is down-regulated in pro-inflammatory conditions [24]. In conclusion, the low sTWEAK levels in inflammation may be due to decreased synthesis and/or eliminated by increasing receptors of TWEAK.
Receiver operating characteristic analysis in our study revealed that a low sTWEAK level and high sCD163/sTWEAK ratio significantly predicted chronic CAD, and low sTWEAK and high sCD163 and CRP levels and sCD163/sTWEAK ratio significantly predicted ACS. However, sTWEAK levels are a significant predictor of both chronic CAD and ACS; significance for chronic CAD was more marked compared to ACS (P<0.0001 vs P=0.001, respectively). Therefore, it was suggested that low sTWEAK levels may be a more significant predictor for presence of chronic CAD.
In the present study, sCD163 levels and the sCD163/sTWEAK ratio were higher in the ACS group than in the control and chronic CAD groups. Several studies have reported that sCD163 levels increased in conditions of acute inflammation [25,26]. Correlation analysis revealed a significant negative correlation between sTWEAK and sCD163 only in patients with ACS. This situation may be attributed to a more significant increase in sCD163 levels in ACS patients. Higher sCD163 levels in patients with ACS agree with the information in the literature, and also it was suggested that the lower sTWEAK levels in our study may be associated with an increase in sCD163 levels. Although sCD163 levels in our study were higher in the chronic CAD group compared to the control group, this difference was not significant. The level in chronic CAD not being significantly different compared to the control group may be ascribed to there being a lower degree of inflammation in chronic CAD compared to ACS. ROC analysis in our study revealed that low sTWEAK and high sCD163 and CRP levels and sCD163/sTWEAK ratio significantly predicted presence of ACS. Since the degree of significance of a high sCD163 level (P<0.0001) was greater at ROC analysis than those of sTWEAK (P=0.001) and CRP (P=0.01), sCD163 levels appear as a more important predictor for presence of ACS than sTWEAK or CRP levels.
The present study firstly investigated both sTWEAK and sCD163 levels in patients with ACS. To the best of our knowledge, only one study has investigated sTWEAK levels in ACS. In that study, Chorianopoulos E. et al. showed that sTWEAK levels were higher in MI patients with ST elevation (173 patients) compared to the control (30 patients) and stable CAD (30 patients) groups, and were lower in stable CAD than in the control group [26]. sCD163 levels were not investigated in that study [26]. Excluding the above mentioned study, there are two other studies reporting elevated sTWEAK levels in inflammatory conditions, involving patients with stroke and chronic renal failure [27,28]. This variation in TWEAK regulation in acute-chronic conditions has not been fully explained. In a study performed by Maecker et al., it was suggested TWEAK plays a critical role in attenuating the transition from innate to adaptive TH1 immunity and TWEAK’s function may have evolved to guard against development of potentially harmful excessive inflammatory and autoimmune responses [29]. It was concluded that TWEAK has a more complex role than expected in inflammation and the immune system, and this opinion needs to be elucidated with further studies [29]. In our study, sTWEAK levels were higher in ACS patients compared to chronic CAD, but this difference was not significant. Further studies analyzing sTWEAK levels in ACS are now needed.
Due to the interaction between sTWEAK and sCD163, the sCD163/sTWEAK ratio has also been used in research involving these parameters. The sCD163/sTWEAK ratio in our study was higher in the ACS group than in the control and chronic CAD groups. Correlation analysis revealed that the sCD163/sTWEAK ratio was significantly correlated with both sTWEAK and sCD163 levels. The sCD163/sTWEAK ratio was reported to be a more significant predictor compared with sCD163 or sTWEAK levels alone in various diseases in previous studies [23]. At ROC analysis in our study, while sCD163 predicted only presence of ACS, sTWEAK levels and the sCD163/sTWEAK ratio predicted both presence of chronic CAD and ACS. At made ROC analysis for ACS, the degree of significance of the sCD163/sTWEAK ratio was greater than that of sTWEAK (P<0.0001 compared to P=0.001, respectively). Additionally in our study, CRP, a well-known inflammatory marker, was only significantly correlated with the sCD163/sTWEAK ratio (in the chronic CAD group). Therefore, similarly to the studies mentioned above, when the sCD163/sTWEAK ratio in our study was compared with the other parameters, it was appeared as a parameter having predictive significance for both chronic CAD and ACS.
The present study firstly investigated the relation between sTWEAK, sCD163 levels and Gensini scoring. No significant difference was observed in sTWEAK, sCD163 and CRP levels and the sCD163/sTWEAK ratio between patients with severe and mild coronary artery involvement. While these parameters were correlated with presence of CAD, no correlation was determined with severity of CAD obtained with Gensini scoring. This finding is compatible with Jelic-Ivanovic et al.’s study [20]. In that study, while a decreased sTWEAK levels were significantly correlated with presence of CAD, no correlation was determined with severity of CAD described in terms of 1, 2 or 3-vessel disease [20].
One potential limitation is the cross-sectional design of the study that limits drawing casal relationship between serum levels of sTWEAK or sCD163 and CAD. We also have no data to assess cardiovascular morbidity and mortality of the study population. The relatively small study sample was another potential limitation of our study which might limit the statistical power of the study. Prospective studies with greater sample size are needed.
Conclusions
It was concluded thatsTWEAK level may be a diagnostic marker of especially chronic CAD, sCD163 level of ACS, and the sCD163/sTWEAK ratio of both chronic CAD and ACS. In addition, it was observed that these parameters did not correlate with severity of disease in chronic CAD.
Disclosure of conflict of interest
None.
References
- 1.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47:C19–31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 2.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 3.Smith SC Jr, Anderson JL, Cannon RO 3rd, Fadl YY, Koenig W, Libby P, Lipshultz SE, Mensah GA, Ridker PM, Rosenson R. CDC/AHA workshop on markers of inflammation and cardiovascular disease: Application to clinical and public health practice: Report from the clinical practice discussion group. Circulation. 2004;110:e550–3. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]
- 4.Blanco-Colio LM, Martín-Ventura JL, Muñóz-García B, Orbe J, Páramo JA, Michel JB, Ortiz A, Meilhac O, Egido J. Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (stweak) as a possible biomarker of subclinical atherosclerosis. Arterioscler ThrombVasc Biol. 2007;27:916–922. doi: 10.1161/01.ATV.0000258972.10109.ff. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Ventura JL, Blanco-Colio LM, Tunon J, Munoz-Garcia B, Madrigal-Matute J, Moreno JA, Vega de Ceniga M, Egido J. Biomarkers in cardiovascular medicine. Rev Esp Cardiol. 2009;62:677–688. doi: 10.1016/s1885-5857(09)72232-7. [DOI] [PubMed] [Google Scholar]
- 6.Munoz-Garcia B, Madrigal-Matute J, Moreno JA, Martin-Ventura JL, Lopez-Franco O, Sastre C, Ortega L, Burkly LC, Egido J, Blanco-Colio LM. Tweak-fn14 interaction enhances plasminogen activator inhibitor 1 and tissue factor expression in atherosclerotic plaques and in cultured vascular smooth muscle cells. Cardiovasc Res. 2011;89:225–233. doi: 10.1093/cvr/cvq278. [DOI] [PubMed] [Google Scholar]
- 7.Saas P, Boucraut J, Walker PR, Quiquerez AL, Billot M, Desplat-Jego S, Chicheportiche Y, Dietrich PY. Tweak stimulation of astrocytes and the proinflammatory consequences. Glia. 2000;32:102–107. [PubMed] [Google Scholar]
- 8.Blanco-Colio LM, Martin-Ventura JL, Munoz-Garcia B, Moreno JA, Meilhac O, Ortiz A, Egido J. Tweak and fn14. New players in the pathogenesis of atherosclerosis. Front Biosci. 2007;12:3648–3655. doi: 10.2741/2341. [DOI] [PubMed] [Google Scholar]
- 9.Wiley SR, Winkles JA. Tweak, a member of the tnf superfamily, is a multifunctional cytokine that binds the tweakr/fn14 receptor. Cytokine Growth Factor Rev. 2003;14:241–249. doi: 10.1016/s1359-6101(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 10.Moreno JA, Munoz-Garcia B, Martin-Ventura JL, Madrigal-Matute J, Orbe J, Paramo JA, Ortega L, Egido J, Blanco-Colio LM. The cd163-expressing macrophages recognize and internalize tweak: Potential consequences in atherosclerosis. Atherosclerosis. 2009;207:103–110. doi: 10.1016/j.atherosclerosis.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Graversen JH, Madsen M, Moestrup SK. Cd163: A signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol. 2002;34:309–314. doi: 10.1016/s1357-2725(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 12.Bover LC, Cardo-Vila M, Kuniyasu A, Sun J, Rangel R, Takeya M, Aggarwal BB, Arap W, Pasqualini R. A previously unrecognized protein- protein interaction between tweak and cd163: Potential biological implications. J Immunol. 2007;178:8183–8194. doi: 10.4049/jimmunol.178.12.8183. [DOI] [PubMed] [Google Scholar]
- 13.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 14.Schaer CA, Schoedon G, Imhof A, Kurrer MO, Schaer DJ. Constitutive endocytosis of cd163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res. 2006;99:943–950. doi: 10.1161/01.RES.0000247067.34173.1b. [DOI] [PubMed] [Google Scholar]
- 15.Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor cd163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Aristoteli LP, Moller HJ, Bailey B, Moestrup SK, Kritharides L. The monocytic lineage specific soluble cd163 is a plasma marker of coronary atherosclerosis. Atherosclerosis. 2006;184:342–347. doi: 10.1016/j.atherosclerosis.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Matsushita N, Kashiwagi M, Wait R, Nagayoshi R, Nakamura M, Matsuda T, Hogger P, Guyre PM, Nagase H, Matsuyama T. Elevated levels of soluble cd163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of cd163 by timp-3. Clin Exp Immunol. 2002;130:156–161. doi: 10.1046/j.1365-2249.2002.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gensini GG. Coronary arteriography: Role in myocardial revascularization. Postgrad Med. 1978;63:121–128. 133–138. doi: 10.1080/00325481.1978.11714729. [DOI] [PubMed] [Google Scholar]
- 19.Oishi Y, Wakatsuki T, Nishikado A, Oki T, Ito S. Circulating adhesion molecules and severity of coronary atherosclerosis. Coron Artery Dis. 2000;11:77–81. doi: 10.1097/00019501-200002000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Jelic-Ivanovic Z, Bujisic N, Spasic S, Bogavac-Stanojevic N, Spasojevic-Kalimanovska V, Kotur-Stevuljevic J. Circulating stweak improves the prediction of coronary artery disease. Clin Biochem. 2009;42:1381–1386. doi: 10.1016/j.clinbiochem.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Kralisch S, Ziegelmeier M, Bachmann A, Seeger J, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Serum levels of the atherosclerosis biomarker stweak are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis. 2008;199:440–444. doi: 10.1016/j.atherosclerosis.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Munoz-Garcia B, Martin-Ventura JL, Martinez E, Sanchez S, Hernandez G, Ortega L, Ortiz A, Egido J, Blanco-Colio LM. Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques modulation by atorvastatin. Stroke. 2006;37:2044–2053. doi: 10.1161/01.STR.0000230648.00027.00. [DOI] [PubMed] [Google Scholar]
- 23.Urbonaviciene G, Martin-Ventura JL, Lindholt JS, Urbonavicius S, Moreno JA, Egido J, Blanco-Colio LM. Impact of soluble tweak and cd163/tweak ratio on long-term cardiovascular mortality in patients with peripheral arterial disease. Atherosclerosis. 2011;219:892–899. doi: 10.1016/j.atherosclerosis.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Chicheportiche Y, Fossati-Jimack L, Moll S, Ibnou-Zekri N, Izui S. Down-regulated expression of tweak mrna in acute and chronic inflammatory pathologies. Biochem Biophys Res Commun. 2000;279:162–165. doi: 10.1006/bbrc.2000.3913. [DOI] [PubMed] [Google Scholar]
- 25.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble cd163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in hiv-infected patients. J Infect Dis. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chorianopoulos E, Jarr K, Steen H, Giannitsis E, Frey N, Katus HA. Soluble tweak is markedly upregulated in patients with st-elevation myocardial infarction and related to an adverse short-term outcome. Atherosclerosis. 2010;211:322–326. doi: 10.1016/j.atherosclerosis.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Inta I, Frauenknecht K, Dorr H, Kohlhof P, Rabsilber T, Auffarth GU, Burkly L, Mittelbronn M, Hahm K, Sommer C, Schwaninger M. Induction of the cytokine tweak and its receptor fn14 in ischemic stroke. J Neurol Sci. 2008;275:117–120. doi: 10.1016/j.jns.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Carrero JJ, Ortiz A, Qureshi AR, Martin-Ventura JL, Barany P, Heimburger O, Marron B, Metry G, Snaedal S, Lindholm B, Egido J, Stenvinkel P, Blanco-Colio LM. Additive effects of soluble tweak and inflammation on mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:110–118. doi: 10.2215/CJN.02790608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maecker H, Varfolomeev E, Kischkel F, Lawrence D, LeBlanc H, Lee W, Hurst S, Danilenko D, Li J, Filvaroff E, Yang B, Daniel D, Ashkenazi A. Tweak attenuates the transition from innate to adaptive immunity. Cell. 2005;123:931–944. doi: 10.1016/j.cell.2005.09.022. [DOI] [PubMed] [Google Scholar]


