Abstract
Custom-cast posts and cores are usually used to treat endodontically treated teeth. However, several researches have underlined how these devices may be a much higher elastic modulus than the supporting dentine and the difference in the modulus could lead to stress concentrating in the cement lute, leading to failure. The role of the cement seems to play a fundamental role in order to transfer the strength during the chewing phases. Aim of this research is to record the rate of cytotoxicity of five different dual-cured resin cements used for fiber posts cementation. We tested the cytotoxicity of this five materials on MG63 osteoblast-like cells through two different methods: MTT ([3-4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide succinate) assay which tests for mitochondrial enzyme activity6 and xCELLigence® system.
Keywords: Fiber post cementation cytotoxicity
Introduction
The aim of the endodontic treatment is represented by the morpho-functional restoration of the tooth, through the use of appropriate restorative materials. Before proceeding to the anatomical reconstruction of the tooth, is important to have obtained cleaning and filling of the endodontic system by shaping of channels, to reach the killing of bacteria responsible for any periapical pathology [1]. The endodontic space under therapy should not be considered only as a system of channels to clean, shape and fill, but as a space that interfaces with the adjacent tissues [2]. Endodontic materials should remain inside the root canal space and should not extrude in periapical tissues, but in reality the clinic shows us how these can be in contact for a long period with the periodontal tissues [3,4]. For this reason it is important to study the direct cytotoxicity of these materials. This problem is also present during the cementation of endodontic fiber posts that may be in close contact with the periapical and lateral periodontium [5]. The purpose of this study was to evaluate the rate of cytotoxicity of five different dual-cured resin cements used for fiber posts cementation. We tested the cytotoxicity of this five materials on MG63 osteoblast-like cells through two different methods: MTT ([3-4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide succinate) assay which tests for mitochondrial enzyme activity [6] and xCELLigence® system for evaluating the kinetics of cell adhesion [6,7].
Materials and methods
Cell cultures
MG63 osteoblast-like cells, originally isolated from a human osteosarcoma, were used. MG63 were cultured in 200 ml culture flasks each containing a 15 ml mixture of 90% Ham’s F12/Dulbecco’s modified Eagle’s medium (DMEM-F12) complemented with 2 mM glutamine, 10% foetal bovine serum, 200 U mL-1 penicillin and 200 μg mL-1 streptomycin and then placed in standard cell culture conditions at 37°C in a 5% CO2 humidified air environment until confluence had been achieved. Cells were trypsinized and then seeded, for the following assays, on material disks in 96-well plates at a density of 4×104 cell per 150 μl.
Preparation of test cements
The following materials were tested: Biscem, C & B, Duolink (Bisco Inc., Schaumburg, USA ®); Virage Plus and Virage dual (Sweden & Martina, Padova, IT ®). The materials were prepared according to the manufacturer’s instructions under aseptic conditions. Materials were inserted into a round metal appliance designed for production of discs measuring 5 mm wide and 3 mm high. Materials were allowed to set for 24 h in a humid atmosphere and aseptic conditions. For cyto-compatibility assays, samples were sterilized by incubation for 2 h in 200 U mL-1 penicillin, 200 μg mL-1 streptomycin, 250 μg mL-1 fungizon and 50 mg L-1 gentamycin. Twelve samples of each material to be tested were disinfected by immersion in ethanol and left to dry in the flow cabinet [8,9]. These were added to the culture wells using sterile forceps. All the samples were subsequently incubated at 37°C with 5% CO2 in DMEM following a 1 h ultraviolet exposure [8-10]. The elutes of materials were prepared separately and were incubated in DMEM-F12 at 37°C in a 5% CO2 for 1, 2, 3 and 7 days [11]. Confluent MG63 were counted using a hematocytometer (4×104 cell) seeded onto 96 well plates and then incubated with the supernatant of each cements and for each time-points. Cell viability was evaluated by MTT assay after 24 h. Negative controls were performed in empty (not cell-containing) wells and positive controls were performed in wells with unexposed MG63.
MTT assay
The cytotoxic effects of each materials were assessed by measuring the reduction in cell metabolic activity using the colorimetric assay as initially described [12]. After 1, 2, 3 and 7 days of growth of MG63 cells on the cement disk surfaces, the cells were analysed. Unexposed control cultures were maintained under the same conditions. Briefly, the cells were washed once with PBS, and MTT solution (0.5 mg/mL) was added to each well. After incubation at 37°C under 5% CO2 for 3 h, the blue formazan formed by the reduction of the MTT was dissolved in 150 μl of IPA/HCl (0,4 N) by vigourously pipetting. The amount of formazan was determined by measuring absorbance at 570 and 650 nm for background using a microplate reader (model Multiscan Go UV-Vis, Thermo Scientific).
For each point, three wells were analysed, and three independent experiments were performed, so for every materials we tested 9 samples. Results were expressed as a percentage of viability, namely the ratio of absorbance of exposed to unexposed cells, according to the following equation:
Viability (%) = (Ast570 - Ast650)/(Asc570 - Asc650) · 100
Where Ast570 is the absorbance (at 570 nm) of the extract from incubated cells with tested material, Asc570 the absorbance (at 570 nm) of the extract from control cells and Asc650/Ast650 the absorbance at the reference wavelength for control and treated cells, respectively. Cytotoxicity responses were rated as severe (<30%), moderate (30-60%), slight (60-90%) or non-cytotoxic (>90%) based on the activity relative to values obtained for the controls [13].
xCELLigence
The RTCA instrument is composed of a RTCA impedance analyzer, a computer with RTCA software for controlling the system operation, a 48-well electronic microtiter plate (E-Plate) and the RTCA station, which accommodates the E-Plate and it is placed inside the tissue culture incubator. The presence of cell culture medium or buffer and the application of a low voltage create an electric field between the electrodes, which can be impeded by cell presence. The electronic readout of cell-sensor impedance is displayed in real-time as CI, a value directly influenced by cell attachment, spreading, and/or cell proliferation. The CI value at each time point is defined as Rn-Rb/Rb, where Rn is the cell-electrode impedance of the well with the cells and Rb is the background impedance of the well with only medium. Fifty microliters of cell culture medium was added to each well for the impedance background measurement. After adding 4×104 cells and extract, the final volume was 200 μL. The E-Plates were incubated at 37°C with 5% CO2 and monitored on the RTCA system at 2-minute time intervals for up to 72 hours with or without treatment. To facilitate the statistical evaluation of the results, we performed at least two repeats of each experimental condition as recommended in the technical manual of xCELLigence [14,15].
Statistical analysis
Data were analyzed using GraphPad Prism software version 6.00 for Windows (GraphPad Prism Software, San Diego, CA, USA). Average values were expressed as mean ± s.d. Statistical significance between different groups was determined by repeated-measures ANOVA test and Tukey test. A p value <0.05 was accepted as statistically significant.
Results
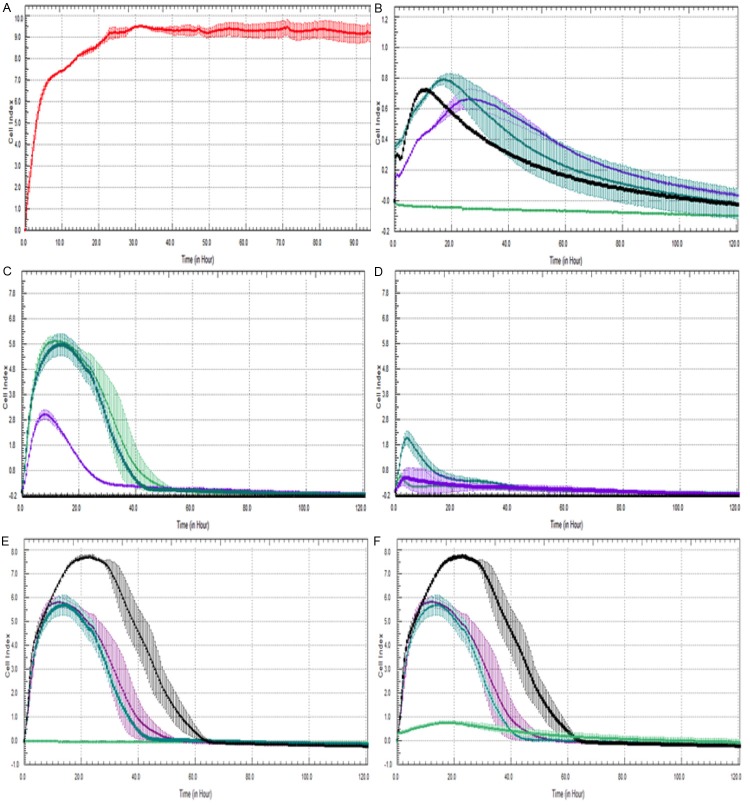
The cell viability of human osteosarcoma cells in contact with the dishes was evaluated by means of MTT assay (Figure 1A).
Figure 1.
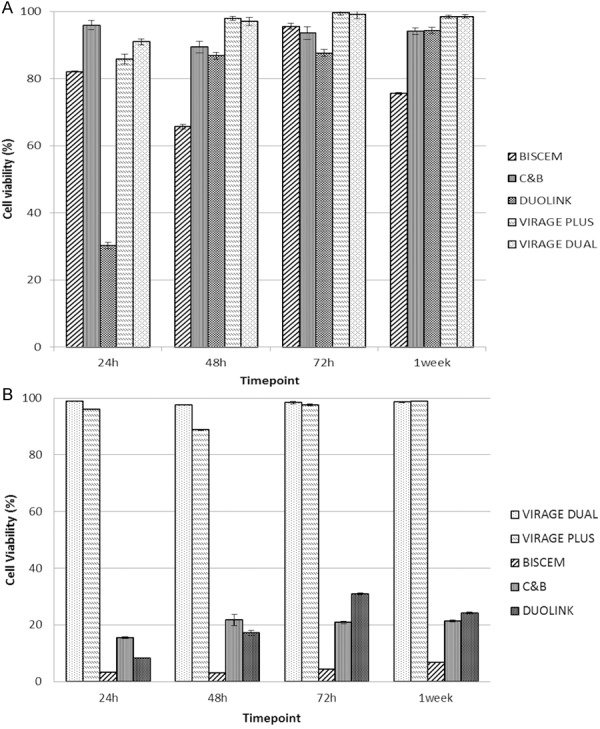
A. Results of the MTT assay with MG63 exposed to Biscem, C & B, Duolink, Virage Plus and Virage Dual at different time point. After 24 h we noticed a slide and non-cytotoxic effect for all materials, excepted for Duolink. This material determines a little decrease of cell viability at 24 h (P<0.05), but this effect tends to decrease in the remaining time point. In fact, from 48 h all materials shows a highly biocompatibility. B. Results of the MTT assay with MG63 exposed to Biscem, C & B, Duolink, Virage Plus and Virage Dual eluates at different time point.
As previously described in literature (rif. Biblio wataha 2003 dental materials), it needs an evaluation of cements eluates to better understand the biocompatibility rates of dental materials. For this reason, we performed an eluates cytotoxicity evaluation at different time-points.
At all time-points of incubation, a statistically significant high viability in comparison with control (DMEM only) was observed for Virage dual and Virage plus (Figure 1B).
For Biscem, C & B and Duolink, extract dilution in DMEM decreased the material’s biocompatibility (Figure 1B). Compared with extracts of the other tested materials at the same dilution, all materials have presented a lower biocompatibility to the MG63 osteoblasts-like cells in vitro. To confirm the results above mentioned, we performed an eluates analysis using xCELLigence®, system who evaluated the kinetics of cell adhesion and the cell index, parameter proportional to cell proliferation [16].
Discussions
Cytotoxicity assays are the initial screening tests used to evaluate the biocompatibility of materials [17]. The combination of these tests with: genotoxicity, mutagenicity, carcinogenicity and microbial analysis allow obtaining parameters that characterize biocompatibility [18]. The aim of this study was to evaluate the cytotoxicity activity of five dual-cured sealers used for fiber posts cementation through two different tests: MTT and Xcelligence® assays. We evaluated the rate of cytotoxicity of Dual Virage, Virage Plus, Biscem, C & B and Duolink on MG63 osteoblasts-like cells both on dry samples of cements (set group), that on the eluates derived from them (fresh group). At 24 hours, results from set group revealed a low rates of cytotoxicity for all sealers except Duolink. However, this rate tended quickly to decrease and, after 48 h all sealers results highly biocompatibility (Figure 2). Furthermore, the cytotoxicity remained low even after a week of contact with cells. Trials on eluates, with MTT and Xcelligence®, revealed a very low rate of cytotoxicity for Virage and Virage Plus dual, whereas C & B, Biscem and Duolink shown to be more cytotoxic. Biscem, Duolink rates of cytotoxicity could be due to the presence of TEGDMA as reported in previously studies [19,20]. Results appear to be steady after a week of observation.
Figure 2.
Cell Index of control group (A) and with MG63 exposed to Biscem (B), c & b (C), Duolink (D) Virage Plus (E) and Virage Dual (F) eluates at different timepoint 24 h (-), 48 h (-), 72 h -), 7 days (-) Results obtained with xCelligence analysis confirm the biocompatibility rates of the above-mentioned materials.
Conclusions
Virage Dual and Virage Plus revealed very low cytotoxicity, but further studies needs to understand effects of these cements on different types of cells culture.
Disclosure of conflict of interest
None.
References
- 1.Sjogren U, Hagglund B, Sundqvist G, Wing K. Factors affecting the long-term results of endodontic treatment. J Endod. 1990;16:498–504. doi: 10.1016/S0099-2399(07)80180-4. [DOI] [PubMed] [Google Scholar]
- 2.Estrela C, Holland R, Estrela CR, Alencar AH, Sousa-Neto MD, Pecora JD. Characterization of successful root canal treatment. Braz Dent J. 2014;25:3–11. doi: 10.1590/0103-6440201302356. [DOI] [PubMed] [Google Scholar]
- 3.Da Silva D, Endal U, Reynaud A, Portenier I, Orstavik D, Haapasalo M. A comparative study of lateral condensation, heat-softened gutta-percha, and a modified master cone heat-softened backfilling technique. Int Endod J. 2002;35:1005–1011. doi: 10.1046/j.1365-2591.2002.00610.x. [DOI] [PubMed] [Google Scholar]
- 4.Pommel L, Camps J. In vitro apical leakage of system B compared with other filling techniques. J Endod. 2001;27:449–451. doi: 10.1097/00004770-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Brackett MG, Lewis JB, Kious AR, Messer RL, Lockwood PE, Brackett WW, Wataha JC. Cytotoxicity of endodontic sealers after one year of aging in vitro. J Biomed Mater Res B Appl Biomater. 2012;100:1729–1735. doi: 10.1002/jbm.b.32739. [DOI] [PubMed] [Google Scholar]
- 6.Alanezi AZ, Jiang J, Safavi KE, Spangberg LS, Zhu Q. Cytotoxicity evaluation of endosequence root repair material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e122–125. doi: 10.1016/j.tripleo.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Toy E, Yuksel S, Ozturk F, Karatas OH, Yalcin M. Evaluation of the genotoxicity and cytotoxicity in the buccal epithelial cells of patients undergoing orthodontic treatment with three light-cured bonding composites by using micronucleus testing. Korean J Orthod. 2014;44:128–135. doi: 10.4041/kjod.2014.44.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang Y, Zheng Q, Zhou X, Gao Y, Huang D. A comparative study on root canal repair materials: a cytocompatibility assessment in L929 and MG63 cells. ScientificWorldJournal. 2014;2014:463826. doi: 10.1155/2014/463826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willershausen I, Wolf T, Kasaj A, Weyer V, Willershausen B, Marroquin BB. Influence of a bioceramic root end material and mineral trioxide aggregates on fibroblasts and osteoblasts. Arch Oral Biol. 2013;58:1232–1237. doi: 10.1016/j.archoralbio.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Koulaouzidou EA, Economides N, Beltes P, Geromichalos G, Papazisis K. In vitro evaluation of the cytotoxicity of ProRoot MTA and MTA Angelus. J Oral Sci. 2008;50:397–402. doi: 10.2334/josnusd.50.397. [DOI] [PubMed] [Google Scholar]
- 11.Ko H, Yang W, Park K, Kim M. Cytotoxicity of mineral trioxide aggregate (MTA) and bone morphogenetic protein 2 (BMP-2) and response of rat pulp to MTA and BMP-2. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e103–108. doi: 10.1016/j.tripleo.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Dahl JE, Frangou-Polyzois MJ, Polyzois GL. In vitro biocompatibility of denture relining materials. Gerodontology. 2006;23:17–22. doi: 10.1111/j.1741-2358.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 14.Rakers S, Imse F, Gebert M. Real-time cell analysis: sensitivity of different vertebrate cell cultures to copper sulfate measured by xCEL Ligence((R)) Ecotoxicology. 2014;23:1582–1591. doi: 10.1007/s10646-014-1279-6. [DOI] [PubMed] [Google Scholar]
- 15.Roshan Moniri M, Young A, Reinheimer K, Rayat J, Dai LJ, Warnock GL. Dynamic assessment of cell viability, proliferation and migration using real time cell analyzer system (RTCA) Cytotechnology. 2014;67:379–86. doi: 10.1007/s10616-014-9692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toy E, Malkoc S, Corekci B, Bozkurt BS, Hakki SS. Real-time cell analysis of the cytotoxicity of orthodontic brackets on gingival fibroblasts. J Appl Biomater Funct Mater. 2014;12:248–55. doi: 10.5301/JABFM.5000165. [DOI] [PubMed] [Google Scholar]
- 17.Severin I, Dahbi L, Lhuguenot JC, Andersson MA, Hoornstra D, Salkinoja-Salonen M, Turco L, Zucco F, Stammati A, Dahlman O, Castle L, Savolainen M, Weber A, Honkalampi-Hamalainen U, Von Wright A. Safety assessment of food-contact paper and board using a battery of short-term toxicity tests: European union BIOSAFEPAPER project. Food Addit Contam. 2005;22:1032–1041. doi: 10.1080/02652030500183425. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 19.Ulker HE, Sengun A. Cytotoxicity Evaluation of Self Adhesive Composite Resin Cements by Dentin Barrier Test on 3D Pulp Cells. Eur J Dent. 2009;3:120–126. [PMC free article] [PubMed] [Google Scholar]
- 20.Ergun G, Egilmez F, Yilmaz S. Effect of reduced exposure times on the cytotoxicity of resin luting cements cured by high-power led. J Appl Oral Sci. 2011;19:286–292. doi: 10.1590/S1678-77572011000300019. [DOI] [PMC free article] [PubMed] [Google Scholar]