Abstract
IL-6 is a cytokine secreted by glioma cells and plays an important role in the tumor growth. However, the impact of IL-6 on the invasiveness and prognosis of glioma is still unclear. In this study, immunohistochemistry was performed to determine the expression of IL-6 in 86 glioma tissues, and ELISA to measure IL-6 in the serum and cerebrospinal fluid (CSF) of these patients. Results showed, as ccompared with normal controls, the IL-6 in the glioma, CSF and serumincreased remarkably, and increased with the elevation of glioma grade. In addition, IL-6 in the supernatant was also detectable in glioma cell lines U251, U87, A172 and T98G. Transwell invasion assay showed that the invasiveness of glioma U87 cells and U251 cells increased remarkably after exogenous IL-6 treatment. Survival analysis indicated higher IL-6 before surgery and significantly reduction in IL-6 after operation in the serum and CSF predicted a poor prognosis. Thus, we speculate that, the poor prognosis of glioma is related to the IL-6 autocrine in the glioma and the IL-6 induced tumor growth and invasion. IL-6 may serve as a therapeutic target for glioma patients and IL-6 in the CSF and serum of glioma may be used to predict the prognosis of these patients.
Keywords: Glioma, IL-6, cerebrospinal fluid
Introduction
Glioma is the most common malignant tumor in the central nervous system (CNS) and accounts for about 70% of tumors in the CNS. Although the microsurgical therapy, radiotherapy and chemotherapy and other treatments have been applied in the therapy of glioma, the therapeutic efficacy is still poor, and especially in patients with poorly differentiated glioma, the survival time is short, and the 5-year survival rate is only 5% [1]. The high invasiveness of glioma is a major cause of post-operative recurrence and a poor prognosis [2]. To date, no biomarkers have been found to be closely related to the occurrence, growth, invasion and prognosis of glioma, which brings difficulties to the clinical treatment and evaluation of glioma [3,4]. The cerebrospinal fluid (CSF) is the most close to the CNS anatomically, and thus examination of CSF has been routinely used in the evaluation of CNS related diseases. Glioma may disrupt the blood brain barrier (BBB), and thus proteins secreted by the tumor and even malignant cells may be detectable in the CSF and peripheral blood. Detection of tumor specific proteins in the CSF and peripheral blood by ELISA is crucial for the investigations of tumor microenvironment and tumor markers. IL-6 is a cytokine participating in the immunoregulation and inflammatory reaction. IL-6 plays an important rolein various malignant tumors includingglioma [5-7]. Thereis evidence showing that IL-6 expressed in the glioma may promote the glioma growth to affect the prognosis of glioma patients [8,9]. However, the source of IL-6 in the glioma,its impact on the invasiveness and the role of IL-6 in the CSF and peripheral blood in the prognosis of glioma are still unclear. In this study, IL-6 expression was detected in different grades of glioma, the IL-6 in the CSF and serum was also measured in patients with glioma, and its role in the tumor invasiveness andprognosis was further evaluated. Our findings may provide evidence for for the clinical treatment on the glioma.
Materials and methods
Subjects
From January 2008 to October 2010, 86 patients diagnosed with glioma and having complete clinical information were recruited into glioma group. Glioma was diagnosed by post-operative pathological examination in the General Hospital of Chinese Armed Police Force, herein. Patients with infectious diseases, nervous system diseases, tumors of other organs, autoimmune diseases, andliver and kidney dysfunction, trauma or other chronic diseases were excluded from the present study. According to WHO grading systems of tumors of CNS, glioma in these patients was classified as grade I~IV. Re-examination was done at 1 month after discharge, and follow-up by telephone was done with 60 months. In addition, 18 healthy subjects aged 18-65 years and receiving routine physical examination were enrolled into control group, and the clinical information of subjects in both groups at baseline is shown in Table 1.
Table 1.
Patient’s information in this study
| Variable | Glioma group (%) (n=86) | Control group (%) (n=18) |
|---|---|---|
| Sex | ||
| Male | 37 (43) | 8 (44.4) |
| Female | 49 (57) | 10 (55.6) |
| Age | ||
| 18-60 years | 55 (64) | 13 (72.2) |
| ≥61 years | 31 (36) | 5 (27.8) |
| WHO grade | ||
| I-II | 18 (20.9) | |
| III | 25 (29.1) | |
| IV | 43 (50) |
The immunohistochemical staining of tumor specimen
Tissues were embedded in paraffin and cut into 5-μm sections which were then deparaffinized and boiled in citrate buffer (pH=6.0; Beijing Tideradar Technology Co., Ltd) for antigen retrieval. Then, these sections were incubated with 0.3% H2O2 (ZSGB Biotechnology Co., Ltd., Beijing) to inactivate endogenous catalase, and blocked in 10% BSA (Shanghai Mai Biotechnology Co., Ltd., Shanghai) at room temperature. After incubation with primary antibody against IL-6 (1:100; ZSGB Biotechnology Co., Ltd., Beijing) in a humidified environmentat 4°C overnight, biotin-conjugated peroxidase was added (ZSGB Biotechnology Co., Ltd., Beijing), followed incubation at room temperature for 1 h. In blank control, the primary antibody was replaced with PBS. Visualization was done with DAB in 0.3% H2O2 for 2 min, and sections were then observed under a light microscope.
Collection of blood and CS FandIL-6 measurement
The collection of blood and CSF vialumbar puncture was done according to the standard protocols. In glioma group, CSF was collected at 24 h before surgery, and blood was collected at 24 h before surgery and 1 month after surgery. In control group, CSF and blood were also collected. The CSF and blood were centrifuged at 4°C for 10 min at 4 000 rpm/min, and the supernatant was harvested and stored at -80°C immediately. IL-6 was detected bydouble-antibody sandwich ELISA according to the manufacturer’s instructions (HUYU Biological Technology Co., Ltd., Shanghai). The optical density (OD) of each well was detected for the delineation of standard curve with OD difference between samples and blank control as vertical ordinate and standard concentration as horizontal ordinate. The ODof samples was determined according to the standard curve.
Cell culture
The glioma cell lines U251 cells, U87 cells, A172 cells and T98G cells were maintained in DMEM containing 10% new-born calf serum (pH7.2-7.4) at 37°C in a humidified environment of 5% CO2. The cell growth was observed under an inverted microscope. When the cell confluence reached 70-80%, cells were digested with 0.125% trypsin and passaged. When the cell confluence reached 50-60%, the medium was refreshed with serum-free medium and maintained for 24 h. The supernatant was harvested for ELISA.
Transwell invasion assay
The upper chambers of Transwell chamber with the Makrolon micro-porous membrane (8-μm pore-size; American Costar Company) were placed into 24-well plates, and then 10 μg/mL type I collagen (American SIGMA Company) was added to these upper chambers for coating, followed by air drying. After addition of DMEM containing 10% serum, cells (U87A cells and A172 cells) maintained in serum free DMEM were harvested and transferred into upper chambers, followed by incubation for 12 h at 37°C in a humidified environment of 5% CO2.Then, the upper chambers were collected and washed in PBS. A cotton swab was used to remove the non-migrated cells, and the membrane was fixed in 4% paraformaldehyde for 10 min, followed by 0.25% crystal violet staining. The membrane was observed under a microscope and 8 fields were randomly selected for the counting of migrating cells.
Prognostic analysis
The relationship of IL-6 in the serum and CSF before surgery and decrement of serum IL-6 (percentage) with the survival time was evaluated, aiming to investigate the association between IL-6 and prognosis of glioma patients.
Statistical analysis
All data are expressed as mean ± standard deviation (SD), and statistical analysis was performed with SPSS version 17.0. IL-6 in the serum and CSF and data of immunohistochemistry and Transwell invasion assay were compared with Mann-Whitney test. IL-6 before and after surgery was compared with paired t test. Spearman correlation analysis was used for the evaluation of correlation, and prognosis analysis was done with Long rank (Mantel-Cox) test. A value of P<0.05 was considered statistically significant.
Results
IL-6 expression in glioma
As compared to normal brain tissues,IL-6 expression in glioma increased markedly. Moreover, IL-6 increased with the progression of glioma: the higher the grade of glioma, and the higher the IL-6 expression was. Of note, the IL-6 expression was comparable in glioma of grade III and grade IV (Figure 1).
Figure 1.
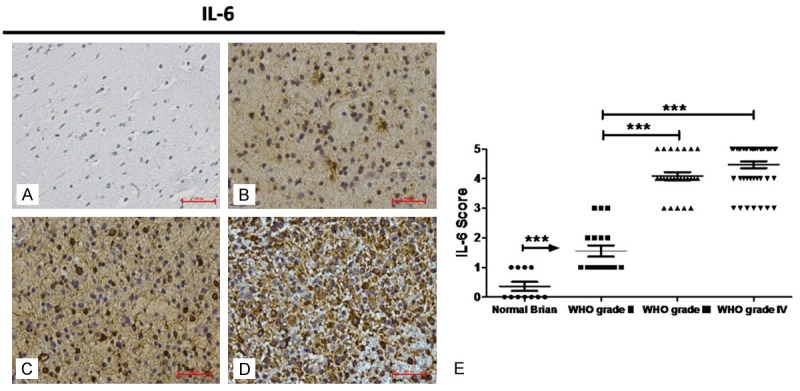
Immunohistochemistry for IL-6 in human glioma. IL-6 protein expression was detected by immunohistochemistry in normal brain tissues (A) and gliomas [WHO grade II (B), WHO grade III (C) and WHO grade IV (D)] (scale bar: 100 μm). IL-6 expression was detected in the brain of 18 patients with brain trauma cases and without a history of neurological diseases, 18 patients with WHO grade II glioma, 25 patients with WHO grade III glioma and 43 patients with glioblastomas of WHO grade IV. Staining intensities were scored on a scale from 0 to 5 where 0 refers to the absence of staining and 5 to the maximum staining (E) (*P<0.05; **P<0.01; ***P<0.0001).
IL-6 in the blood and CSF
As compared to control group, IL-6 in the serum and CSF of glioma patients increased notably and this increase was related to the grade of glioma: the higher the grade of glioma, the higher the IL-6 levels were. In addition, IL-6in the serum and CSF showed a positive relationship before surgery in glioma patients, and IL-6 in the serum and CSF reduced markedly after surgery (Figure 2).
Figure 2.
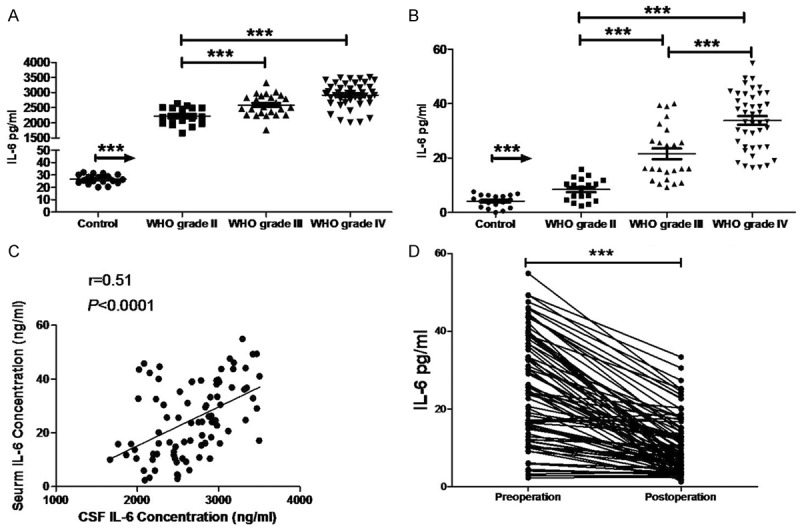
IL-6 contents of the CSF (A) and serum (B) of glioma patients and healthy controls were determined by ELISA. IL-6 content of the CSF was positively correlated with that of the serum (C). The serum IL-6 (D) significantly decreased after surgery (***P<0.0001).
Association between IL-6 and prognosis of glioma patients
Univariate analysis showed IL-6 in the serum and CSF of 86 glioma patients before surgery and the decrement of serum IL-6 after surgery were closely related to the prognosis of glioma patients (Figure 3).
Figure 3.
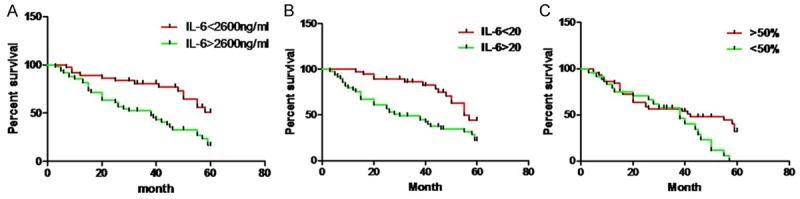
Relationship between survival time and serum IL-6 within 60 months after surgery in glioma patients. IL-6 contents of the CSF (A) and serum (B) before surgery and the decrement of serum IL-6 after surgerywere significantly related to the overall survival (Kaplan-Meier method).
IL-6 expression ofglioma cell lines and its role of IL-6 inglioma invasion
ELISA showed that IL-6 was detectable in the supernatant of glioma cell lines (U251 cells, U87 cells, T98G cells and A172 cells). Among these cell lines, IL-6 content was the highest in the supernatant of A172 cells, and the lowest in the supernatant of U87 cells. Incubation with exogenous IL-6 (100 ng/ml) could markedly increase the invasion of U87 cells and A172 cells (Figure 4).
Figure 4.
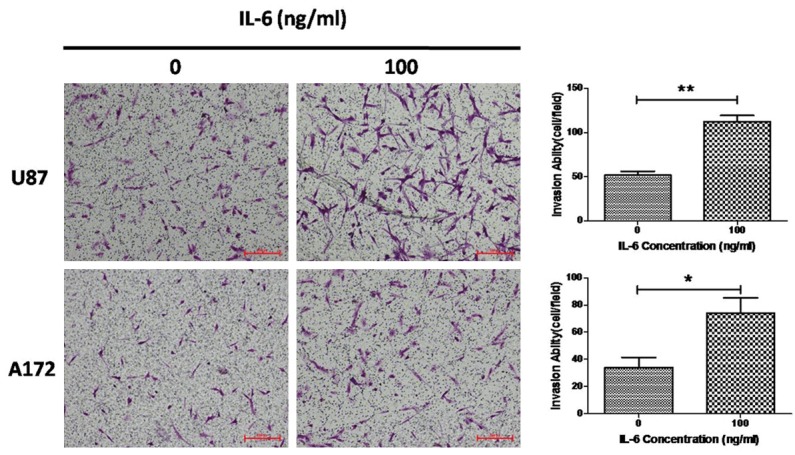
Transwell invasion assay of U87 cells and A172 cells. IL-6 promotes the invasion of U87 cells and A172 cells. Data were from three independent experiments (*P<0.05; **P<0.01).
Discussion
It has been confirmed that IL-6 is acytokineplaying an important role in the inflammation, and can regulate acuteinflammatory reaction of acute inflammation, modulate the differentiation and activation of B and T lymphocytes and exert crucial effectson the growth of some types of cells [10]. Recent studies indicate that not only inflammatory cellsbut tumor cells can synthesize and release IL-6. IL-6 is highly expressed in multiple solid tumors, and able to regulate some pathological processes related to the growth, invasion and apoptosis of cancer cells [5,6,11,12]. In the present study, results showed IL-6 expression was at a high level inglioma and showed an increased tendency with the increase of glioma grade, which was consistent with previous findings [13-15]. Furthermore, our results also revealed IL-6in the CSF and peripheral blood of glioma patients also increased dramatically, and exhibited an increased tendency with the increase of glioma grade. Of note, IL-6 in the CSF was positive related to that in the serum. This implies that the more malignant the glioma, the higher the IL-6 expression and secretion are, and IL-6 may be released into the blood and CSF via the disruptedBBB, affecting the tumor microenvironment and the wholebody. In our study, the prognostic analysis showed IL-6 in the CSF and peripheral blood before surgerywas significantly associated with the prognosis of glioma patients. At 1 month after surgery, IL-6 in the peripheral blood reduced to different extents, and this decrement was also related to the prognosis of these patients. Thus, IL-6 in the CSF andserum before and after surgerymay be used for the pre-operative assessment of the degree of malignancyand the post-evaluation of prognosis of glioma patients.
The expression and secretion of IL-6 in the tumor cells are regulatedby many factors, which mainly include the feedbackof NF-κB, Notch, S1PR1, and STAT3 phosphorylation [16-18]. IL-6 can increase STAT3 phosphorylation to elevate the proliferation and anti-apoptosis of tumor cells. IL-6 may alsopromote the infiltration of immune cells into the tumor to regulate tumor related inflammation [12]. Moreover,IL-6 is able to promote angiogenesis in the tumor by regulating VEGF expression [19]. Chen et al [11]found that IL-6 could enhance the tumor invasion in an EMT dependent mechanism. High invasiveness is a main characteristic of glioma. To further confirm the role of IL-6 in the invasion of glioma, IL-6 was detected in the supernatant of U251 cells, U87 cells, T98G cells and A172 cells. Results showed that IL-6 was detectable in the supernatant of these glioma cell lines. After incubation with exogenous IL-6, the invasiveness increased significantly in U87 cells and A172 cells. This suggests that glioma cells can secret IL-6 to promote theinvasion of glioma in an autocrine dependent manner.
Taken together, glioma has a high IL-6 expression, and the higher the grade of glioma, the higher the IL-6 contents of the CSF and peripheral blood are. In addition, IL-6 in the CSF and peripheral blood before surgery is closely related to the prognosis of glioma patients. Serum IL-6 reduces significantly at 1 month after surgery, and its decrement may be used to predict the prognosis of glioma patients. Thus, IL-6 in the CSF and peripheral blood before and after surgery may be used for the prediction of clinical prognosis. In vitro experiments reveal IL-6 secreted by glioma cells may significantly increase the invasiveness of glioma cells in an autocrine dependent manner. The regulatory network of IL-6 is very complex, and thus the specific mechanisms underlying the regulatory role of IL-6 in the invasion of glioma cells are warranted to be further studied.
Disclosure of conflict of interest
None.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Kislin KL, McDonough WS, Eschbacher JM, Armstrong BA, Berens ME. NHERF-1: modulator of glioblastoma cell migration and invasion. Neoplasia. 2009;11:377–387. doi: 10.1593/neo.81572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Febbo PG, Ladanyi M, Aldape KD, De Marzo AM, Hammond ME, Hayes DF, Iafrate AJ, Kelley RK, Marcucci G, Ogino S, Pao W, Sgroi DC, Birkeland ML. NCCN Task Force report: Evaluating the clinical utility of tumor markers in oncology. J Natl Compr Canc Netw. 2011;9(Suppl 5):S1–32. doi: 10.6004/jnccn.2011.0137. quiz S33. [DOI] [PubMed] [Google Scholar]
- 4.Berghoff AS, Stefanits H, Heinzl H, Preusser M. Clinical Neuropathology Practice News 4-2012: levels of evidence for brain tumor biomarkers. Clin Neuropathol. 2012;31:206–209. doi: 10.5414/NP300511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X, Mao Y, Wang J, Zu L, Hao M, Cheng G, Qu Q, Cui D, Keller ET, Chen X, Shen K, Wang J. IL-6 secreted by cancer-associated fibroblasts induces tamoxifen resistance in luminal breast cancer. Oncogene. 2014 doi: 10.1038/onc.2014.158. [DOI] [PubMed] [Google Scholar]
- 6.Wang G, Ye Y, Zhang X, Song J. Bradykinin stimulates IL-6 production and cell invasion in colorectal cancer cells. Oncol Rep. 2014;32:1709–1714. doi: 10.3892/or.2014.3366. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Q, Zhang X, Zhang L, Li W, Wu H, Yuan X, Mao F, Wang M, Zhu W, Qian H, Xu W. The IL-6-STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McFarland BC, Hong SW, Rajbhandari R, Twitty GB Jr, Gray GK, Yu H, Benveniste EN, Nozell SE. NF-kappaB-induced IL-6 ensures STAT3 activation and tumor aggressiveness in glioblastoma. PLoS One. 2013;8:e78728. doi: 10.1371/journal.pone.0078728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kesanakurti D, Chetty C, Dinh DH, Gujrati M, Rao JS. Role of MMP-2 in the regulation of IL-6/Stat3 survival signaling via interaction with alpha5beta1 integrin in glioma. Oncogene. 2013;32:327–340. doi: 10.1038/onc.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83–89. doi: 10.1016/j.cytogfr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Gao Q, Han S, Pan F, Fan W. The CCL2/CCR2 axis enhances IL-6-induced epithelial-mesenchymal transition by cooperatively activating STAT3-Twist signaling. Tumour Biol. 2015;36:973–981. doi: 10.1007/s13277-014-2717-z. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Lai W, Zhang Y, Liu L, Luo X, Zeng Y, Wu H, Lan Q, Chu Z. Tumor-associated macrophage-derived IL-6 and IL-8 enhance invasive activity of LoVo cells induced by PRL-3 in a KCNN4 channel-dependent manner. BMC Cancer. 2014;14:330. doi: 10.1186/1471-2407-14-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotfilder M, Knupfer H, Mohlenkamp G, Pennekamp P, Knupfers M, Van Gool S, Wolff JE. Interferon-gamma increases IL-6 production in human glioblastoma cell lines. Anticancer Res. 2000;20:4445–4450. [PubMed] [Google Scholar]
- 14.Li R, Li G, Deng L, Liu Q, Dai J, Shen J, Zhang J. IL-6 augments the invasiveness of U87MG human glioblastoma multiforme cells via up-regulation of MMP-2 and fascin-1. Oncol Rep. 2010;23:1553–1559. doi: 10.3892/or_00000795. [DOI] [PubMed] [Google Scholar]
- 15.Michaud-Levesque J, Bousquet-Gagnon N, Beliveau R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp Cell Res. 2012;318:925–935. doi: 10.1016/j.yexcr.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 16.Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, Bornmann W, Bromberg JF. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9:R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Invest. 2007;117:3660–3663. doi: 10.1172/JCI34237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, Karin M. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piperi C, Samaras V, Levidou G, Kavantzas N, Boviatsis E, Petraki K, Grivas A, Barbatis C, Varsos V, Patsouris E, Korkolopoulou P. Prognostic significance of IL-8-STAT-3 pathway in astrocytomas: correlation with IL-6, VEGF and microvessel morphometry. Cytokine. 2011;55:387–395. doi: 10.1016/j.cyto.2011.05.012. [DOI] [PubMed] [Google Scholar]


