Abstract
As drug abuse carries a societal stigma, patients do not often report their history of drug abuse to the healthcare providers. However, drug abuse is highly co-morbid with a host of other health problems such as psychiatric disorders and skin diseases, and majority of individuals with drug use disorders seek treatment in the first place for other problems. Therefore, it is very important for physicians to be aware of clinical signs and symptoms of drug use. Recently diagnostic value of dermatologic tissue alterations associated with drug abuse has become a very particular interest because skin changes were reported to be the earliest noticeable consequence of drug abuse prompting earlier intervention and treatment. Although hair is an annex of skin, alterations on hair structure due to drug use have not been demonstrated. This study represents the first report on ultra-structural hair alterations of drug abusers. We have investigated ultra-structure of the hair samples obtained from 6 cocaine, 6 heroin, 7 cannabis and 4 lysergic acid diethylamide (LSD) abusers by scanning electron microscope (SEM). SEM analysis of hair samples gave us drug-specific discriminating alterations. We suggest that results of this study will make a noteworthy contribution to cutaneous alterations associated with drug abuse which are regarded as the earliest clinical manifestations, and this SEM approach is a very specific and effective tool in the detection of abuse of respective drugs, leading early treatment.
Keywords: Cocaine, heroin, cannabis, lysergic acid diethylamide, LSD, drug abuse, hair, anatomy, scanning electron microscope
Introduction
Drug abuse is one of the most serious health problems worldwide with an estimated between 162 million and 324 million users of illicit drugs. Heroin, cocaine and other drugs kill around 0.2 million people each year. 1% of the causes of death were attributed to illicit drug use, worldwide. 10-13% of drug users are problem users with drug dependence and/or drug-use disorders. Health problems associated with drug abuse impair family life and productive employment, diminish the quality of life and may threaten survival. Illicit drug use also undermines the social fabric of the community. It weakens economic and social development and contributes to crimes, instability, insecurity and the spread of infectious diseases such as HIV, hepatitis B and C [1,2].
Although some drugs are known to be relatively safer than others [3,4], the use of every illicit drug is associated with legal consequences and social stigma. Social disqualification of drug abusers by society is very common due to society’s moral condemnations and criminaliz-ation of drug abuse [5-7]. Several studies indicated that drug use disorders are more highly stigmatized than other health conditions [6,8]. Furthermore, social stigma was reported to be a significant barrier for accessing health care and substance use treatment services [5,7-10].
Individuals using illicit drugs often choose to conceal their drug use problems to avoid negative impacts of social stigma. However, drug abuse is a serious public health issue, and to reduce and eliminate the psychological, physical and social consequences of drug abuse, it is very important to recognize the signs and symptoms of drug abuse early. Drug abuse is highly co-morbid with a host of other health problems such as psychiatric disorders and skin diseases, and majority of individuals with drug use disorders seek treatment in the first place for other problems. Healthcare providers play a crucial role in the identification of these problems and the accessibility to treatment. Therefore although patients may not report their history of drug use or abuse, it is very important for physicians to be aware of clinical signs and symptoms of drug use [11,12].
It is known that most of illicit drugs affect both skin and other organ systems. Recent studies indicated that diagnostic value of dermatologic tissue alterations associated with drug abuse has become a very particular interest because skin changes were reported to be the earliest noticeable consequence of drug abuse prompting earlier intervention and treatment of drug abuse [13-15]. Although hair is an annex of skin, to our knowledge, alterations on hair structure due to drug use have not been demonstrated before. Therefore, in this paper, we have investigated ultra-structure of the hair samples obtained from cocaine, heroin, cannabis and lysergic acid diethylamide (LSD) abusers by scanning electron microscope (SEM) and discussed diagnostic value of the results. Our study provides the first detailed morphological evaluation of hair samples from respective drug abusers.
Material and methods
Hair samples
Scalp hair specimens were obtained from 6 cocaine, 6 heroin, 7 cannabis and 4 LSD abusers. For reference, drug-free hair samples from 3 healthy volunteers were also used. All individuals had no chronic disease and were not under any chemotherapy. Drug tests for those individuals were already carried out and confirmed by Turkish Doping Control Center. Hair samples were taken from the posterior vertex. 12 hair specimens from each user were examined. Hair cut into 1 cm long segments and before SEM analysis routine light microscopic procedure was performed to the tissue specimen. All experimental protocols were approved by the Non-interventional Clinical Researches Ethics Board of Hacettepe University.
SEM analysis
The tissue samples were fixed in 2.5% gluter aldehyde for 24 hours, washed in phosphate buffer (pH: 7.4), post-fixed in 1% osmium tetroxide for one hour, washed in phosphate buffer (pH: 7.4), dehydrated in increasing concentrations of acetone, critical point dried and mounted on metal stubs with a double sided adhesive band. Electron microscopy analyses have been carried out on a Carl Zeiss EVO 50 EP scanning electron microscope (SEM) in Hacettepe University Department of Geological Engineering. Samples have been sputter coated with Au prior to analyses (BIO-RAD). Operating conditions are as follows: 15 kV accelerating voltage, 20-40 pA beam current and a working distance of 10 mm [16-18].
Results
This study represents the first detailed morphological investigation of the hair samples from cocaine, heroin, cannabis and LSD abusers by light microscope and SEM, and results were evaluated by comparing with the ultra-structure of drug-free healthy hair samples. It is well known that the hair shaft is gradually damaged by ageing. Once the hair shaft leaves the skin and grows longer, it undergoes some degree of degeneration depending on the extent of environmental and cosmetic damage. Weathering effects can be seen more often as the distance from the scalp increases [19]. In SEM analysis of healthy hair, distal segments from above the scalp slight amounts of hair damage were detected such as irregular, partly broken, detached cuticle cells. Split ends were also observed. These observations were in accordance with weathering aging of hair [19]. To eliminate these natural damages, we have investigated all hair samples segmented from scalp to 4-8 cm distance, according to hair sample length. The percentage of structural alterations for hair samples in each group are presented as descriptive statistics.
Ultra-structure of healthy hair
In SEM examination, all drug-free hair shafts (Figure 1) were observed to be intact, homogeneous and regular. Cortex, which is the major site of keratinization and ensures the shaft rigidity, was intact in all samples. Cuticle, which forms the hair surface, was also intact, homogeneous and cuticle cells were typically arranged in a similar fashion to roof tiles. No damaged area was detected on the hair shaft. Rarely squamous debris was observed on the cuticle (2.8%). Diameter of hair samples were measured between 48 and 112 μm.
Figure 1.
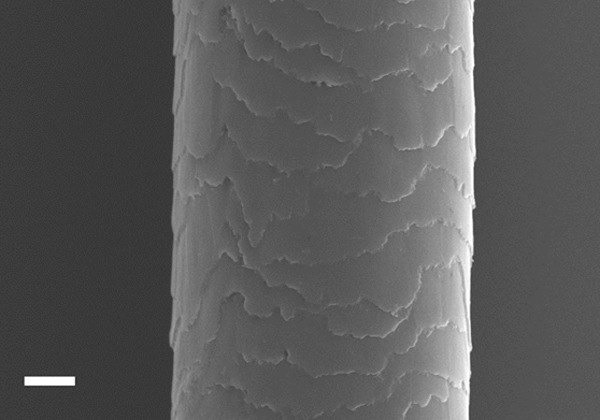
Scanning electron micrograph showing the ultra-structure of drug free healthy hair shaft. Scale: 10 μm.
Ultra-structural alterations of hair obtained from cocaine users
In the examination of the hair shafts of cocaine abusers, it was found that the keratinized structure was damaged in all individuals and 97.2% of their hair samples, and hair shafts were detected as ribbon-like and irregular (Figure 2A). Electron microscopic examination of hair also reveals that cuticle layer, which is the most outer layer of hair shaft, was almost disappeared in 95.8% of the samples (Figure 2A-F). The pathognomonic feature of the hair samples collected from all cocaine users was the extraordinary balloon-like enlargements on the hair shaft (88.9%), which were also observed under light microscope (Figure 2D-F). In addition to these findings, hair shafts from cocaine abusers were very thin, (8-)15-31 μm in diameter and fragile.
Figure 2.
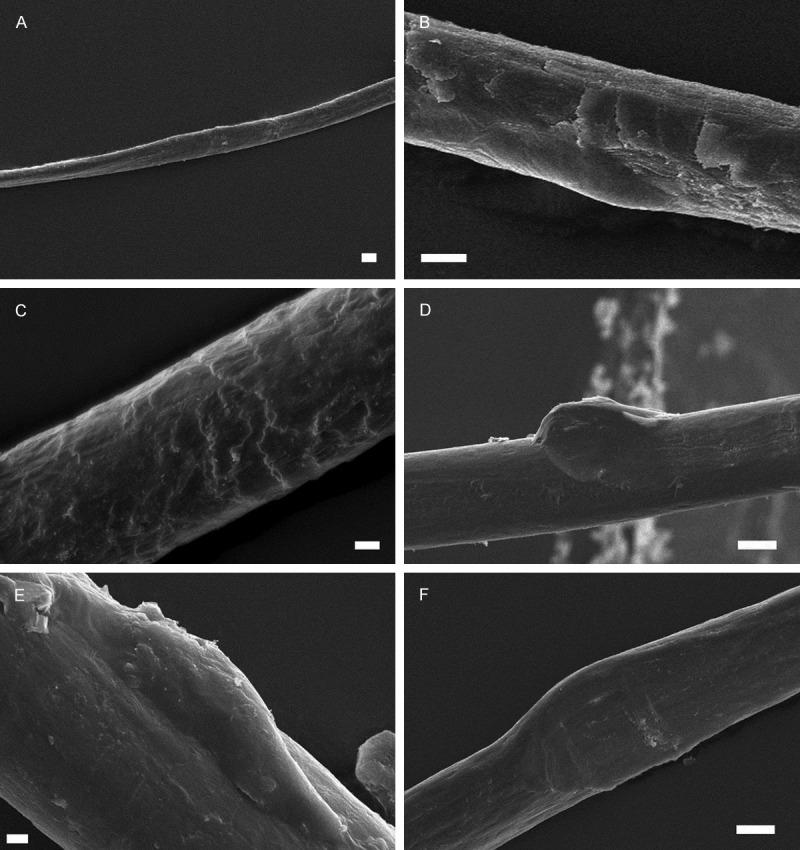
Scanning electron micrograph showing the ultra-structure of hair samples from cocaine abusers: A: Ribbon-like hair fiber. A-F: Cuticular damage and non-cuticular pattern. D-F: Balloon-like enlargement of hair shaft. Scale: 10 μm A, B, D and F, 2 μm C and E.
Ultra-structural alterations of hair obtained from heroin users
As shown in Figure 3, in the examination of the hair specimen obtained from heroin abusers, it was observed that the keratinized structure of the hair shafts was normal (Figure 3A). All hair shafts were detected intact and regular. Cuticle layer was also regular and cuticle cells were partially broken and detached in all samples. The squamous debris was frequently observed on the cuticle (95.8%) (Figure 3A-D). In addition to these findings, interestingly, in the SEM examinations of the hair shaft, numerous pores were observed over cuticular surface of hair samples of all heroin users with a frequency of 91.7% (Figure 3E and 3F). Hair shaft diameter was more than 50 μm.
Figure 3.
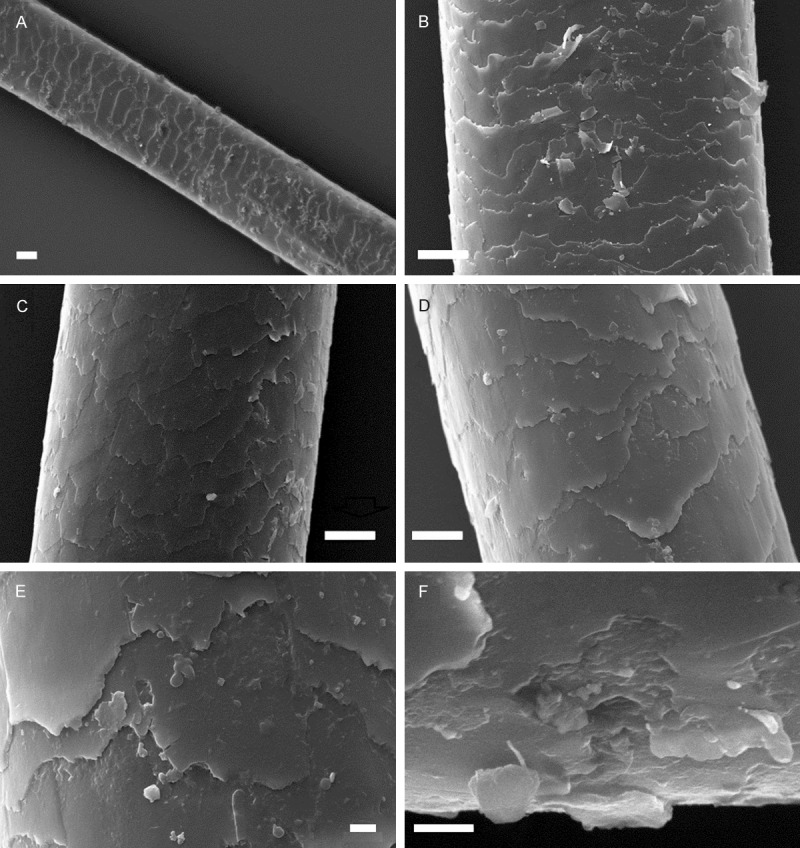
Scanning electron micrograph showing the ultra-structure of hair samples from heroin abusers. A-D: Intact and regular hair shaft and squamous debris. E and F: Pores over the cuticular surface of hair shaft. Scale: 20 μm A, 10 μm B-D, 2 μm E and F.
Ultra-structural alterations of hair obtained from cannabis users
SEM examination of hair specimen of cannabis abusers showed that cuticle layer was appeared as intact and regular. Cuticle scales were tightly associated in all hair samples (Figure 4A-D), however, cuticle surface were usually indistinct (83.3%) (Figure 4A and 4C). Rarely squamous debris was observed on the cuticle (4.8%). As shown in Figure 4C and 4D, hair shafts have local node-shaped enlarged areas which were also observed under light microscope as pathognomonic feature of 81% of the hair samples of all cannabis users. Hair shaft diameter was measured as more than 55 μm.
Figure 4.
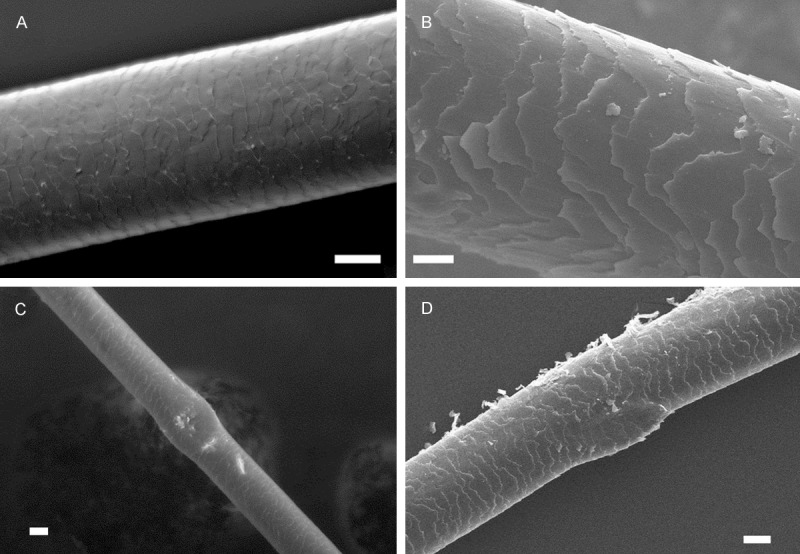
Scanning electron micrograph showing the ultra-structure of hair samples from cannabis abusers. A-D: Tightly associated, intact cuticle. A and C: Indistinct cuticular surface. C and D: Node-shaped enlarged area of hair shaft. Scale: 20 μm A and D, 10 μm B, 30 μm C.
Ultra-structural alterations of hair obtained from LSD users
As shown in Figure 5, in the examination of many hair specimen of LSD users, cuticle layer was usually destroyed (97.9%), cuticle cells were obviously lifted from the hair shaft in 97.9% of the hair samples. They were very fragile, broken and detached (95.8%) (Figure 5A). The squamous debris was observed on the cuticle (97.9%) (Figure 5A-D). In addition to these findings, during sample collection and preparation we have observed that hair fibers were generally very weak and fragile. Hair shaft diameter was measured as more than 17-47 (-75) μm.
Figure 5.
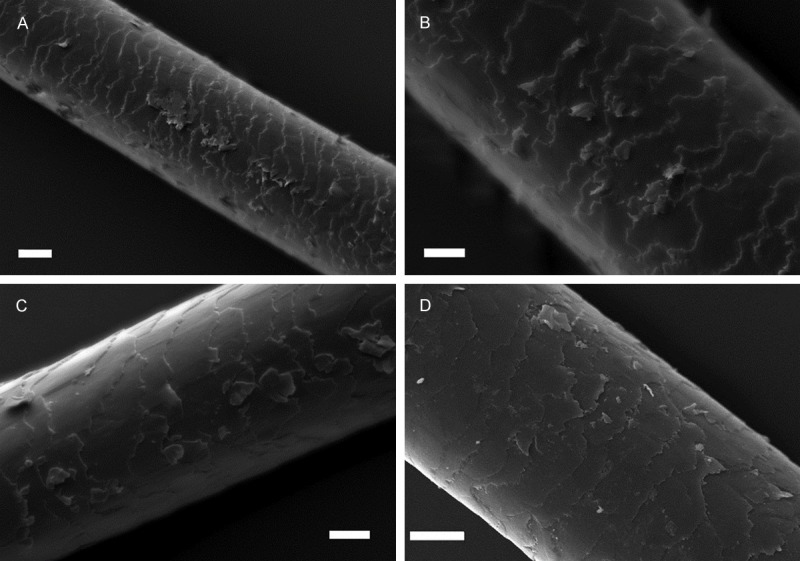
Scanning electron micrograph showing the ultra-structure of hair samples from LSD abusers. A: Destroyed area on the cuticle. A-D: Cuticle cells which were detached and lifted from the hair shaft. Scale: 10 μm A-D.
Discussion
Drug abuse is a major public health problem all over the world. The negative consequences of drug abuse affect not only individuals who abuse drugs but also their families and society as a whole. As society often accept drug abuse as a moral and criminal issue, rather than a health concern, patients abusing illicit drugs do not often disclose or admit having a drug use problem to avoid their exposition to public eye. However, early start of drug abuse treatment is a crucial factor in improving successful treatment and in reduction of psychological, physical and social consequences with beneficial effect on primary prevention in community [11,12]. Recent studies indicated that skin changes associated with drug abuse are regarded as the earliest clinical manifestation of this disorder and understanding these cutaneous changes allows for earlier intervention and treatment. On this account, major cutaneous signs of drug use for the most commonly abused drugs have already been reported [13-15].
Literature survey revealed that, although hair is an appendage of skin, alterations on hair structure due to drug use have not been demonstrated before. However, hair is a very important and powerful biological material for diagnosis many diseases, in cosmetology, toxicology, forensic science as well as drug analysis. It is known that, illicit drug sregardless of how it’s administered (orally, intra-venously, smoking, snorting, inhalation etc.) are accumulated into hair by several processes such as incorporation into the growing hair shaft from blood circulation, absorption from sweat, sebum and the external environment such as cigarette smoke [20-22]. Relationship with drugs and hair melanin, which is thought to be the most likely binding site for some drugs, is considered to be another possible mechanism of drug accumulation. Boumba and co-workers also reported that one of the single or multiple processes mentioned above can explain the drug incorporation into hair [23].
Microscopic examination of hair addresses to answer various clinical, toxicological and forensic issues. In our previous studies, we have shown that SEM examinations of hair samples obtained from patients with certain syndromes and disorders revealed pathognomonic morphological alterations in the hair [16-18]. As a part of our ongoing studies on structural alterations of hair, in this study, we have evaluated morphological changes in the hair obtained from drug abusers by a high resolution SEM. As shown in Table 1, SEM examinations revealed that the most common drugs like cocaine, heroin, cannabis and LSD usage yield into various alterations on hair structure. According to our results, damaged keratinized structure as well as ribbon-like and very thin (~8 μm) hair shaft was observed only in hair samples from cocaine abusers as distinctive characters. Moreover, while hair fibers were detected as regular in heroin and LSD users, balloon-like and node-shaped enlarged areas were observed in the hair fibers of cocaine and cannabis abusers, respectively. While cuticle layer was intact in heroin and cannabis abusers, it was destroyed in both cocaine and LSD abusers. Cuticle cells were significantly lifted from hair shaft only in LSD abusers; on the other hand, cuticle layer was almost disappeared only in cocaine abusers. In addition, most of hair samples of LSD abusers were thinner (~17 μm) than that of heroin and cannabis abusers. As a diagnostic tool, occurrence of pores on cuticle in heroin users was also significant. Consequently, as shown in Table 1, SEM examination of hair samples obtained from cocaine, heroin, cannabis and LSD abusers revealed drug specific ultra-structural alterations that may have a great value to differentiate abuse of respective drugs.
Table 1.
Comparison of significant structural alterations observed in hair samples of cocaine, heroin, cannabis and LSD abusers. Percentage of alterations in hair samples are provided (%)
| Structural alterations | Healthy (%) | Cocaine (%) | Heroin (%) | Cannabis (%) | LSD (%) |
|---|---|---|---|---|---|
| Cortex | Intact (-) | Damaged (97.2) | Intact (-) | Intact (-) | Intact (-) |
| Fibre enlargements | - | Balloon-like (88.9) | - | Node-shaped (81) | - |
| Cuticle layer | Intact (-) | Disappeared (95.8) | Intact (-) | Intact (-) | Damaged (97.9) |
| Cuticle cells | Distinct (-) | - | Distinct (-) | Indistinct (83.3) | Distinct (-) |
| - | - | - | - | Lifted from hair shaft (97.9) | |
| - | - | Partially broken (100) | Intact (-) | Broken (95.8) | |
| - | - | Partially detached (100) | Associated (-) | Detached (95.8) | |
| Squamous debris on cuticle | ~ Absent (2.8) | Absent (-) | Present (95.8) | ~ Absent (4.8) | Present (97.9) |
| Pores on cuticle | Absent (-) | Absent (-) | Present (91.7) | Absent (-) | Absent (-) |
| Fibre fragility | Normal | Fragile | Normal | Normal | Fragile |
| Fibre diameter (μm) | 48-112 | (8-) 15-31 | >50 | >55 | 17-47 (-75) |
According to literature survey, only few microscopic reports related with hair structure of drug abusers have been published. In authentic drug positive hair samples the opiate content had declined after they had been stored in an aqueous environment for some weeks. In this study, SEM and transmission electron microscopy (TEM) analysis of hair samples also showed long term water caused alterations on hair fiber in cortex [24]. Kimura and co-workers reported a laser microscopic method in order to show methamphetamine distribution in hair [25]. In another study, Stout and co-workers discussed the effects of various decontamination methods, which carry out before chemical analysis of hair sample in drug screening, on the surface structure of drug-free hair specimen to design of an effective decontamination strategy [26].
To our knowledge, this is the first study reporting the ultra-structural hair alterations of drug abusers. As hair is an appendage of skin, drug specific discriminating alterations on the hair of cocaine, heroin, cannabis and LSD abusers detected in this study will make a noteworthy contribution to cutaneous alterations associated with drug abuse, which are regarded as the earliest clinical manifestations prompting earlier intervention and treatment of drug use [13-15], and this SEM approach is a very spesific and effective tool in the detection of abuse of respective drugs, leading early treatment.
SEM, which is three dimensional examination technique revealing easily comparable images, is indispensable for diagnosis in various tissues. As it is routinely used in various diseases ongoing with dermatological manifestations such as Chediak-Higashi syndrome [16], dyskeratosis congenita [17] and hereditary trichodysplasia [18] its usage in hair analysis of possible cocaine, heroin, cannabis and LSD abusers may result in valuable contributions to scientific literature.
On the other hand, when considering social stigma, analysis of hair follicles has some advantages. Hair sample collection is non-invasive, less embarrassing, can easily be performed under conditions that prevent adulteration and substitution, and stored for years in properly conditions. Segmental analysis of hair also provides the detection of historical use of drug [23,27,28].
In conclusion, this study demonstrated the ultra-structural changes of hair obtained from cocaine, heroin, cannabis and LSD abusers which will make a noteworthy contribution to cutaneous signs of drug abuse. As a future extension of this study a more comprehensive assessment of SEM analysis of hair of other illicit drug abusers may provide even greater information.
Acknowledgements
This work supported by Hacettepe University Scientific Research Projects Coordination Unit (Project No: 04A101008). The authors would like to thank Aytekin Temizer, Pharm. PhD, who was professor in Department of Analytical Chemistry, Faculty of Pharmacy, Hacettepe University and former head of Turkish Doping Control Center, which is a Hacettepe University unit, for providing hair samples and H. Evren Çubukçu, PhD, who is assistant professor in Department of Geological Engineering, Faculty of Engineering, Hacettepe University, for his great help during SEM studies.
Disclosure of conflict of interest
None.
References
- 1.World Drug Report 2013. United Nations Publication; United Nations Office on Drugs and Crime. Sales No. E.13.XI.6. [Google Scholar]
- 2.World Drug Report 2014. United Nations Publication; United Nations Office on Drugs and Crime. Sales No. E.14.XI.7. [Google Scholar]
- 3.Gable RS. Comparison of acute lethal toxicity of commonly abused psychoactive substances. Addiction. 2004;99:686–696. doi: 10.1111/j.1360-0443.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- 4.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369:1047–1053. doi: 10.1016/S0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 5.Ahern J, Stuber J, Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88:188–196. doi: 10.1016/j.drugalcdep.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Room R. Stigma, social inequality and alcohol and drug use. Drug Alcohol Rev. 2005;24:143–155. doi: 10.1080/09595230500102434. [DOI] [PubMed] [Google Scholar]
- 7.Radcliffe P, Stevens A. Are drug treatment services only for ‘thieving junkie scumbags’? Drug users and the management of stigmatised identities. Soc Sci Med. 2008;67:1065–1073. doi: 10.1016/j.socscimed.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Ronzani TM, Higgins-Biddle J, Furtado EF. Stigmatization of alcohol and other drug users by primary care providers in Southeast Brazil. Soc Sci Med. 2009;69:1080–1084. doi: 10.1016/j.socscimed.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Digiusto E, Treloar C. Equity of access to treatment, and barriers to treatment for illicit drug use in Australia. Addiction. 2007;102:958–969. doi: 10.1111/j.1360-0443.2007.01842.x. [DOI] [PubMed] [Google Scholar]
- 10.Luoma JB, Twohig MP, Waltz T, Hayes SC, Roget N, Padilla M, Fisher G. An investigation of stigma in individuals receiving treatment for substance abuse. Addict Behav. 2007;32:1331–1346. doi: 10.1016/j.addbeh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Johnson MD, Heriza TJ, St Dennis C. How to spot illicit drug abuse in your patients. Postgrad Med. 1999;106:199–218. doi: 10.3810/pgm.1999.10.1.721. [DOI] [PubMed] [Google Scholar]
- 12.Muhrer JC. Detecting and Dealing with Substance Abuse Disorders in Primary Care. The Journal for Nurse Practitioners. 2010;6:597–605. [Google Scholar]
- 13.Brewer JD, Meves A, Bostwick JM, Hamacher KL, Pittelkow MR. Cocaine abuse: dermatologic manifestations and therapeutic approaches. J Am Acad Dermatol. 2008;59:483–487. doi: 10.1016/j.jaad.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 14.Fink B, Landthaler M, Hafner C. Skin alterations due to abuse of illegal drugs. Journal Der Deutschen Dermatologischen Gesellschaft. 2011;9:633–638. doi: 10.1111/j.1610-0387.2011.07699.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu SW, Lien MH, Fenske NA. The effects of alcohol and drug abuse on the skin. Clin Dermatol. 2010;28:391–399. doi: 10.1016/j.clindermatol.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Çelik HH, Töre HG, Balcı S, Bağçiçek G, Özdemir B. Scanning electron microscopic examination of the hair in Chédiak-Higashi syndrome. Case Rep Clin Pract Rev. 2003;4:252–255. [Google Scholar]
- 17.Celik HH, Erbil H, Tatar I, Ozdemir MB. Light and scanning electron microscopic investigation of the changes in hair with Dyskeratosiscongenita. Saudi Med J. 2007;28:296–297. [PubMed] [Google Scholar]
- 18.Özdemir B, Akdoğan I, Ergin Ş, Tatar İ, Çelik H, Mete E, Ergin Ç. Scanning electron microscopic examination of hair in tineacapitis caused by Trichophytontonsurans. Anatomy. 2010;4:35–38. [Google Scholar]
- 19.Trueb RM. Pharmacologic interventions in aging hair. Clin Interv Aging. 2006;1:121–129. doi: 10.2147/ciia.2006.1.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson GL. Mechanisms of drug incorporation into hair. Forensic Sci Int. 1993;63:19–29. doi: 10.1016/0379-0738(93)90256-a. [DOI] [PubMed] [Google Scholar]
- 21.Thorspecken J, Skopp G, Potsch L. In vitro contamination of hair by marijuana smoke. Clin Chem. 2004;50:596–602. doi: 10.1373/clinchem.2003.026120. [DOI] [PubMed] [Google Scholar]
- 22.Tsanaclis L, Nutt J, Bagley K, Bevan S, Wicks J. Differentiation between consumption and external contamination when testing for cocaine and cannabis in hair samples. Drug Test Anal. 2014;6(Suppl 1):37–41. doi: 10.1002/dta.1623. [DOI] [PubMed] [Google Scholar]
- 23.Boumba VA, Ziavrou KS, Vougiouklakis T. Hair as a biological indicator of drug use, drug abuse or chronic exposure to environmental toxicants. Int J Toxicol. 2006;25:143–163. doi: 10.1080/10915810600683028. [DOI] [PubMed] [Google Scholar]
- 24.Potsch L, Skopp G, Becker J. Ultrastructural alterations and environmental exposure influence the opiate concentrations in hair of drug addicts. Int J Legal Med. 1995;107:301–305. doi: 10.1007/BF01246877. [DOI] [PubMed] [Google Scholar]
- 25.Kimura H, Mukaida M, Mori A. Detection of stimulants in hair by laser microscopy. J Anal Toxicol. 1999;23:577–580. doi: 10.1093/jat/23.7.577. [DOI] [PubMed] [Google Scholar]
- 26.Stout PR, Ropero-Miller JD, Baylor MR, Mitchell JM. Morphological changes in human head hair subjected to various drug testing decontamination strategies. Forensic Sci Int. 2007;172:164–170. doi: 10.1016/j.forsciint.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Xiang P, Shen M. Determination of clozapine in hair and nail: the role of keratinous biological materials in the identification of a bloated cadaver case. J Forensic Leg Med. 2014;22:62–67. doi: 10.1016/j.jflm.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Namera A, Konuma K, Saito T, Ota S, Oikawa H, Miyazaki S, Urabe S, Shiraishi H, Nagao M. Simple segmental hair analysis for alpha-pyrrolidinophenone-type designer drugs by MonoSpin extraction for evaluation of abuse history. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;942-943:15–20. doi: 10.1016/j.jchromb.2013.10.021. [DOI] [PubMed] [Google Scholar]


