Abstract
Hepatocellular carcinoma (HCC) accounts for 80% to 90% of liver cancers and it is one of the most prevalent carcinomas throughout the world. Traditional chemotherapy is often developed chemoresistance HCC patients.Matrine is an active component oftraditional Chinese medicine (TCM) and is a promising alternative HCC drug. In this study, the therapeutic effects and the underlying molecular mechanisms of matrine on the human HCC cell lineHep G2 were investigated. High dosage of matrine (1.0 mg/mL) could significantly (P < 0.05) inhibit cell proliferation by 48.39 ± 3.32%, under which cell shrinkage and disruption were observed. Flow cytometry assay showed that the proportion of G1/G0 cells significantly increased, while that of S and G2/M cells significantly decreased after treatment of matrinefor 48 h. These results indicated that cell arrest by matrine appeared. Up-regulation of the hepato-specific miR122a followed by down expression of its targetcyclin G1 (CG1) gene by low concentration of matrine (0.2 mg/mL) was detected using was observed using quantitative real-time PCR, immunohistochemistry (IHC) and western blot assays. In conclusion, matrineinducescell arrest and apoptosis with recovery expression of the hepato-specific miR122a in human hepatocellular carcinoma Hep G2 cell line.
Keywords: Matrine, traditional Chinese medicine (TCM), hepato-specific miR122a, hepatocellular carcinoma (HCC), cyclin G1 (CG1)
Introduction
Liver cancer is a major health problem, it’s the fourth most common cause of cancer death according the World Health Organization (WHO) [1]. There were 22,620 new cases and 18,160 deaths related to liver cancer in 2009 in the United States. China accounts for 53% of all liver cancer death all around the world. More than 90% of the primary liver cancer in China is hepatocellular carcinoma (HCC). It is the second leading cancer killer that mainly affects middle-aged people-those in the prime of their most productive years [2].
Though liver resection is an appropriate option for HCC therapy, it only applies to small percentages of the patients with early stage of HCC [3]. Traditional chemotherapy remains an important therapeutic strategy forhuman cancers. However, most chemotherapeutic drugs for HCC treatment are cytotoxic agents with a high risk of side effects, such as adriamycin (ADM), cisplatin, 5-fluorouracil (5-FU) and doxorubicin [4,5]. Furthermore, chemoresistance is developed in HCC patients, which presents a major obstacle to the long-term efficacy of chemotherapeutic treatments [6]. Therefore, alternative treatments should be developed to improve the efficiency of HCC therapy.
Traditional Chinese medicine (TCM) is appreciated for its 5000-year-old history and still holds an important position in primary health care in China. TCM could complement Western medicine by using modern techniques, thus, increasing interest in TCM is observed in the Western world. Sophora flavescens Ait (SF) is a widely used TCM for a series of diseases including viral hepatitis, cardiac arrhythmia and skin inflammations in China [7]. The active components of SF are various alkaloids, among whichmatrinehas been characterized as the major bioactive component [8,9].
As an alkaloid, matrine has favorable medical value. Its antiviral activity promises the use in treatment of chronic hepatitis B [10]. It was reported that intramuscular injection of matrine improvedthe clinical symptoms of chronic hepatitis B patients, recovered liver functions and changed serum conversion from positive to negative HBVDNA [11]. It was also shown that matrinehad antifibrosis activityto inhibit the platelet-derived growth factor and transform growth factor-beta actions in hepatic stellate cells [12]. Recent studies showed that matrine is effective in inhibiting cell growth and inducing differentiation in human leukemia K562 cells [9,13,14]. Matrine is also a differentiation inducer in SMMC-7721 cells [15]. In human multiple myeloma cells and gastric cancer MKN45 cells, matrine could induce tumor cell apoptosis by interrupting cell-cell adhesion and inhibiting cancer metastasis [16,17]. Matrinecould potentially prevent tumor invasion [18,19]. Consequently,matrine could be a promising alternative anticancer drug for HCC treatment. At present, matrine has been used for HCC treatment in murine [7], however, the antitumor therapeutic efficacy and the underlying molecular mechanisms ofmatrine with respect to the physiological and pharmacological effects on human HCC have not been well characterized.
In this study, the therapeutic effects and the underlying molecular mechanisms of matrine on the human HCC cell line Hep G2 were investigated, with respect to the inhibitory effect on cell proliferation, changes of cell morphology, induction of cell apoptosis, and expression of thehepato-specific miR122a and its target cyclin G1 (CG1) gene.
Materials and methods
Cell line and matrine treatment
The human HCC cell lines Hep G2 [American Type Culture Collection (ATCC) number HB-8064] was purchased from Cancer Research Institute of China Medical University (Shenyang, China). The cells were cultured with Iscove’s modified Dulbecco’s medium (IMDM) with 10% fetal bovine serum and gentamicin. Matrine was obtained from Xian Botany Garden (Shanxi, China), and its purity was > 99% as assessed by HPLC. Matrine stock solution was prepared in dd H2O at 10 mg/mL. Log-phase growing cells were seeded at 1 × 105 cells/mL and exposed to matrine at concentrations ranging from 0.0 (negative control) to 1.0 mg/mL for 48 h.
MTT assay
The effects of matrine on cell viability was assessed by MTT assay as described previously [20]. Briefly, cells were plated at a density of 3000 cells per well into 96-well plates. At the end of treatment, the supernatant was removed, and 20 µL of the tetrazolium compound, MTT, and 270 mL of fresh IMDM medium were added. After incubation for 4 h at 37°C, 120 µL of DMSO was placed in each well to dissolve the tetrazolium crystals. Finally, the absorbance at a wavelength of 570 nm was recorded using a multi-well plate reader (Tecan, Maennedorf, Switzerland). Each experiment was performed four times. Results are expressed as the percentage growth inhibition with respect to the untreated cells.
Microscopic inspection
Digested cell culture (3 × 105 cells/mL) was added to a 24-well plate (0.9 mL for each well) and incubated for 12 h. Then 0.1 mL matrine of low (0.2 mg/mL) or high concentration (1.0 mg/mL) per well was added. Cells were incubated for 48 h before observation. The cells were examined using an Olympus IX70 inverted microscopy, DVC1310 digital video camera and QED camera with standalone 145 software.
Flow cytometry (FCM) analysis
Hep G2 cells at log phrase were collected at a final concentration of 2 × 105 cells/ml, and were incubated in 6-well plate for 12 h (2.7 mL for each well). Then 0.3 mL matrine of low (0.2 mg/mL) or high concentration (1.0 mg/mL) per well was used to induce the cells for 48 h. Simultaneously, 0.3 mL cell culture, as negative control, was cultured for 48 h, collected, washed with PBS, and fixed with 70% ethanol, in sequence. Cells were centrifuged to eliminate ethanol, washed with PBS, and stained with propidium iodide (PI) in dark for 30 min before FCM analysis. Finally, BD FACSCalibur (BD, USA) was used to detect cell cycle. Cells were sampled using sampling software CellQuest 3.0. The proportion of cells in different phrases were quantified by Mod Fit LT 3.0 [21]. Each experiment was performed four times.
Measurement of apoptosis
1 × 106 cells were treated with various concentrations of matrinefor 48 h, then collected by centrifugation. Pellets were lysed by DNA lysis buffer (10 mM Tris, pH 7.5, 400 mM EDTA, and 1% Triton X-100) then centrifuged. The supernatant obtained wasincubated overnight with proteinase K (0.1 mg/mL) and then withRNase (0.2 mg/mL) for 2 h at 37°C. After extraction with phenol chloroform (1:1), DNA was separated in 2% agarose gel andvisualized by UV after staining with ethidium bromide. Quantitative assessment of apoptotic cells was assessed by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphatenick endlabeling (TUNEL) method, which examines DNA-strandbreaks during apoptosis by using BD ApoAlert DNA Fragmentation Assay kit.
Immunohistochemical staining
Immunohistochemical staining was performed using the highly specific affinity-purified poly clonal anti-CG1 antibody. Negative controlwas performed using nonimmune serum instead of the primary anti body. Briefly, the sections were washed in phosphate buffered saline followed by preincubation with 1.5% normal goat serum in phosphate buffer within a moist chamber for 4 h at room temperature. Those sections were then incubated overnight with anti-CG1 antibody at a final concentration of 2 µg/mL. After being washed with 6 changes of phosphate-buffered saline containing 0.02% Triton X-100 over 15 min, the slides were processed for immunostaining with the avidin-biotinylated peroxidase complex method (Vector Laboratories, Burlingame, CA) according to the user manual. The tissue sections were briefly counterstained with Mayer’s hematoxylin before mounting. Cultured cells were grown on sterile coverslips in tissue culture dishes overnight, fixed with 45% acetone/10% formaldehyde in 0.1 M phosphate buffer for 5 min, and then processed for immunohistochemicalassay as described above.
Quantitative real-time PCR assay
The expression of mature has-mir-122a (P/N: 4373151) in Hep G2 cells was assayed by the Taqman MicroRNA Assays (Applied Biosystems) according to Gramantieri et al [22] with some modifications. Each sample was analyzed in triplicate. Reverse transcription reaction was done starting from 10 ng of total RNA and using the looped primers. Quantitative real-time PCR was done using the standard Taqman MicroRNA Assays protocol on the 7500 Real-Time PCR System (Applied Biosystems, USA). The 20 µL PCR included 1.33 µL reverse transcription product, 1 × Taqman Universal PCR Master Mix, No Amp Erase UNG (P/N 4324018; Applied Biosystems), 0.2 µmol/L Taqman probe, 1.5 µmol/L forward primer, and 0.7 µmol/L reverse primer. The reactions were incubated in a 96-well plate at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The ∆∆Ct method for relative quantitation of gene expression was used to determine miRNA expression levels. The ∆∆Ct was calculated by subtracting the Ct of U6 RNA from the Ct of the miRNA of interest. The ∆∆Ct was calculated by subtracting the ∆Ct of the reference sample (non-treated Hep G2) from the ∆Ct of each sample. Fold change was generated using the equation 2-∆∆Ct. A pool of three reference samples was used for the standard curve calculation and as reference sample for the ∆∆Ct. The Taqman MicroRNA Assays for U6 RNA (RNU6B, P/N: 4373381; Applied Biosystems) was used to normalize the relative abundance of miRNA.
Western blot analysis
Cell lysates were prepared in RIPA buffer (50 mmol/L Tris-HCl buffer, pH 7.4, 150 mmol/L NaCl, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) supplemented with 1 × Halt protease inhibitor cocktail and 1 × Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL). A Bio-Rad protein assay (Bio-Rad) was used to determine protein concentrations. Proteins were separated on 10-12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Whatman, Boston, MA). Membranes were first hybridized with specific primary antibodies and then with HRP-conjugated secondary antibodies (Cell Signaling Technology). Protein bands were visualized using a commercial Immobilon Western Chemiluminescent HRP Substrate detection reagent (Millipore, Billerica, MA). The chemiluminescence of proteins transferred to PVDF membranes was detected with ECL Plus (GE Healthcare Amersham, Piscataway, NJ). Relative protein expression values were quantitatively determined via densitometry with ImageJ software.
Statistical analysis
All data were analyzed with statistical software package SPSS 16.0. The results were expressed as means ± SD. The statistical significance of the studies was determined by the parametric unpaired student’s t test. Differences with P < 0.05 are considered significant and P < 0.01 isconsidered highly significant.
Results
Growth inhibition of matrine on Hep G2 cells
To test the growth-modulatory effects of matrine on Hep G2 cells, a wide concentration range of matrine (0.2, 0.5, 0.8 and 1.0 mg/mL) was used. After 48 h’ treatment of matrine, significant inhibition of cell growth was observed at high concentration of 0.8 and 1.0 mg/mL compared with the control groups, with an average growth inhibition rate of 44.03 ± 4.18 and 48.39 ± 3.32%, respectively (Figure 1A). However, low concentration of matrine (0.2 and 0.5 mg/mL) had no significant effect on cell growth in comparison with the control groups. These results indicated that the growth inhibitory effect of matrine on Hep G2 cells was concentration dependent (Figure 1A).
Figure 1.
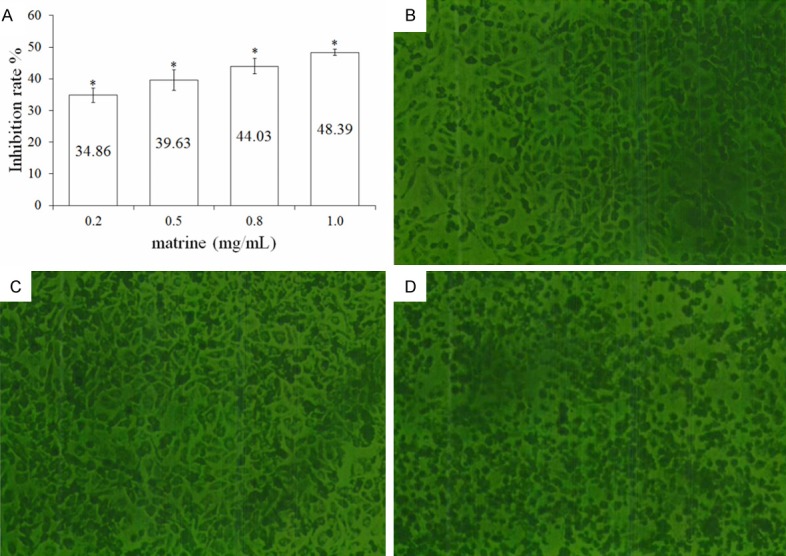
Growth inhibitory effect and cellular morphological changes of matrine (0.2, 0.5, 0.8 and 1.0 mg/mL) on Hep G2 cells. (A) Growth inhibitorydata are expressed as means ± SD, n = 4. *P < 0.05 represents significant differences in comparison with the control group. Results are expressed as the percentage growth inhibition with respect to the untreated cells. (B-D) The cellular morphological changes after treatments of matrine at different concentrations for 48 h was observed by FIMS (100 ×). Compared with the control group (B), no clear morphological change was observed in cells treated by low concentration of matrine (0.2 mg/mL) (C). While clear morphological changes including cell shrinking, disruption, and destruction were observed in cells treated by high concentration of matrine (1.0 mg/mL) compared with the control group (D).
Cellular morphological changes
After 48 h treatment of matrine, the cell morphology was observed by FIMS. At low concentration (0.2 mg/mL) of matrine (Figure 1C), the treated cells did not show clear difference in cellular morphology from the control groups (Figure 1B); while clear morphological changes including cell shrinking, disruption, and destruction were observed in cells treated by high concentration of matrine (1.0 mg/mL) compared with the control group (Figure 1D). High concentration of matrine could lead to the decrease of cell colony, which was also indicated by the degradation of cell diopter (Figure 2D).
Figure 2.
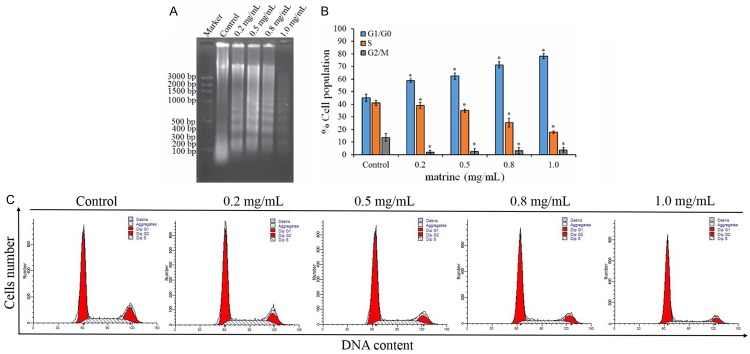
The effects ofmatrine on cell-cycle arrest and apoptosis in Hep G2 cells. A. The DNA fragmentation of Hep G2 induced by matrine, as determined by electrophoresis assay at 48 h with various concentrations of matrine treatment. B. Quantitative evaluations of terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick endlabeling (TUNEL) assay by flow cytometry. C. The distribution of cellcycle in matrine-treated cells. Each value is the mean ± SD of three determinations. *P < 0.05 represents significant differences between control and matrine-treated cells.
Cell apoptosis induced by matrine
To examine the mechanism responsible for matrine-mediated cellproliferation inhibition, cell-cycle distribution was evaluated usingflow cytometry analysis. Compared with the control groups, significant (P < 0.05) increase of the cell ratio of G1/G0 phrase, while significant (P < 0.05) decrease of that of S and G2/M phase, was observed; most cells stayed at G1 phase after treatment of matrine at low and high concentrations (0.2 and 1.0 mg/mL) (Table 1). The proportion of diploid slightly increased with a decrease of aneuploid; additionally, cell debris increased largely compared with the control group.
Table 1.
The changes of cell cycle induced by matrine (X ± S)
| Group | Cell debris (%) | Apoptosis (%) | Diploid (%) | Aneuploid (%) | |
|---|---|---|---|---|---|
| Control | 1.88 ± 0.2167 | 0.0267 ± 0.0252 | 94.7867 ± 4.5027 | 5.2133 ± 4.5027 | |
| Matrine (mg/mL) | 0.2 | 18.8967 ± 3.9119 | 0.0400 ± 0.0693 | 98.7367 ± 1.4318 | 1.2401 ± 1.4673 |
| 1.0 | 46.9533 ± 5.0537 | 0.06934 ± 0.0723 | 99.9101 ± 0.1558 | 0.0901 ± 0.1558 | |
The effect of matrine on the induction of apoptosis in Hep G2 cells by DNA fragmentation assay was also conducted. Agarose gel electrophoresis at 48 h showed that matrinetreatmentresults in the formation of DNA fragments in Hep G2 cells (Figure 2A). A quantitative evaluation was alsomade using TUNEL to detect DNA-strand breaks. Compared tothe control cells, at 48 h 1.0 mg/mL matrine induced 33.1% of apoptotic cells in Hep G2 cells (Figure 2B). The results showed that treating cells with matrine caused a significant inhibition of cell-cycle progression in Hep G2 cells (Figure 2C), resulting in a clear increase of the percentage of cells in the G1 phase when compared with the control.
Up-regulation of the hepato-specific miR-122a in matrine treated cells
To study the correlation between the matrine treatment and the expression of the hepato-specific miR-122a, the relative expression of the miR-122a was detected among the samples. As shown by Figure 3, an obviously up-regulated expression of miR-122a in the matrine treated Hep G2 cells was determined by quantitative real-time PCR assay. This up-regulation was further enhanced by increasing the concentration of matrine, and reached its peak, about 4.39 folds, when the matrine concentration increased to 1.0 mg/mL.
Figure 3.
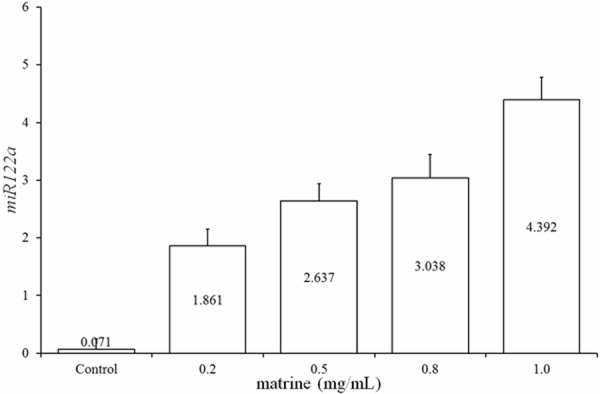
Relative expression of the hepato-specific miR122a in Hep G2 cells treated with different matrine concentrations standardized by negative control (non-matrine treated). *, Significant difference (P < 0.05).
Down-regulation of the miR-122a target CG1 in matrine treated cells
Immunohistochemical staining showed that almost all cell nuclei and brown cytoplasmic particles were sepia-colored after treatment of matrine at low concentration (0.2 mg/mL) for 48 h (Figure 4B). The mean OD of each group showed the expression level of the CG1 gene. The mean OD of the control group was 0.5367 ± 0.0235; that of the matrine-treated group was 0.3888 ± 0.0826. The mean OD of the matrine-treated group was highly significant (P < 0.01) higher than that of the control group (Figure 4C), suggesting that low concentration of matrine was enough to inhibit CG1 gene expression. These results were also confirmed by western blot assay (Figure 4D). The band of the control was clearly brighter (about 1.5 folds) than that of the matrine treated cells (Figure 4D).
Figure 4.
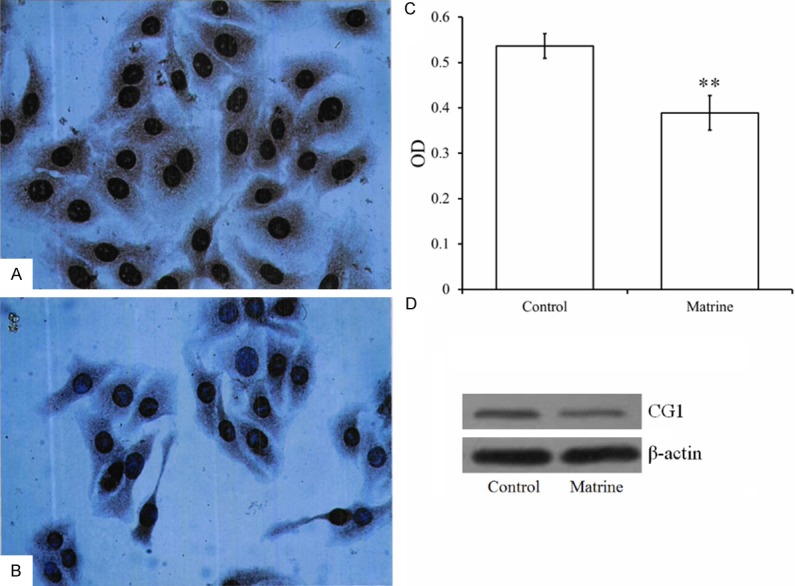
The expression of CG1 gene in the Hep G2 cells. Low concentration (0.2 mg/mL) of matrine could significantly (P < 0.01) inhibited the expression of CG1 genes (B) compared with the control group (A), as indicated by immunohistochemical staining (400 ×), the mean OD (C) and western blot (D). **P < 0.01 represents highly significant differences in comparison with the control group.
Discussion
Though traditional chemotherapy remains a main method for cancer therapy, cancer cells often develop drug resistance significantly lowering the efficiency of chemotherapeutic treatment. Furthermore, given the poor prognosis associated with some liver cancers and limited treatment options outside of surgery, patients may seek alternative treatments, including TCM products, alone or in combination with standard of care [1]. In recent years, the potential of natural products from medicinal plants used in TCM has been recognized by the scientific community in the Western world. Many natural products and derivatives thereof belong to the standard repertoire of cancer chemotherapy [23]. Novel TCM-derived anti-cancer drugs including arsenic trioxide, camptothecin, cantharidin, homoharringtonine,podophyllotoxin, vinblastine and vincristine (see reference [23]). Evidences showed that these anti-cancer TCMs function as 1) apoptosis inducer to inhibit cell growth through the genes involving in regulation of cell proliferation, angiogenesis or apoptosis [24,25]; 2) immune-enhancer to improve immunological function or strengthening resistance against both tumor and viruses [26].
The results presented in this study suggested that matrine has an important role in suppressing tumor cell growth in the human HCC cell line Hep G2. Matrine decreases the survival of Hep G2 cells in a dose-dependent manner. We found that low concentration (0.2 mg/mL) of matrine was sufficient to inhibit Hep G2 cell growth; this cell growth arrest was concentration dependent, i.e. elevated inhibitory effect was observed when dose was increased (Figure 1A). However, the cell morphology did not significantly change at low concentration (0.2 mg/mL) until dose was increased to 1.0 mg/mL (high concentration) (Figure 2). In accompany with cell growth arrest, Hep G2 cell apoptosis was induced after matrine treatment for 48 h, as indicated by phrase changes compared with untreated control with respect to cell cycle detected by FCM assay (Figure 2). Significant (P < 0.05) decrease of the S and G2/M phase fraction followed by increase of the cell ratio of G1/G0 phrase after treatment of matrine at low and high concentrations (0.2 and 1.0 mg/mL) was in agreement with the MTT assay (Table 1; Figure 1A). These results were similar to the inhibitory effect of matrine on the murine H22 cell proliferation [7]. Differing from the dose effect of matrine on murine H22 cells in which obvious inhibition occurred when 0.5 mg/mL matrine was used for 48 h, low concentration (0.2 mg/mL) for 48 h was sufficient to induced cell apoptosis in human Hep G2 cells in our study. These results indicated that low concentration of matrine inhibited Hep G2 cell proliferation byretarding cell growth to prolong cell cycle.
The proportion of cell apoptosis in this study was slightly low (Table 1), indicating that the inhibitory effect of matrine on Hep G2 cells was obtained mainly by cell cycle retardation (cell growth extension and proliferation deceleration) rather than cell apoptosis. Clear increase of G1/G0 cells and decrease of S and G2/M cells implied that the inhibitory effect of matrine on Hep G2 proliferation might be mainly attributed to cell arrest in G1 phrase. However, further studies are required to confirm the results and to investigate the underlying mechanism.
The hepato-specific miR122a is often found down-regulated in all HCC-derived cell lines [22]. Using qPCR technique, the expression of the hepato-specific miR122a was determined in the present study. Compared with the non-matrine treated Hep G2 cells (control), dose-dependent up-regulation of miR122a in all matrine-treated groups (Figure 3) suggesting a correlation between matrine and miR122a in the human Hep G2. These clues were further enhanced by the down-regulation of CG1, a target of the hepato-specific miR122a [22], by western blot assay (Figure 4D). Low concentration of matrine (0.2 mg/mL) inhibited CG1, about 1.5 fold lower than the control, which inversely correlated with the expression of miR122a, about 1.861 folds as Figure 3 shown. These results indicated that the antitumor TCM matrinecould inducecell arrest and apoptosis with recovery expression of the hepato-specific miR122a in human hepatocellular carcinoma Hep G2 cell line.
Disclosure of conflict of interest
None.
References
- 1.Wu P, Dugoua JJ, Eyawo O, Mills EJ. Traditional Chinese medicines in the treatment of hepatocellular cancers: a systematic review and meta-analysis. J Exp Clin Cancer Res. 2009;28:1–13. doi: 10.1186/1756-9966-28-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Li L, Lu F. [Mortality of primary liver cancer in China from 1990 through 1992] . Zhonghua Zhong Liu Za Zhi. 1999;21:245–249. [PubMed] [Google Scholar]
- 3.Xu Y, Xia F, Ma L, Shan J, Shen J, Yang Z, Liu J, Cui Y, Bian X, Bie P, Qian C. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011;310:160–169. doi: 10.1016/j.canlet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Avila MA, Berasain C, Sangro B, Prieto J. New therapies for hepatocellular carcinoma. Oncogene. 2006;25:3866–3884. doi: 10.1038/sj.onc.1209550. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 6.Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu P, Chen KK, Lopez JP, Poon RT, Fan ST. Octamer 4 (Oct4) mediates chemotherapeutic drug resistance in liver cancer cells through a potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology. 2010;52:528–539. doi: 10.1002/hep.23692. [DOI] [PubMed] [Google Scholar]
- 7.Ma L, Wen S, Zhan Y, He Y, Liu X, Jiang J. Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Medica. 2008;74:245–251. doi: 10.1055/s-2008-1034304. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Huang J. [Recent research progress of anti-tumor mechnism matrine] . Zhongguo Zhong Yao Za Zhi. 2004;29:115–118. [PubMed] [Google Scholar]
- 9.Yan Z, Jikai J, Xiaoshan L, Qing J, Zhang L. Differentiation and apoptosis in K562 erythroleukemia cells induced by matrine. Natural Med. 1998;52:295–299. [Google Scholar]
- 10.Li CQ, Zhu YT, Zhang FX, Fu LC, Li XH, Cheng Y, Li XY. Anti-HBV effect of liposome-encapsulated matrine in vitro and in vivo . World J Gastroenterol. 2005;11:426–428. doi: 10.3748/wjg.v11.i3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Zhu M, Shi R, Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am J Chin Med. 2003;31:337–354. doi: 10.1142/S0192415X03001107. [DOI] [PubMed] [Google Scholar]
- 12.Zhang JP, Zhang M, Zhou JP, Liu FT, Zhou B, Xie WF, Guo C. Antifibrotic effects of matrine on in vitro and in vivo models of liver fibrosis in rats. Acta Pharmacol Sin. 2001;22:183–186. [PubMed] [Google Scholar]
- 13.Zhang L, Jiang J, Tam J, Zhang Y, Liu XS, Xu XR, Liu BZ, He YJ. Effects of matrine on proliferation and differentiation in K-562 cells. Leukemia Res. 2001;25:793–800. doi: 10.1016/s0145-2126(00)00145-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Huang G, Wang Z, Guo Y, Zhang H. [Effects of matrine on the relative molecules expression of proliferation and apoptosis in K562 cells] . Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2001;23:333–336. [PubMed] [Google Scholar]
- 15.Wang Y, Peng C, Zhang G, Liu Y, Li H, Shan J. [Study on invasion and metastasis related factors in differentiation of SMMC-7721 cells induced by matrine] . Zhong Yao Cai. 2003;26:566–569. [PubMed] [Google Scholar]
- 16.Han Y, Zhang S, Wu J, Yu K, Zhang Y, Yin L, Bi L. Matrine induces apoptosis of human multiple myeloma cells via activation of the mitochondrial pathway. Leuk Lymphoma. 2010;51:1337–1346. doi: 10.3109/10428194.2010.488708. [DOI] [PubMed] [Google Scholar]
- 17.Luo C, Zhu Y, Jiang T, Lu X, Zhang W, Jing Q, Li J, Pang L, Chen K, Qiu F, Yu X, Yang J, Huang J. Matrine induced gastric cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules of Bcl-2 family. Toxicology. 2007;229:245–252. doi: 10.1016/j.tox.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Dai Z, Gao J, Wu W, Wang X, Li ZF, Kang HF, Liu XX, Ma XB. [Effect of matrine injections on invasion and metastasis of gastric carcinoma SGC-7901 cells in vitro] . Zhong Yao Cai. 2007;30:815–819. [PubMed] [Google Scholar]
- 19.Zhang L, Wang T, Wen X, Wei Y, Peng X, Li H, Wei L. Effect of matrine on HeLa cell adhesion and migration. Eur J Pharmacol. 2007;563:69–76. doi: 10.1016/j.ejphar.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Danielsen T, Hvidsten M, Stokke T, Solberg K, Rofstad E. Hypoxia induces p53 accumulation in the S-phase and accumulation of hypophosphorylated retinoblastoma protein in all cell cycle phases of human melanoma cells. Brit J Cancer. 1998;78:1547. doi: 10.1038/bjc.1998.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gramantieri L, Ferracin M, Fornari F, Veronese A, Sabbioni S, Liu CG, Calin GA, Giovannini C, Ferrazzi E, Grazi GL, Croce CM, Bolondi L, Negrini M. Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 2007;67:6092–6099. doi: 10.1158/0008-5472.CAN-06-4607. [DOI] [PubMed] [Google Scholar]
- 23.Efferth T, Li PC, Konkimalla VSB, Kaina B. From traditional Chinese medicine to rational cancer therapy. Trends Mol Med. 2007;13:353–361. doi: 10.1016/j.molmed.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- 25.Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, Ross DD, Funk JO. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol. 2003;64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 26.Ooi LS, Wang H, Luk CW, Ooi VE. Anticancer and antiviral activities of Youngia japonica (L. ) DC (Asteraceae, Compositae) J Ethnopharmacol. 2004;94:117–122. doi: 10.1016/j.jep.2004.05.004. [DOI] [PubMed] [Google Scholar]


