Abstract
The study aim was to explore the clinical efficacy and safety of inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) in combined with idiopathic pulmonary fibrosis and emphysema. 45 patients with combined idiopathic pulmonary fibrosis and emphysema (CPFE) who were treated with ICS/LABA (Group A), 24 patients with CPFE who were treated without ICS/LABA (Group B) and 35 patients with idiopathic pulmonary fibrosis (IPF) (Group C) were enrolled into this study. Then, clinical efficacy and safety of ICS/LABA was analyzed through lung function scores and lung high-resolution computed tomography (HRCT) scans. Compared with baseline levels, the FEV1%, FVC% and DLCO% levels were increased 11.2%, 13.53% and 12.8% respectively in group A, but declined 14.21%, 16.8% and 21.25% respectively in group B, meanwhile, lung HRCT score was declined 9.31 in group A but increased 14.87 in group B, and there was significant difference between group A and group B (P<0.01). Furthermore, the acute outbreak frequency was 44.4% and 75% in group A and B respectively within 12 months (P<0.05); moreover, CPI index and HRCT score were both lower in group A than those in group B in acute episode period (P<0.05), but there was no significant difference of PO2 value between group A and B (P>0.05). The incidence of adverse reaction was higher in group A than that in group B during this study, but there was no significant difference (P>0.05). ICS/LABA therapy could improve lung function condition in patients with CPFE and declined acute out-break frequency and severity of diseases during acute episode period.
Keywords: ICS/LABA, CPFE, efficacy, safety
Introduction
Idiopathic pulmonary fibrosis (IPF) is a clinicopathological syndrome characterized by pulmonary interstitial fibrosis caused by diffuse alveolitis and anatomical disorder in alveolus, and the histopathological characteristics of IPF mainly presented usual interstitial pneumonia, but the cause of pulmonary fibrosis is still unknown. Furthermore, IPF is a chronic and ultimately fatal pulmonary disease characterized by a progressive decline in lung function [1] and the poor prognosis of IPF is usually associated with age, sex, smoking history, lung function and lung computed tomography (CT) scores. In addition, the prognosis of patients with combined pulmonary fibrosis and emphysema was much poorer than those with IPF but without emphysema [2-6]. Moreover, Prof. Wiggnins and his team firstly reported eight cases with combined Idiopathic pulmonary fibrosis and emphysema (CPFE) in 1990 [7]. Then, Prof. Cottin also reported 61 patients with CPFE who had special imaging features through chest high-resolution computed tomography (HRCT) analysis in 2005 [8]. Recently, some studies have found that CPFE was relatively independent particularity in many aspects, such as pathogenesis, imaging features and prognosis [9,10], but there was not any report about treatment of CPFE in our country. Our aim was to explore treatment method of CPFE primarily in order to estimate the clinical efficacy and safety of inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) in treatment of CPFE.
Material and methods
Selection of patients
A prospective cohort study were performed in this paper. 73 patients with CPFE were enrolled into this study from January 2009 to December 2013, including 62 males and 11 females, with the mean age of 64±7 years old, and all patients conformed diagnostic criteria of IPF under non-invasive condition that was a collaborative effort of the ATS/ERS in 2011 [11]. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Harbin Medical University. Written informed consent was obtained from all participants. Diagnostic criteria of CPFE referred to following criteria [8] that 1) chest HRCT showed pulmonary fibrosis and emphysema and clear low density zone, a very thin wall (<1 mm) or no wall, and/or multiple bullae (>1 cm) with upper zone; 2) presence of a diffuse parenchymal lung disease with significant pulmonary fibrosis on CT scan, defined as reticular opacities with peripheral and basal predominance, honeycombing, architectural distortion and/or traction bronchiectasis or bronchiolectasis; focal ground-glass opacities and/or areas of alveolar condensation may be associated but should not be prominent. Meanwhile, all cases were excluded the known other connective tissue diseases, including hypersensitivity pneumonitis, sarcoidosis, pneumoconiosis, pulmonary histiocytosis, lymphangioleiomyomatosis and eosinophilic pneumonia. Interpretive criteria of clinical character of the acute attack CPFE was used as the reference standard for chronic obstructive pulmonary disease (COPD) and IPF. Here, all cases were divided into three groups, including group A that used ICS/LABA to treat patients, group B that patients were not treated by ICS/LABA, group C that patients with IPF at the same time, place and conditions as control group. Group C included 24 males and 11 females, with the mean age of 65 years old (65±5). Furthermore, therapeutic drugs were chosen strictly, for example, ICS and LABA were used for over 3 months in group A and should conform to related therapeutic measures of GOLD COPD (2011 version) [12]. Meanwhile, group B and control group were treated without ICS and LABA. Besides, patients whose conditions got worse in three groups should be recorded thoroughly.
Assessment of chest HRCT scoring
Chest HRCT scoring, pulmonary ventilation and related indexes of diffuse function were firstly performed in three groups, and starting time (0 month) was as onset time, then the inspection results were analyzed for every 3 months, including clinical symptoms, adverse reactions. Chest HRCT analysis was as follows: 1) emphysema evaluation used visual grade method [13] as follows and was scored by two CT observers independently: 0=non-emphysema, 1-8 score=mild emphysema, 9-16 score=moderate emphysema and 17-24 score=severe emphysema; 2) pulmonary fibrosis was scored by two CT observers independently at the origin of the great vessels, the carina and 1cm above the right hemidiaphragm and then calculated the ratio of fibrosis zone and corresponding lobe area respectively. Meanwhile, ground-glass opacification, intralobular fibrosis, microcystic honeycombing and macrocytic honeycombing were also calculated [14]. The score criterion was as follows: 0=no change; 1=lesions range ≤5%; 2=lesions range from 6% to 24%; 3=lesions range from 25%-49%; 4=lesions range from 50%-74% and 5=lesions range ≥75%. Then all pulmonary fibrosis and emphysema scores were adjusted through multiplied by 4.8. Moreover, total HRCT scores of CPFE were pulmonary fibrosis scores + emphysema scores. forced vital capacity (FVC)%, forced expiratory volume in one second (FEV1)%, diffusion capacity for carbon monoxide (DLCO)% and pulmonary function composite physiologic index (CPI) were as dynamic monitoring indexes [15]. Finally, the average values of dynamic monitoring results were analyzed.
End point
Our research cycle was 12 months and set the study termination as end point, if some patients were dead during this study, death time would be the end point.
Statistical analysis
Statistical analysis was performed by SAS 9.13 software and measurement data results were presented as mean ± SD. Multiplied test results used multivariate analysis of variance, once the results had statistically significant; the data were analyzed by one-way analysis of variance. Count data were presented as frequency and percentage and analyzed by chi-square test. Rank sum test was used to analyze ranked data, P<0.05 denoted a significant statistical difference.
Results
In total, 103 subjects completed the study. 8 patients were excluded, including 4 patients in CPFE group and 4 patients in IPF control group due to some reasons. Table 1 showed the changes of all kinds of test indexes and differential analysis 12 months after treatment in group A and B. FEV1%, FVC% and DLCO% were 65.27%, 65.89% and 57.29% respectively in group A, and compared with before the treatment, FEV1%, FVC% and DLCO% were increased 11.2%, 13.53% and 12.8% respectively; and in group B, FEV1%, FVC% and DLCO% were 42.92%, 40.83% and 34.25%, which were decreased 14.21%, 16.8% and 21.25% respectively compared with before the treatment. The changes of lung function were significant differences both in two groups (P<0.01, Figures 1, 2, 3 and 4). Furthermore, above indexes for 6 months, 9 months and 12 months in group A and B were compared and were significant differences (P<0.05). Additionally, HRCT score was 27.29 in group A, which was decreased 9.31 compared with before the treatment, but in group B, the HRCT score was 46.08, which was increased 14.87 compared with before the treatment. There was statistically significant difference in two groups (P<0.01).
Table 1.
Differential analysis of lung function and chest HRCT in patients with CPFE 12 months after treated with ICS/LABA
| Group | FEV1% | FVC% | DLCO% | HRCT score | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Treatment group (n=45) | Control group (n=24) | Treatment group (n=45) | Control group (n=24) | Treatment group (n=45) | Control group (n=24) | Treatment group (n=45) | Control group (n=24) | |
| 0 month | 54.07±8.69 | 57.13±10.04* | 52.36±8.83 | 57.63±10.75* | 46.49±7.91 | 55.5±6.66# | 36.6±5.47 | 31.21±6.73 |
| 3 months | 55.57±8.69 | 53.04±10.03* | 56.27±8.21 | 54.67±8.24* | 50.47±7.36 | 50.5±6.87* | 31.16±5.23 | 33.54±5.88 |
| 6 months | 60.32±5.84 | 49.29±9.45# | 60.31±7.63 | 49.83±8.83# | 53.29±7.68 | 45.96±6.78# | 28.51±4.94 | 36.75±5.08 |
| 9 months | 62.77±4.54 | 44.96±8.64# | 63.11±7.53 | 45.42±7.51# | 55.16±7.47 | 40.13±7.24# | 27.53±5.05 | 40.63±3.81 |
| 12 months | 65.27±4.86 | 42.92±7.86# | 65.89±8.05 | 40.83±7.81# | 57.29±7.17 | 34.25±7.01# | 27.29±5.13 | 46.08±2.17 |
| F value | 35.63618 | 26.02683 | 17.83554 | 40.70971 | ||||
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||
There was no significant difference between two groups (P>0.05);
There was significant difference between two groups (P<0.05).
Figure 1.
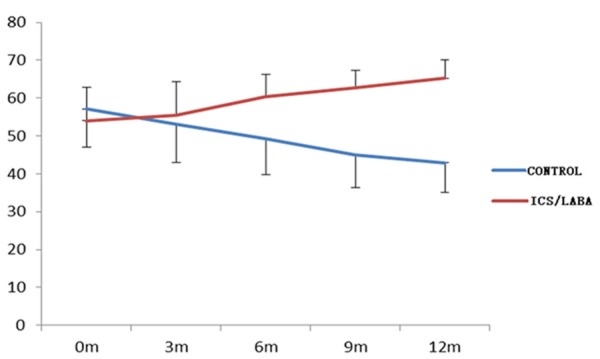
Variation tendency of FEV1 with time in two groups.
Figure 2.
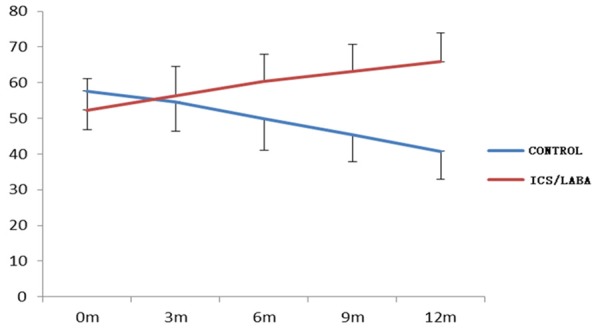
Variation tendency of FVC with time in two groups.
Figure 3.
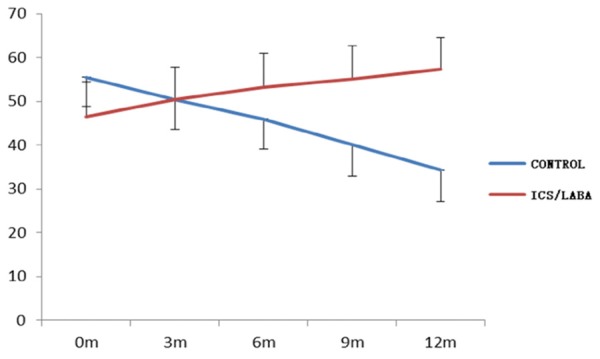
Variation tendency of DLCO with time in two groups.
Figure 4.
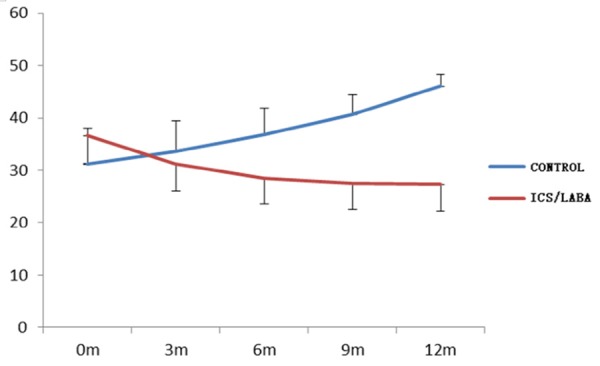
Variation tendency of HRCT with time in two groups.
Comparative analysis of CPI and chest HRCT
Table 2 showed that after observing for 12 months, CPI index was 61.42 and 51.1 in CPFE group and IPF group respectively, which were increased 14.19 and 3.13 respectively, compared before 12 months. Meanwhile, HRCT score was 46.08 and 36.84 in CPFE group and IPF group respectively, which were increased 14.87 and 7.1 respectively, compared before 12 months, the increase of speed was faster in CPFE group than that in IPF group (P<0.01, Figures 5, 6). These results indicated that the progress of disease was faster in CPFE group than that in IPF group.
Table 2.
Comparison of CPI and HRCT in patients with CPFE and IPF
| Group | CPI | Chest HRCT | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CPFE control group | IPF control group | F | p | CPFE control group | IPF control group | F | p | |
| 0 month | 47.23±7.98 | 47.97±7.93 | 1.462423 | 0.22789 | 31.21±6.73 | 29.74±3.04 | 4.9779 | >0.0001 |
| 3 months | 47.24±7.4 | 48.42±7.8 | 3.76982 | 0.05351 | 33.54±5.88 | 30.32±2.97 | 37.43908 | <0.0001 |
| 6 months | 51.47±7.65 | 49.02±7.7 | 16.11451 | <0.0001 | 36.75±5.08 | 32.77±3.13 | 57.10978 | <0.0001 |
| 9 months | 56.13±7.01 | 50.28±7.79 | 91.78243 | <0.0001 | 40.63±3.81 | 35.97±3.34 | 78.36473 | <0.0001 |
| 12 months | 61.42±7.83 | 51.1±7.6 | 284.9828 | <0.0001 | 46.08±2.17 | 36.84±3.28 | 308.7724 | <0.0001 |
| F value | 146.3116 | 314.041547 | ||||||
| P value | <0.0001 | <0.0001 | ||||||
Figure 5.
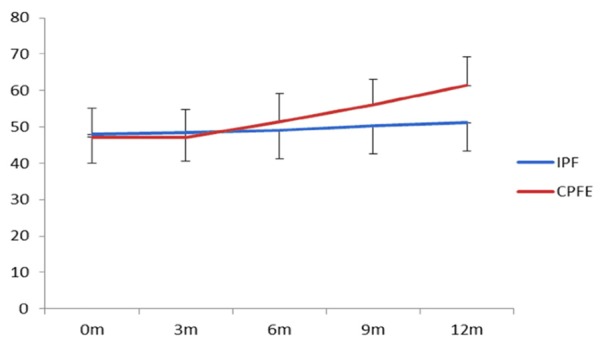
Variation tendency of CPI with time in two groups.
Figure 6.
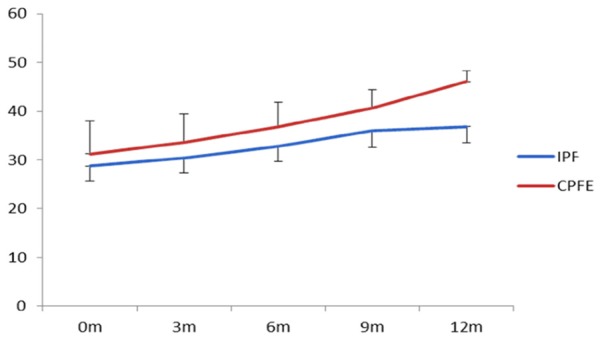
Variation tendency of HRCT with time in two groups.
Changes of all indexes of patients with CPFE during acute aggravating period
Table 3 showed that the acute seizure frequency was 44.4% and 75% in group A and B respectively within 12 months, but the frequency was lower in group A than that in group B, which was a significant difference (P<0.05). Besides, there were significant differences of CPI up-index and HRCT scores between two groups (P<0.05), but there was no significant difference of PO2 value between two groups (P>0.05). These results demonstrated that ICS/LABA therapy obviously reduced acute seizure frequency of patients with CPFE and made patients not suffer from severe disease during acute outbreak period.
Table 3.
Comparative analysis of indexes of patients with CPFE during acuteaggravating period in treatment group and non-treatment group
| Group | CPFE treatment group (n=45) | CPFE control group (n=24) | Statistics | P value |
|---|---|---|---|---|
| Frequency | 20 (44.4%) | 18 (75%) | 5.9062 | 0.0151 |
| PO2 descent (mmHg) | 3.7014 | 0.0544 | ||
| <10 | 10 (22.2%) | 4 (16.67) | ||
| 10-20 | 6 (13.33%) | 6 (25%) | ||
| >20 | 4 (8.9%) | 8 (33.3%) | ||
| CPI up-index | 9.4375 | 0.0021 | ||
| 1-5 | 11 (24.4%) | 3 (12.5%) | ||
| 5-10 | 7 (15.56) | 5 (20.83%) | ||
| >10 | 2 (4.4%) | 10 (41.67%) | ||
| HRCT up-value | 5.4802 | 0.0192 | ||
| <5 | 4 (8.89%) | 2 (8.33%) | ||
| 5-10 | 13 (28.89%) | 7 (29.16%) | ||
| >10 | 4 (8.89%) | 12 (50%) |
Possible related adverse reactions during treatment of ICS/LABA
Table 4 showed that treatment of ICS/LABA increased risks of gastroesophageal reflux, cardiovascular diseases, gastric mucosal lesion and gastrointestinal bleeding, somnipathy and oral fungus infection, but there were no significant differences between two groups (P>0.05), but treatment of ICS/LABA could reduce incidence rate of cardiopulmonary disease, which suggested this therapy might delay the progress of disease.
Table 4.
Impairment condition after inhaled corticosteroids
| Adverse events | CPFE control group | CPFE treatment group | Chi-square value | P value |
|---|---|---|---|---|
| Pneumonia | 5 (20.83%) | 9 (19.57%) | 0.02 | 0.9 |
| Gastroesophageal reflux | 3 (12.5%) | 12 (26.67%) | 1.85 | 0.17 |
| BMI<18 | 4 (16.67%) | 4 (8.7%) | 0.99 | 0.32 |
| BMI>25 | 3 (12.5%) | 5 (10.87%) | 0.04 | 0.84 |
| Secondary diabetes or aggravation of daibetes | 6 (25%) | 6 (13.04%) | 1.59 | 0.21 |
| Spontaneous fracture or caput femoris necrosis | 2 (8.33%) | 1 (2.17%) | 1.46 | 0.23 |
| Secondary erythremia | 6 (25%) | 11 (23.91%) | 0.01 | 0.92 |
| Cerebrovascular diseases | 6 (25%) | 9 (19.57%) | 0.28 | 0.6 |
| Cardiovascular diseases | 3 (12.5%) | 8 (17.39%) | 0.28 | 0.59 |
| Cardiopulmonary diseases | 8 (33.33%) | 2 (4.35%) | 10.82 | 0 |
| Gastric mucosal lesion and gastrointestinal bleeding | 4 (16.67%) | 12 (26.09%) | 0.79 | 0.37 |
| Somnipathy or anxiety | 2 (8.33%) | 8 (17.39%) | 1.06 | 0.3 |
| Oral fungus infection | 3 (12.5%) | 13 (28.26%) | 2.22 | 0.14 |
| Newly developed tuberculosis | 2 (8.33%) | 1 (2.17%) | 1.46 | 0.23 |
Discussion
ATS/ERS referred to using HRCT analysis to diagnose IPF as noninvasive diagnostic method in new revised IPF therapy guideline, which was great helpful to study IPF further. However, there were still not specific measures to cure IPF [5]. Recently, with the high incidence rate of IPF [16] and short median survival time [11], it was necessary to explore the effective methods to treat IPF. Since pulmonary HRCT became a noninvasive diagnostic method to diagnose IPF, many reports about CPFE have been studied, nearly, some researchers have primary studies on pathogenesis and prognosis through specific imaging features of CPFE [17]. The results demonstrated that CPFE was a special disease and different from IPF. This study hopes to explore effective therapeutic method for CPFE and get more information about clinical characteristics of CPFE.
This study chose drugs on the basis of pathogenesis and clinical characteristics of IPF and therapeutic measures of COPD. Although exact pathogenesis of IPF was still not known, many hypotheses indicated that cell injury caused by inflammation and exceed repairmen of fibroblast played much more important role in pathogenesis of IPF [18]. Glucocorticoid promoted inflammatory resolution through inhibiting angiotelectasis and suppressed proliferation of fibroblast in the late stage of inflammation. Furthermore, Glucocorticoid have used widespread in COPD and IPF [3]. Therefore, inhaled corticosterioids were chosen as objectives. CPFE presented classic emphysema feature in imageology and lung function showed the changes of FEV1, FVC and DLCO [19,20], and airway constriction and lesion happened on the process of CPFE, which implied that the pathomechanism of CPFE was similar with COPD. Thus, we combined ICS and LABA to treat CPFE and assessed the effect of these two drugs on CPFE.
Our study proved that ICS/LABA not only slowed the progression of disease, but improve clinical characteristics. Results showed that FEV1%, FVC% and DLCO% improved 11.2%, 13.53 and 12.8% respectively in ICS/LABA treatment group after 12 months, and lung HRCT scores declined 9.31, which were significant different to non-treatment group. This demonstrated ICS/LABA was effective. Additionally, patients with CPFE presented ventilation function lesions through comparisons of above indexes. Meanwhile, patients might suffer from severe CO2 retention, but we spent short time to study so as not to collect specimens to perform relative biochemistry test. This study made full use of lung HRCT scores system and calculated score coefficient of emphysema and pulmonary fibrosis after revision, which was beneficial to reflect the CPFE condition and data statistics. Data showed that CPFE imaging scores declined 9.31 in group A, but increased 14.87 in control group after 12 months according to HRCT score system. Thus, results of image examination confirmed our treatment was effective.
Moreover, this study analyzed clinical characteristics of CPFE in acute outbreak periods. Results implied that ICS/LABA could effectively decreased acute attack times, and data from PO2, FEV1%, FVC% and DLCO% also proved that ICS/LABA could alleviate the severity of CPFE in acute attack periods.
We also analyzed adverse events and found that ICS/LABA increased adverse reactions risks, including gastroesophageal reflux, hypertension disease, coronary heart disease, gastrointestinal mucous membrane hemorrhage and oral fungus infection, but could not cause pneumonia, recurrence or infection of pulmonary tuberculosis. Firstly, drugs’ mechanisms might cause adverse events, and doses or administration routes also led to adverse reactions. This problem should be studied further.
Besides, this study also observed whether CPFE had independent clinical characteristics. We collected 12 months of data, including in lung function and lung HRCT scores between CPFE control group and IPF control group and found FEV1%, FVC% and DLCO% values were declined faster in CPFE group than those in IPF group during 12 months courses (P<0.05), which was consistent with previous references [20,21], moreover, the change of lung HRCT score was synchronous with the change of lung function, thus, we concluded that CPFE might be a pulmonary interstitial disease and independent of IPF
Our study has certain limitations. First, the study’s sample size was relatively small. However, we performed long-term follow-up to allow the evaluation of efficacy and safety of ICS/LABA in the specific population. Second, our study was nonrandomized clinical research and may have some selected bias.
In summary, our results indicated that ICS/LABA therapy could improve lung function condition in patients with CPFE and declined acute out-break frequency and severity of diseases during acute episode period.
Acknowledgements
We thank Weihua Liu for her contribution to data analysis.
Disclosure of conflict of interest
None.
References
- 1.Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch JP 3rd. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–430. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Egan JJ, Martinez FJ, Wells AU, Williams T. Lung function estimates in idiopathic pulmonary fibrosis: the potential for a simple classification. Thorax. 2005;60:270–273. doi: 10.1136/thx.2004.035436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay SE, Kazerooni EA, Toews GB, Lynch JP 3rd, Gross BH, Cascade PN, Spizarny DL, Flint A, Schork MA, Whyte RI, Popovich J, Hyzy R, Martinez FJ. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998;157:1063–1072. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 4.Rudd RM, Prescott RJ, Chalmers JC, Johnston ID. British Thoracic Society Study on cryptogenic fibrosing alveolitis: response to treatment and survival. Thorax. 2007;62:62–66. doi: 10.1136/thx.2005.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collard HR, King TE Jr, Bartelson BB, Vourlekis JS, Schwarz MI, Brown KK. Changes in clinical and physiologic variables predict survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:538–542. doi: 10.1164/rccm.200211-1311OC. [DOI] [PubMed] [Google Scholar]
- 6.Choi SH, Lee HY, Lee KS, Chung MP, Kwon OJ, Han J, Kim N, Seo JB. The value of CT for disease detection and prognosis determination in combined pulmonary fibrosis and emphysema(CPFE) PLoS One. 2014;9:e107476. doi: 10.1371/journal.pone.0107476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med. 1990;84:365–369. doi: 10.1016/s0954-6111(08)80070-4. [DOI] [PubMed] [Google Scholar]
- 8.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF Groupe d'Etude et de Recherche sur les Maladies Orphelines Pulmonaires (GERM OP) Combined pulmonary fibrosis and emphysema:a distinct underrecognized entity. Eur Repir J. 2005;26:586–593. doi: 10.1183/09031936.05.00021005. [DOI] [PubMed] [Google Scholar]
- 9.Mejia M, Carrillo G, Rojas-Serrano J, Estrada A, Suárez T, Alonso D, Barrientos E, Gaxiola M, Navarro C, Selman M. Idiopathic pulmonary fibrosis and emphysema decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136:10–15. doi: 10.1378/chest.08-2306. [DOI] [PubMed] [Google Scholar]
- 10.Roliani P, Mura M, Mattia P, Ferlosio A, Farinelli G, Mariotta S, Graziano P, Pezzuto G, Ricci A, Saltini C, Orlandi A. HRCT and histopathoiogical evaluation of fibrosis and tissue destruction in IPF associated with pulmonary emphysema. Respir Med. 2008;102:1753–61. doi: 10.1016/j.rmed.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.GOLD Executive Committee. Global strategy for the diagnosis, management, and prevention of cheonic obstructive pulmonanry disease (Rrvised2011) [EB/OL] [2012-01-16] [Google Scholar]
- 13.Takasugi JE, Godwin JD. Radiology of chronic obstruct ion pulmonary disease. Radio Clin Nor Am. 1998;36:29. doi: 10.1016/s0033-8389(05)70006-3. [DOI] [PubMed] [Google Scholar]
- 14.Antoniou KM, HanselI DM, Rubens MB, Marten K, Desai SR, Siafakas NM, Nicholson AG, du Bois RM, Wells AU. Idiopathic pulmonary fibrosis: outcome in relation to smoking status. Am J Respir Crit Care Med. 2008;177:190–194. doi: 10.1164/rccm.200612-1759OC. [DOI] [PubMed] [Google Scholar]
- 15.Barbea JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonanryarteries of smokers. Am J Respir Crit Care Med. 2001;164:709–713. doi: 10.1164/ajrccm.164.4.2101023. [DOI] [PubMed] [Google Scholar]
- 16.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, Rottoli P, Saltini C, Selman M, Strange C, Wood B. An offcial American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 17.Todd NW, Judy J, Lavanis S, Franks TJ, Galvin JR, Deepak J, Britt EJ, Atamas SP. Centrilobular emphysema combined with pulmonary fibrosis results in improved survival. Fibrogenesis Tissue Repair. 2011;4:6. doi: 10.1186/1755-1536-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, Sakamoto H, Iwasaki H. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005;10:449–455. doi: 10.1111/j.1440-1843.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 19.Heathcote KL, Cockcroft DW, Fladeland DA, Fenton ME. Normal expiratory flowrate and lung volumes in patients with combined emphysema andinterstitial lung disease: a case series and literature review. Can Respir J. 2011;18:e73–e76. doi: 10.1155/2011/354325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt SL, Nambiar AM, Tayob N, Sundaram B, Han MK, Gross BH, Kazerooni EA, Chughtai AR, Lagstein A, Myers JL, Murray S, Toews GB, Martinez FJ, Flaherty KR. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J. 2011;38:176–183. doi: 10.1183/09031936.00114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akagi T, Matsumoto T, Harada T, Tanaka M, Kuraki T, Fujita M, Watanabe K. Coexistent emphysema delays thedecrease of vital capacity in idiopathic pulmonary fibrosis. Respir Med. 2009;103:1209–1215. doi: 10.1016/j.rmed.2009.02.001. [DOI] [PubMed] [Google Scholar]


