Abstract
Objective: This study is to investigate the expression of miRNA-1233 in placental tissue from patients with hypertensive disorder complicating pregnancy (HDCP) and its role in disease pathogenesis. Methods: The expression levels of miRNA-1233 and HoxB3 in placental tissue from HDCP patients and normal control subjects, as well as in the in vitro trophoblast cells, were detected with real-time PCR and Western blot analysis. The proliferation and invasion abilities of trophoblast cells were assessed by the cell counting kit (CCK)-8 and transwell chamber assays, respectively. Dual-luciferase reporter assay was performed to evaluate the interaction between miRNA-1233 and Hoxb3. Results: Real-time PCR showed that, compared with the control group, the expression levels of miRNA-1233 were significantly elevated in placental tissue from HDCP patients. On the other hand, both the mRNA and protein expression levels of HoxB3 were significantly decreased in the HDCP group. Moreover, the mRNA and protein expression levels of HoxB3 were significantly declined by the transfection of miRNA-1233 mimics in trophoblast cells. Bioinformatics analysis and the dual-luciferase reporter gene assay showed that, miRNA-1233 targeted HoxB3 in the 3’-UTR and suppressed the gene expression. In addition, the results from the CCK-8 and transwell chamber assays showed that, the transfection of miRNA-1233 significantly decreased the proliferation and invasion abilities of the trophoblast cells. Conclusion: In placental tissue from HDCP patients, up-regulated miR-1233 could suppress the expression of HoxB3, and then inhibit the invasion of trophoblast cells, which might contribute to the disease pathogenesis.
Keywords: Hypertensive disorder complicating pregnancy (HDCP), microRNA (miRNA)-1233, HoxB3, trophoblast cells
Introduction
Hypertensive disorder complicating pregnancy (HDCP) is a specific disease for pregnant women, which is characterized by hypertension, proteinuria, and edema [1]. HDCP severely affects the life and health of the pregnant and puerperal women, as well as the newborn [2-4]. At present, the pathogenesis of HDCP has not been clearly known. Studies show that the clinical symptoms of HDCP would gradually disappear after the expulsion of placenta [5]. Moreover, trophoblast cells have been found to be important for the pathogenesis of HDCP [6]. The vascular structure and function of the placenta in HDCP are associated with multiple abnormalities, which might be caused by reduced placental trophoblast invasion [7].
MicroRNA (miRNA) is a class of post-transcriptional regulators for target genes in various species, including humans. miRNA has also been involved in the cell migration and invasion [8-11]. Studies have shown that, altered miRNA expression profiles are closely related to the pathophysiology of placenta [12,13]. The abnormal expression of miR-106a-363 gene cluster in the placenta has been reported in patients with preeclampsia [12]. Moreover, miRNA-20a has been found to be highly expressed in the placenta of HDCP patients, which could inhibit the expression of FOXA1 and block the trophoblast invasion in the placenta [13]. These findings suggest that miRNAs play important roles in the development of HDCP. However, the expression and role of miRNA-1233 in the placenta from patients with HDCP have not yet been fully elucidated.
In this study, the role of miRNA-1233 in the pathogenesis of HDCP and the related mechanisms were investigated. The expression levels of miRNA-1233 and HoxB3 in placental tissue were detected. The effects of miRNA-1233 on the expression of HoxB3 in trophoblast cells in vitro, and on the cell proliferation and invasion abilities, were also investigated and analyzed.
Materials and methods
Patients and sample collection
Totally 45 placenta samples were collected from HDCP patients who had underwent cesarean section in our hospital, from Dec 2013 to Dec 2014. In these HDCP subjects, these were 25 cases of gestational hypertension (PIH; high blood pressure at 20 weeks of pregnancy, without proteinuria), 15 cases of mild preeclampsia (mPE, systolic pressure ≥ 140 mmHg or diastolic pressure ≥ 90 mmHg, 24-h proteinuria ≥ 300 mg), and 5 cases of severe preeclampsia (sPE; systolic pressure ≥ 160 mmHg or diastolic pressure ≥ 100 mmHg, 24-h proteinuria ≥ 2 g). These subjects were aged between 22-37 years old, with a mean age of 26.6 and a median age of 27 years old. Another 30 placenta samples were collected from normal parturient women to be used as controls. These normal control subjects were aged between 23-33 years old, with a mean age of 28.5 and a median age of 27 years old.
Trophoblast cell culture and transfection
Bewo trophoblast cell line was purchased from the Cell Bank of Chinese Academy of Sciences, Shanghai, China. These cells were planted onto a 24-well plate at a density of 1 × 105/well. The cells were cultured with antibiotic-free F12K medium (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco) for 24 h. 1.25 µL miRNA-1233 mimics (RiboBio, Guangzhou, Guangdong, China) or miRNA-NC (RiboBio), together with 1 µL Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA), was added into 50 µL Opti-Mem (Invitrogen). After standing at room temperature for 20 min, the mixture was added to incubate the cells for 6 h, and the cells were incubated with F12K medium containing 10% FBS for another 48 h or 72 h before the following assessments.
Quantitative real-time PCR
The mRNA expression levels of target genes in placental tissue and the trophoblast cells (48-h transfection) were detected with quantitative real-time PCR. Total RNA was extracted from tissues or cells with the Trizol agent (Sangon Biotech, Shanghai, China). Reverse transcription PCR was performed to obtain the cDNA templates (Boruike Biotech, Chengdu, Sichuan, China). For the detection of miRNA-1233 in placental tissue, and for the detection of miRNA-1233 and HoxB3 in transfected trophoblast cells, the primers for PCR were as follows: miRNA-1233, forward 5’-TGGGAGGCCAGGGCAC-3’ and the reverse universal primer within the kit; HoxB3, forward 5’-TGGACCTGGAGGATGAGAGG-3’ and reverse 5’-CATCGAAGCCGAAGCCATTG-3’; U6, forward 5’-CTCGCTTCGGCAGCACA-3’ and reverse 5’-AACGCTTCACGAATTTGCGT-3’. The 30 µL PCR system consisted of 5 µL template, 10 µL qRT-PCR-Mix (KAPA Biosystems, Woburn, MA, USA), 0.5 µL primer each, and 13 µL ddH2O. The reaction conditions were as follows: pre-denaturation at 95°C for 10 min; 95°C for 30 s, 65°C for 30 s, 72°C for 1 min, for totally 40 cycles. The relative expression levels of target genes were calculated with the 2-ΔΔCt method.
Western blot analysis
The protein expression levels of HoxB3 in placental tissue and the trophoblast cells (72-h transfection) were detected with the Western blot analysis. For the extraction of tissue proteins, 100 mg placental tissue was first triturated in liquid nitrogen, and the following procedures were the same with the extraction of cell proteins. Cells were lysed with RIPA lysis buffer containing PMSF protease inhibitors, and the total protein sample was collected by centrifugation at 10000 rpm for 10 min. Protein concentration was determined, and 10 µL protein sample was separated with 10% SDS-PAGE and then transferred onto a PVDF membrane. The membrane was blocked with 5% fat-free milk at room temperature for 1 h, and then incubated with rabbit anti-human anti-HoxB3 (1:1000 dilution; Abcam, Boston, MA, US) or mouse anti-human anti-GAPDH (1:5000 dilution; Bioworld Technology, Minneapolis, MN, USA) primary antibody at 4°C overnight. Then the membrane was incubated with HRP-conjugated goat anti-rabbit (1:2000 dilution) or goat anti-mouse (1:5000 dilution) secondary antibody at room temperature for 1 h. The protein bands were visualized by the electrochemiluminescence (ECL) method.
Cell counting kit (CCK)-8 assay
The proliferation of trophoblast cells was assessed by the CCK-8 assay. These cells were planted onto 96-well plates at a density of 2 × 103/well. 20 μL CCK-8 reaction solution was into each well at 0, 24, 48, and 72 h, respectively, followed by incubation at 37°C for 1 h. Absorbance (OD) at 490 nm was read, and the growth curve was obtained accordingly.
Transwell chamber assay
The cell invasion ability was assessed with the matrigel-coated ECM 550 transwell chamber (Merck Millipore, Billerica, MA, USA). Trophoblast cells (approximately 1 × 105), in 200 μL serum-free F12K medium, were added into the top transwell chamber. Then 500 μL F12K medium containing 10% FBS was added into the lower chamber. After 48 h, the lower chamber was fixed with 4% formaldehyde at room temperature for 10 min and then washed with PBS. The cells were subjected to the Giemsa staining, and the migrating cells were observed with microscopy. The stained cells were counted from 5 randomly selected fields, and the cell invasion ability was evaluated.
Dual-luciferase reporter assay
According to the bioinformatics analysis, the potential target sequences for miRNA-1233 in the 3’ untranslated region (3’-UTR) of HoxB3 and its mutated sequences (wild type HoxB3 3’-UTR: 5’-UUAGAGGGCUCA-3’; mutant HoxB3 3’-UTR: 5’-UUAGATGTCACT-3’) were synthesized, and cloned into the pMIR-REPORTER luciferase plasmids (Promega, Madison, WI, USA). The reconstructed plasmid (0.8 mg) and miRNA-1233 mimics (50 nM) were co-transfected into Bewo trophoblast cells with Lipofectamine 2000. After 24 h, cells were lysed, and the luciferase was detected with the GloMax 20/20 luminometer. The Renilla luciferase plasmid was used as the internal control.
Statistical analysis
Data are expressed as mean ± SD. SPSS 19.0 software was used for statistical analysis. The t-test was performed for the group comparison. P < 0.05 was considered as statistically significant.
Results
Expression of miRNA-1233 and HoxB3 in placental tissue
To investigate the role of miRNA-1233 in the development of HDCP, the expression levels of miRNA-1233 in placental tissue were detected with real-time PCR. Our results showed that, compared with the control group, the expression level of miRNA-1233 was significantly elevated in the HDCP group (P < 0.05) (Figure 1A). To further investigate the involvement of HoxB3 in the disease pathogenesis, the mRNA and protein expression levels of HoxB3 in placental tissue were determined by the real-time PCR and Western blot analysis, respectively. Real-time PCR showed that, compared with the control group, the mRNA expression level of HoxB3 was significantly decreased in the HDCP group (P < 0.05) (Figure 1B). Moreover, within these HDCP patients, the mRNA expression levels of HoxB3 exhibited a decreasing trend along with the increasing severities of the disease (in the order of PIH, mPE, and sPE) (Figure 1C). Similar results were obtained for the Western blot analysis. Our results showed that, compared with the control group, the protein expression level of HoxB3 was significantly declined in the HDCP group (P < 0.05) (Figure 1D). Taken together, these results suggest that, the expression level of miRNA-1233 is increased, while the expression level of HoxB3 is decreased, in placental tissue from HDCP patients. The negative relationship between miRNA-1233 and HoxB3 expression levels might be associated with the disease pathogenesis.
Figure 1.

Expression levels of miRNA-1233 and HoxB3 in placental tissue. (A, B) The expression levels of miRNA-1233 (A) and the mRNA expression levels of HoxB3 (B) in placental tissue from HDCP patients and normal controls were detected with real-time PCR. (C) The mRNA expression levels of HoxB3 within HDCP patients with different severities (i.e., PIH, mPE, and sPE) were analyzed and compared. (D) The protein expression levels of HoxB3 in placental tissue from HDCP patients and normal controls were detected with the Western blot analysis. Compared with the control group, *P < 0.05, **P < 0.01; compared with the former group, &P < 0.05.
Effect of miRNA-1233 transfection on HoxB3 expression in trophoblast cells
To investigate the effect of miRNA-1233 on the expression levels of HoxB3 in trophoblast cells, these cells were first transfected with miRNA-1233 mimics, and then the mRNA and protein expression levels of Hoxb3 were assessed with the real-time PCR and Western blot analysis, respectively. Our results from the real-time PCR showed that, compared with the control group, the mRNA expression level of HoxB3 was significantly decreased in trophoblast cells transfected with miRNA-1233 (P < 0.05) (Figure 2A). Moreover, the Western blot analysis also showed that, the miRNA-1233 transfection significantly decreased the protein expression level of HoxB3 in trophoblast cells (P < 0.05) (Figure 2B). These results suggest that miRNA-1233 transfection could significantly decrease the expression levels of HoxB3 in trophoblast cells in vitro.
Figure 2.
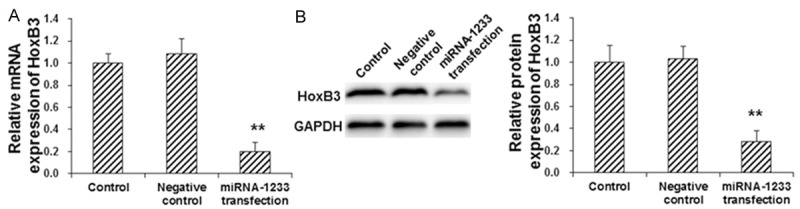
Effect of miRNA-1233 transfection on HoxB3 expression in trophoblast cells. Bewo trophoblast cells were first transfected with miRNA-1233 mimics, and then the mRNA (A) and protein (B) expression levels of Hoxb3 in these cells were assessed with the real-time PCR and Western blot analysis, respectively. Compared with the control group, *P < 0.05, **P < 0.01.
Interaction between miRNA-1233 and the 3’-UTR of HoxB3
To further confirm the interaction between miRNA-1233 and HoxB3, the bioinformatics analysis and the dual-luciferase reporter gene assay were performed. Our results from the bioinformatics analysis showed that HoxB3 might be one of the target genes for miRNA-1233. Moreover, the dual-luciferase reporter gene assay showed that, the fluorescence intensity was significantly declined, when the miRNA-1233 mimics and the pMIR-REPORT luciferase reporter plasmid containing the wild-type 3’-UTR of HoxB3 were co-transfected into the Bewo trophoblast cells. However, in the cells transfected with pMIR-REPORT luciferase reporter plasmid containing the mutant 3’-UTR of HoxB3, no significant differences were observed in the fluorescence intensity (P > 0.05) (Figure 3). These results suggest that miRNA-1233 targets HoxB3 in the 3’-UTR and suppresses the gene expression, which might contribute to the pathogenesis of HDCP.
Figure 3.
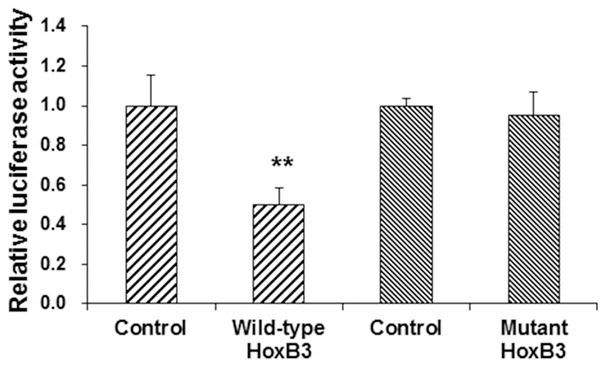
Interaction between miRNA-1233 and the 3’-UTR of HoxB3. Dual-luciferase reporter gene assay were performed to investigate the interaction between miRNA-1233 and HoxB3. The pMIR-REPORT luciferase reporter plasmid containing the wild-type or mutant 3’-UTR of HoxB3 were co-transfected with the miRNA-1233 mimics into Bewo trophoblast cells for 24 h. Then the cells were lysed, and the luciferase was detected. Compared with the control group, **P < 0.01.
Proliferation and invasion abilities of miRNA-1233-transfected trophoblast cells
In order to investigate the effects of miRNA-1233 on the proliferation and invasion abilities of trophoblast cells, the CCK-8 and transwell chamber assays were performed, respectively. Our results from the CCK-8 assay showed that, compared with the control group, the transfection of miRNA-1233 significantly decreased the proliferation of trophoblast cells (P < 0.05) (Figure 4A). On the other hand, the transwell chamber assay showed that, the number of migrating cells was significantly lower in the miRNA-1233 transfection group, compared with the control group (P < 0.05) (Figure 4B), indicating the decreased invasion ability of trophoblast cells transfected with miRNA-1233. These results suggest that the miRNA-1233 transfection could significantly decrease the proliferation and invasion abilities of trophoblast cells in vitro.
Figure 4.
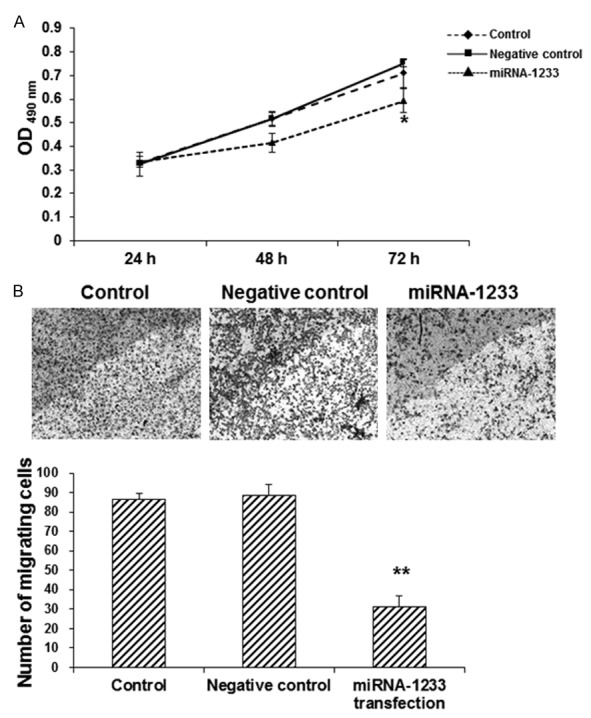
Effects of miRNA-1233 transfection on the proliferation and invasion abilities of trophoblast cells. A. The CCK-8 assay was performed to investigate the proliferation of trophoblast cells with or without miRNA-1233 transfection, at 24, 48, and 72 h, respectively. B. The transwell chamber assay was performed to investigate the invasion ability of trophoblast cells with or without miRNA-1233 transfection. Compared with the control group, *P < 0.05, **P < 0.01.
Discussion
In this study, our results showed that, compared with the normal controls, the expression of miRNA-1233 was significantly higher in placental tissue from HDCP patients. HoxB3 has been shown to be one of the targets of miRNA-1233, which could promote the invasion and metastasis of various tumor cells [14,15]. Therefore, HoxB3 had been investigated in this study. Our results from real-time PCR showed that, the mRNA expression levels of HoxB3 were significantly decreased in placental tissue from HDCP patients. Moreover, the HoxB3 expression exhibited a decreasing trend along with the increasing severities of the disease, from PIH, mPE, to sPE. However, the expression of miRNA-1233 was not associated with the clinical staging of HDCP (data not shown). Furthermore, the protein expression level of HoxB3 was also significantly decreased in placental tissue from the HDCP group. These results suggest a negative relationship between miRNA-1233 and HoxB3 expression levels, indicating that miRNA-1233 might be involved in the regulation of HoxB3 expression levels.
In order to further investigate the effects of miRNA-1233 on the expression of HoxB3, the Bewo trophoblast cells were transfected with miRNA-1233 mimics. Our results from the real-time PCR and Western blot analysis showed that, the mRNA and protein expression levels of HoxB3 in trophoblast cells was decreased by 47% and 29%, respectively, following the transfection of miRNA-1233. These results confirm the regulation of miRNA-1233 on the expression of HoxB3 in trophoblast cells in vitro. Moreover, the dual-luciferase reporter gene assay showed that, the fluorescence intensity in the Bewo trophoblast cells was significantly declined by the co-transfection of miRNA-1233 mimics and pMIR-REPORT luciferase reporter plasmid containing the wild-type 3’-UTR of HoxB3. In contrast, no significant differences were observed in the fluorescence intensity in the cells transfected with pMIR-REPORT luciferase reporter plasmid containing the mutant 3’-UTR of HoxB3. These results confirm that miRNA-1233 targets HoxB3 in the 3’-UTR. On the other hand, the proliferation and invasion abilities of trophoblast cells with or without miRNA-1233 transfection had been also assessed by the CCK-8 and transwell chamber assays, respectively. Our results showed that the miRNA-1233 over-expression could significantly decrease the proliferation and invasion abilities of trophoblast cells in vitro.
In conclusion, our results showed that the expression level of miRNA-1233 was increased, while the expression level of HoxB3 was decreased, in placental tissue from HDCP patients. Moreover, the transfection of miRNA-1233 significantly decreased the expression levels of HoxB3 in trophoblast cells in vitro. In addition, the miRNA-1233 transfection dramatically decreased the proliferation and invasion abilities of these cells. In placental tissue of HDCP patients, up-regulated miR-1233 could suppress the expression of HoxB3, and then inhibit the invasion of trophoblast cells, further affecting the structure and function of the placenta. These findings might contributing to the understanding of the pathogenesis of HDCP, and provide evidence for the diagnosis and treatment of the disease.
Disclosure of conflict of interest
None.
References
- 1.Jaafar J, Pechere-Bertschi A, Ditisheim A. Hypertension In pregnancy: practical considerations. Rev Med Suisse. 2014;10:1645–1649. [PubMed] [Google Scholar]
- 2.Li LX, Liu YL, Wen JG, Li ZZ, Zhao YP. Expression and significance of aquaporin 1 in placenta, placental membranes and peritoneum of patients with hypertensive disorder complicating pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2008;43:497–501. [PubMed] [Google Scholar]
- 3.Zhu J, Li M, Li L. Expression and significance of heat shock protein 70 in maternal serum, umbilical cord blood and placenta of patients with hypertensive disorders complicating pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2014;49:676–680. [PubMed] [Google Scholar]
- 4.Zhou J, Xiao XM, Wu YH. Expression of interferon-gamma in decidual natural killer cells from women with hypertensive disorder complicating pregnancy. J Obstet Gynaecol Res. 2014;40:670–676. doi: 10.1111/jog.12216. [DOI] [PubMed] [Google Scholar]
- 5.Wang RG, You ZL, Liu XL. Effect of ingredients of Astragalus-Salvia compound on vascular endothelial cell in placenta and vascular endothelial growth factor mRNA expression in trophocyte in pregnant rats with inhibited nitric oxide synthesis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:516–519. [PubMed] [Google Scholar]
- 6.Yang S, Li H, Ge Q, Guo L, Chen F. Deregulated microRNA species in the plasma and placenta of patients with preeclampsia. Mol Med Rep. 2015;12:527–534. doi: 10.3892/mmr.2015.3414. [DOI] [PubMed] [Google Scholar]
- 7.Sun LZ, Ma XT, Ge ZP, Han P. Effect of endoplasmic reticulum stress-responsive protein glucose-regulated protein 78, 94 and endoplasmic reticulum apoptosis factor caspase-12 in trophocyte on the pathogenesis of preeclampsia. Zhonghua Fu Chan Ke Za Zhi. 2010;45:891–895. [PubMed] [Google Scholar]
- 8.Song J, Li Y, An RF. Identification of Early-Onset Preeclampsia-Related Genes and MicroRNAs by Bioinformatics Approaches. Reprod Sci. 2015;22:954–963. doi: 10.1177/1933719115570898. [DOI] [PubMed] [Google Scholar]
- 9.Hong F, Li Y, Xu Y. Decreased placental miR-126 expression and vascular endothelial growth factor levels in patients with pre-eclampsia. J Int Med Res. 2014;42:1243–1251. doi: 10.1177/0300060514540627. [DOI] [PubMed] [Google Scholar]
- 10.Lalevee S, Lapaire O, Buhler M. miR455 is linked to hypoxia signaling and is deregulated in preeclampsia. Cell Death Dis. 2014;5:e1408. doi: 10.1038/cddis.2014.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limon P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Li Q, Ren N, Li C, Wang X, Xie M, Gao Z, Pan Z, Zhao C, Ren C, Yang W. Placental miR-106a approximately 363 cluster is dysregulated in preeclamptic placenta. Placenta. 2015;36:250–252. doi: 10.1016/j.placenta.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Zhang Y, Wang H, Wang J, Zhang Y, Wang Y, Pan Z, Luo S. Aberrantly up-regulated miR-20a in pre-eclampsic placenta compromised the proliferative and invasive behaviors of trophoblast cells by targeting forkhead box protein A1. Int J Biol Sci. 2014;10:973–982. doi: 10.7150/ijbs.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Zhu S, Jiang N, Shang Z, Quan C, Niu Y. HoxB3 promotes prostate cancer cell progression by transactivating CDCA3. Cancer Lett. 2013;330:217–224. doi: 10.1016/j.canlet.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Zhu F, Chen P. miR-7 and miR-218 epigenetically control tumor suppressor genes RASSF1A and Claudin-6 by targeting HoxB3 in breast cancer. Biochem Biophys Res Commun. 2012;424:28–33. doi: 10.1016/j.bbrc.2012.06.028. [DOI] [PubMed] [Google Scholar]


