Abstract
Objectives: Diabetic dialysis patients have higher risk of cardiovascular disease (CVD) than general population. While statin treatment is effective in prevention of CVD and all-cause mortality in general population, the use of statin in diabetic dialysis patients remains controversial. Thus, we aimed to assess the effects of statin treatment on prevention of CVD and all-cause mortality in diabetic dialysis patients by meta-analysis. Materials and methods: Pubmed, Embase and Cochrane Library were searched between each database’s inception and July, 2014. Hazard ratio (HR) with 95% confidence interval (CI) for CVD and all-cause mortality was extracted from each study. The pooled analysis was performed using random-effects models by Stata 12.0. Results: Our search yielded five eligible articles including two RCTs and three observational studies. By pooled estimate, statin treatment was associated with a decreased risk of the cardiac endpoint which included cardiac death and nonfatal MI (HR=0.84, 95% CI: 0.78-0.90) and all cardiac events combined (HR=0.89, 95% CI: 0.82-0.96). There was no difference in the overall incidence of fatal or nonfatal stroke (HR=1.24, 95% CI: 0.99-1.53) and all cerebrovascular events combined (HR=1.14, 95% CI: 0.98-1.33) between statin treatment and control group. Finally, statin treatment was associated with a decreased risk of all-cause mortality (HR=0.81, 95% CI: 0.71-0.92). Conclusions: Statin treatment may be beneficial for reducing the risk of cardiac events and all-cause mortality while have no effect on overall cerebrovascular events in diabetic dialysis patients. More RCTs were needed to validate the results.
Keywords: Statin, diabetes, dialysis, meta-analysis
Introduction
Both diabetes and chronic kidney disease (CKD) especially CKD undergoing dialysis have been recognized as independent risk factors for CVD [1]. Therefore, diabetic dialysis patients may have higher risk of CVD than general population. While statin treatment is effective in prevention of CVD and all-cause mortality in general population, the use of statin in diabetic dialysis patients remains controversial [1,2]. Both the original results from two randomized controlled trials (RCTs), namely Die Deutsche Diabetes Dialyse Studie (4D) and A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis (AURORA), failed to show significant effect of statins on prevention of primary cardiovascular outcome, while the 4D study included only diabetic dialysis patients and the AURORA study included dialysis patients with and without diabetes [3,4]. Therefore, Kidney Disease Outcomes Quality Initiative (KDOQI) 2012 guideline has not recommended initiating statin therapy in diabetic dialysis patients [5]. However, subsequent post hoc analyses of the above two RCTs showed that statin treatment may be beneficial for reducing the risk of CVD such as cardiac death or nonfatal myocardial infarction (MI) in diabetic dialysis patients [6-8]. Besides, there are also some observational studies which showed favorable results for statin treatment on CVD and all-cause mortality in diabetic dialysis patients [9,10]. While it is proposed that meta-analyses of interventions should include observational studies in addition to RCTs, more and more meta-analyses which combined RCTs with observational studies have emerged recently [11-13]. Thus, we aimed to evaluate the effects of statin treatment on prevention of CVD and all-cause mortality in diabetic dialysis patients by meta-analysis of RCTs and observational studies published to date.
Materials and methods
Data sources and searches
We searched Pubmed, Embase and Cochrane Library for studies published between each database’s inception and July, 2014. We also searched reference lists of included studies. There were no language restrictions. Two authors (M. Y. and X. S. X.) carried out the search independently. Search terms and strategies for Pubmed were as follows: (diabetes OR diabetic OR “Diabetes Mellitus” [Mesh]) AND (dialysis OR *dialysis OR “Dialysis” [Mesh]) AND (statin OR *statin OR “Hydroxymethylglutaryl-CoA Reductase Inhibitors” [Mesh]). The complete search strategies for Pubmed, Embase and Cochrane Library were documented in Appendix S1.
Study selection
Studies were included in the meta-analysis if they met the following criteria: (1) observational study or randomized controlled trial; (2) patients had diabetes mellitus and underwent hemodialysis or peritoneal dialysis; (3) patients were treated with statins; (4) the outcome of interest was cardiovascular events or cardiovascular mortality or all-cause mortality; (5) there was quantitative data (i.e., events rates, hazard ratio [HR] ). If data were duplicated in more than 1 study, data from the most inclusive report were used. Reviews, commentary articles, and editorials were excluded. Two authors (M. Y. and X. S. X.) independently screened the titles and abstracts of all electronic citations, and full-text articles were retrieved for a comprehensive review and independently re-screened. Discrepancies were resolved by discussion.
Data extraction and quality assessment
We obtained published reports for each trial and extracted standard information to a spreadsheet. Two authors (M. Y. and X. S. X.) carried out data extraction and quality assessment independently. The data we sought included the following study characteristics: study design, country of origin, year of publication, type of dialysis, use of statin, sample size, duration of follow-up. Data on the aforementioned study endpoints were also extracted, including HR and 95% confidence interval (CI). Referring to the report by Deschodt et al, assessment of the methodological quality of the included studies was based on the Methodological Index for Non-Randomized Studies, which consists of 12 criteria, while the criterion ‘randomization’ was added for RCTs [14]. The total quality score ranged from 0 (low quality) to 26 (high quality). Discrepancies were also resolved by discussion.
Statistics
HR extracted from included studies was used in the pooled meta-analysis calculations. The overall pooled-effect estimates were calculated using DerSimonian & Laird random effect models. All pooled estimates were displayed with a 95% CI. Existence of heterogeneity among study effect sizes was examined using the I2 index and the Q-test P value [15]. Heterogeneity was considered as either I2>50% or P<0.05. Begg’s funnel plot and Egger’s test were used to test the possible publication bias. Sensitivity analyses were performed to assess the influence of each study on the summary effect. All tests were 2-sided. A 2-sided P value less than 0.05 was considered statistically significant. All analyses were conducted using Stata 12.0 software (StataCorp, College Station, Texas).
Results
Eligible studies and methodological quality
Based on the search strategy, study selection flow diagram was shown in Figure 1. Finally, five studies were selected. Among them were two RCTs and three observational studies. The two RCTs, namely the 4D study and post hoc analysis of the AURORA study (abbreviated by AURORA study in the following), which included 1986 diabetic dialysis patients, while the three observational studies included 11095 diabetic dialysis patient [3,8-10,16]. One of the observational studies (abbreviated by Chan 2010 study in the following), which based on mathematical modeling and had the same eligibility criteria as the 4D study, included 10288 diabetic dialysis patient [9]. Four studies enrolled diabetic hemodialysis patients while one study enrolled diabetic peritoneal dialysis patients. The study details were shown in Table 1. Results of the assessment of the methodological quality of the included studies were shown in Table 2. The total quality scores of the included studies ranged from 18 ‘moderate’ to 26 ‘excellent’ [3,8-10,16].
Figure 1.
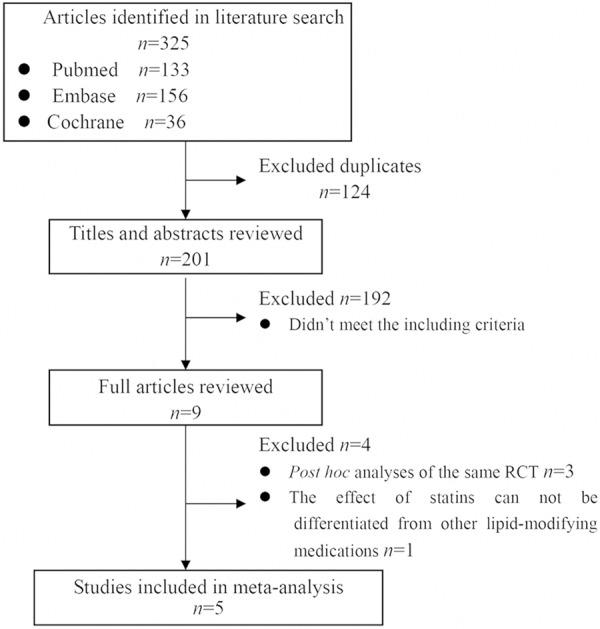
Flow diagram for identification of relevant studies.
Table 1.
Characteristics of the studies included in the meta-analysis*
| Study | Design | Country | Year of publication | Type of dialysis | Use of statin | Number of diabetic patients (statin/control) | Mean follow-up (year) |
|---|---|---|---|---|---|---|---|
| 4D [3] | RCT | Germany | 2005 | HD | Atorvastatin 20 mg per day | 1255 (619/636) | 4 |
| AURORA [8] | RCT | Norway | 2011 | HD | Rosuvastatin 10 mg per day | 731 (388/343) | 2.8 |
| Chan 2010 [9] | OS | USA | 2010 | HD | Average equivalent atorvastatin 33 mg per day | 10288 (5144/5144) | 2 |
| Lee 2011 [10] | OS | Korea | 2011 | PD | Statins (NA for the type of statins) | 362 (181/181) | 2.7 |
| Gotz 2005 [16] | OS | Germany | 2005 | HD | Statins (NA for the type of statins) | 445 (122/323) | 4.3 |
RCT, randomized controlled trial;
OS, observational study; HD, hemodialysis; PD, peritoneal dialysis; NA, not available.
Table 2.
Methodological quality assessment of the included studies
| Study | Clearly stated aim | Inclusion of consecutive patients | Prospective collection of data | Endpoints appropriate to aim of study +ITT | Unbiased assessment of study endpoint(s) | Follow-up period appropriate to aim of study | Loss to follow-up <5% | Prospective calculation of study size | Adequate control group | Contemporary groups | Baseline equivalence of groups | Adequate statistical analyses | Randomization* | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4D [3] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 26 |
| AURORA [8] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 26 |
| Chan 2010 [9] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 24 |
| Lee 2011 [10] | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 2 | 2 | 2 | 2 | 0 | 20 |
| Gotz 2005 [16] | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 0 | 1 | 2 | 1 | 2 | 0 | 18 |
0= not reported; 1= reported but inadequate; 2= reported and adequate;
0 for nonrandomized studies and 2 for randomized studies;
ITT: intention-to-treat.
Meta-analysis of the cardiac events
While AURORA study provided the pooled HR for cardiac endpoint which included cardiac death and nonfatal MI, the 4D study and the Chan 2010 study reported HR for cardiac death or nonfatal MI, respectively [3,8,9]. By pooled estimate, statin treatment was associated with a decreased risk of the cardiac endpoint (HR=0.84, 95% CI: 0.78-0.90; P<0.0001; P heterogeneity =0.455; I2=0.0%; Figure 2). Meta-analysis of only the two RCTs also showed beneficial effect of statin treatment on the cardiac endpoint (HR=0.78, 95% CI: 0.67-0.92; P=0.002; P heterogeneity =0.463; I2=0.0%) (data not shown). By pooled analysis of the 4D study and the Chan 2010 study, statin treatment was associated with a decreased risk of cardiac death (HR=0.83, 95% CI: 0.75-0.91; P<0.0001; P heterogeneity =0.854; I2=0.0%) but statistically insignificantly associated with the risk of nonfatal MI (HR=0.91, 95% CI: 0.79-1.05; P=0.199; P heterogeneity=0.806; I2=0.0%; Figure S1). Only the 4D study and the Chan 2010 study provided the HR for all cardiac events combined which included cardiac endpoint, percutaneous transluminal coronary angioplasty (PTCA), coronary-artery bypass grafting (CABG) as well as other interventions to treat coronary heart disease. Concordant with the results from individual studies, pooled analysis also showed that statin treatment was significantly associated with improved all cardiac events combined (HR=0.89, 95% CI: 0.82-0.96; P=0.002; P heterogeneity=0.374; I2=0.0%; Figure S2).
Figure 2.
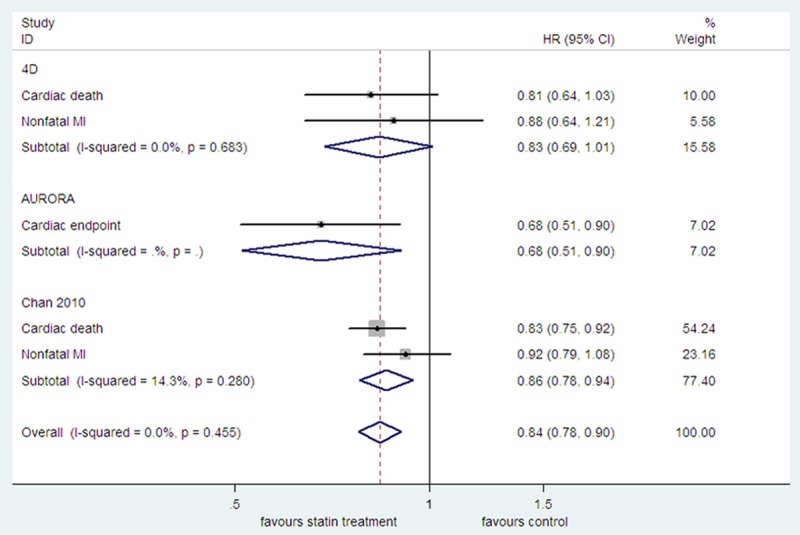
The effect of statin treatment on cardiac endpoint in diabetic dialysis patients.
Meta-analysis of the cerebrovascular events
While AURORA study provided the pooled HR for fatal or nonfatal stroke, the 4D study and the Chan 2010 study reported HR for fatal or nonfatal stroke, respectively [3,8,9]. By pooled estimate, there was no difference in the overall incidence of fatal or nonfatal stroke between statin treatment and control group (HR=1.24, 95% CI: 0.99-1.53; P=0.057; P heterogeneity =0.200; I2=33.2%; Figure 3). Meta-analysis of only the two RCTs also showed the similar result (data not shown). By pooled analysis of the 4D study and the Chan 2010 study, the incidence of fatal stroke in statin treatment group was not significantly different from that in control group (HR=1.31, 95% CI: 0.61-2.79; P=0.488; P heterogeneity =0.044; I2=75.4%), while there was an increased incidence of nonfatal stroke in statin treatment group (HR=1.22, 95% CI: 1.02-1.46; P=0.029; P heterogeneity =0.490; I2=0.0%; Figure S3). Only the 4D study and the Chan 2010 study provided the HR for all cerebrovascular events combined which included ischemic or hemorrhagic stroke, transient ischemic attack (TIA) as well as prolonged reversible ischemic neurologic deficit (PRIND). Consistent with the results from individual studies, pooled analysis also showed that statin treatment had no protective effect on all cerebrovascular events combined (HR=1.14, 95% CI: 0.98-1.33; P=0.079; P heterogeneity =0.887; I2=0.0%; Figure S4).
Figure 3.
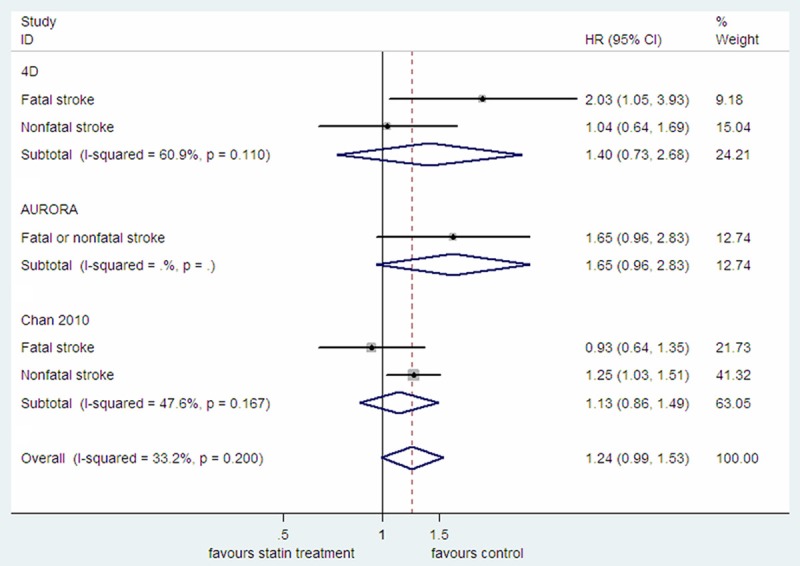
The effect of statin treatment on fatal or nonfatal stroke in diabetic dialysis patients.
Meta-analysis of the all-cause mortality
All the five studies provided the HR for all-cause mortality [3,8-10,16]. By pooled estimate, statin treatment was associated with a decreased risk of all-cause mortality (HR=0.81, 95% CI: 0.71-0.92; P=0.002; P heterogeneity =0.065; I2=54.7%; Figure 4), while meta-analysis of only the two RCTs showed a statistically insignificant protective effect (HR=0.90, 95% CI: 0.80-1.02; P=0.092; P heterogeneity =0.534; I2=0.0%; Figure S5). After excluded the study which focused on diabetic peritoneal dialysis patients, pooled estimate of the four studies which focused on diabetic hemodialysis patients, also showed a beneficial effect for statin treatment (HR=0.84, 95% CI: 0.77-0.92; P<0.0001; P heterogeneity =0.274; I2=22.8%; Figure S6).
Figure 4.
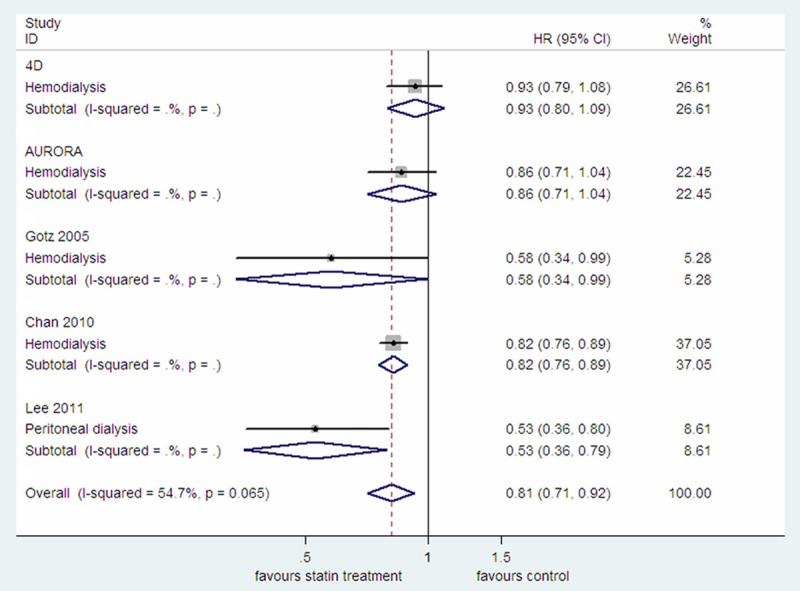
The effect of statin treatment on all-cause mortality in diabetic dialysis patients.
Publication bias and sensitivity analyses
We performed Begg’s funnel plot and Egger’s test to evaluate the possible publication bias. As a result, there was no publication bias in each test for cardiac events, cerebrovascular events and all-cause mortality analysis (data not shown).
The sensitivity analysis of the risk of cardiac endpoint, fatal or nonfatal stroke as well as all-cause mortality, after exclusion of any single study at a time, yielded effect sizes similar in magnitude and direction to the overall estimates. However, the sensitivity analysis of all-cause mortality limited to the four studies which only enrolled diabetic hemodialysis patients, while excluding the Chan 2010 study, yielded effect size that was similar in direction (HR=0.87, 95% CI: 0.74-1.01; P=0.075; P heterogeneity =0.238; I2=30.4%) (data not shown), but different in magnitude and statistical significance.
Discussion
This meta-analysis aimed to assess the effects of statin treatment on cardiovascular events and all-cause mortality in diabetic dialysis patients. The overall findings suggested that statin treatment may be beneficial for reducing the risk of cardiac events and all-cause mortality, while not helpful for improving the risk of cerebrovascular events.
Although there are some differences between RCTs and observational studies, mainly due to confounding or biases that exists in the latter, both types of studies have strengths and weaknesses [17]. Meta-analysis of only RCTs showed lower power and more conservative results than that including observational studies [12]. Integration of results from both types of studies may improve the inference based on only RCTs and enable the most comprehensive summary of the evidence available to date [13,17]. Therefore, we combined the results both from RCTs and observational studies in this meta-analysis.
Cardiovascular disease (CVD) is the leading cause of death in diabetic patients. Results from two meta-analyses suggested that, in non-dialysis diabetic patients, statin treatment may be helpful for primary and secondary prevention of CVD, regardless of the baseline CVD risk or baseline low density lipoprotein cholesterol (LDL-C) [18,19]. Therefore, statin treatment is recommended by the American Diabetes Association for non-dialysis diabetic patients aged ≥40 years, even if they have no overt CVD [20]. Guidelines from the American College of Cardiology and the American Heart Association also have the similar contents [21]. However, the role of statin treatment in diabetic dialysis patients remains controversial. Both the original results from 4D and AURORA study failed to demonstrate the beneficial effects of statin treatment on prevention of CVD [3,4]. But subsequent post hoc analysis of the 4D study suggested that statin treatment may reduce the risk of fatal and nonfatal cardiac events if pretreatment LDL-C was >3.76 mmol/L [6]. post hoc analysis of the AURORA study also showed a protective effect of statin treatment on the cardiac endpoint which included cardiac death and nonfatal MI in diabetic dialysis patients [8]. In the present meta-analysis, pooled analysis of the included studies also revealed that statin treatment was associated with a decreased risk of the cardiac endpoint and all cardiac events combined. Moreover, further analysis suggested that the effect of statin treatment on cardiac endpoint may mainly attribute to the influence of statin treatment on cardiac death but not nonfatal MI. Consequently, statin treatment was also associated with a decreased risk of all cardiac events combined in diabetic dialysis patients. While statin treatment has little or no beneficial effects on CVD in general dialysis patients by meta-analysis [22], the association of statin treatment with a decreased risk of the cardiac endpoint and all cardiac events combined in the present meta-analysis may be due to the higher risk of CVD in diabetic dialysis patients than that in general dialysis patients.
Both diabetes and CKD are risk factors for stroke. In hemodialysis patients, stroke rate peaks at 10-35/1,000 patient years [23]. While statin treatment decreased the risk of stroke in non-dialysis diabetic patients by meta-analysis, both meta-analyses in CKD patients not requiring dialysis and general dialysis patients showed that statin treatment had uncertain effect on stroke [18,22,24]. In the present meta-analysis, statin treatment had no statistically significant effect on the overall incidence of fatal or nonfatal stroke as well as all cerebrovascular events combined. However, further analysis suggested that statin treatment increased the risk of nonfatal stroke. Indeed, the relationship between statin treatment and stroke included intracerebral hemorrhage (ICH) remains controversial [25]. Studies have shown both benefit and detriment. It suggested that whether or not increase the stroke risk with statin use, which may be associated with the underlying etiologies while certain pathologies such as amyloid angiopathy may increase the stroke risk with statin use [25]. In the present meta-analysis, increased risk of nonfatal stroke was also identified with statin treatment in diabetic dialysis patients. However, there were only one RCT and one observational study in our meta-analysis. Therefore, the evidence should be not sufficient to draw firm conclusion. Whether statin treatment increase stroke risk in diabetic dialysis patients, which need to be examined in more RCTs in the future.
All-cause mortality was also a study outcome in the present meta-analysis. It is reported that there was a 9% proportional reduction in all-cause mortality per mmol/L reduction in LDL-C in non-dialysis diabetic patients by meta-analysis [18]. There was also the similar result in CKD patients not requiring dialysis [24]. However, no beneficial effect of statin treatment on all-cause mortality had been identified in general dialysis patients by meta-analysis [22]. In the present meta-analysis, pooled analysis of all the five selected studies or only the four studies which enrolled diabetic hemodialysis patients, consistently showed that statin treatment was associated with a decreased risk of all-cause mortality. However, pooled estimate of only the two RCTs or sensitivity analysis limited to the studies which only enrolled diabetic hemodialysis patient s, while excluding the Chan 2010 study, revealed a statistically insignificant protective effect. Discrepancy in these results may be associated with the sample size included in the analysis. It is reported that the 4D study had insufficient statistical power to detect a small beneficial effect while the statistical power was further diminished by non-study statin treatment among 15% of patients in the placebo arm [26]. On the other hand, the Chan 2010 study was a large-scale study which included a total of 10,288 patients [9]. While the Chan 2010 study was an observational study and got a strong influence on the meta-analysis results due to the size of the study, we should regard the effect of statin treatment on all cause mortality in diabetic dialysis patients with caution when the Chan 2010 study included in the pooled analysis.
There were some limitations to our meta-analysis. First, as mentioned above, only two RCTs were included in this analysis while RCTs were considered the gold standard for estimating effects. The inclusion of a large proportion (approximately 85%) of patients from observational studies should weaken the strength of the results from our meta-analysis. Second, due to efficacy variance in different type of statins, not the same statin was used in the studies which may influence the overall effect estimate. Third, there were no RCTs focused on statin treatment in diabetic peritoneal dialysis patients and only one observation study which enrolled these patients was included in the present analysis. Therefore, there is not enough evidence to assess the effect of statin treatment on diabetic peritoneal dialysis patients.
In conclusion, the results of the present meta-analysis indicated that statin treatment may be beneficial for reducing the risk of cardiac events and all-cause mortality while have no effect on overall cerebrovascular events in diabetic dialysis patients. However, due to strong influence from observational studies in our meta-analysis, more RCTs were needed to validate the results.
Appendix S1. The complete search strategy for Pubmed, Embase and Cochrane Library
For Pubmed database:
(((((((diabetes [Title/Abstract]) OR diabetic[Title/Abstract])) OR “Diabetes Mellitus” [Mesh])) AND (((((dialysis [Title/Abstract]) OR *dialysis [Title/Abstract]) OR hemodialysis [Title/Abstract])) OR (“Renal Dialysis” [Mesh] OR “Dialysis” [Mesh] OR “Peritoneal Dialysis, Continuous Ambulatory”[Mesh] OR “Peritoneal Dialysis” [Mesh] OR “Hemodialysis, Home”[Mesh] OR “Hemodialysis Units, Hospital” [Mesh]))) AND ((((statin [Title/Abstract]) OR *statin [Title/Abstract])) OR (“Hydroxymethylglutaryl-CoA Reductase Inhibitors” [Mesh] OR “Hydroxymethylglutaryl-CoA Reductase Inhibitors” [Pharmacological Action]))) AND ((((randomized [Title/Abstract]) OR random* [Title/Abstract])) OR (“Randomized Controlled Trial” [Publication Type] OR “Randomized Controlled Trials as Topic” [Mesh]))
For Embase database:
No. Query Results
#1. ‘diabetes’:ab,ti
#2. ‘diabetic’:ab,ti
#3. ‘diabetes mellitus’/exp/mj
#4. ‘diabetes’:ab,ti OR ‘diabetic’:ab,ti OR ‘diabetes mellitus’/exp/mj
#5. ‘dialysis’:ab,ti
#6. ‘peritoneal dialysis’:ab,ti
#7. ‘hemodialysis’:ab,ti
#8. ‘dialysis’/exp/mj
#9. ‘dialysis’:ab,ti OR ‘peritoneal dialysis’:ab,ti OR ‘hemodialysis’:ab,ti OR ‘dialysis’/exp/mj
#10. ‘statin’:ab,ti
#11. ‘hydroxymethylglutaryl-coa reductase inhibitors’/exp/mj
#12. ‘statin’:ab,ti OR ‘hydroxymethylglutaryl-coa reductase inhibitors’/exp/mj
#13. ‘diabetes’:ab,ti OR ‘diabetic’:ab,ti OR ‘diabetes mellitus’/exp/mj AND (‘dialysis’:ab,ti OR ‘peritoneal dialysis’:ab,ti OR ‘hemodialysis’:ab,ti OR ‘dialysis’/exp/mj) AND (‘statin’:ab,ti OR ‘hydroxymethylglutaryl-coa reductase inhibitors’/exp/mj)
For Cochrane Library:
ID Search
#1. diabetes:ti,ab,kw or diabetic:ti,ab,kw (Word variations have been searched)
#2. MeSH descriptor: [Diabetes Mellitus] explode all trees
#3. #1 or #2
#4. dialysis:ti,ab,kw or hemodialysis:ti,ab,kw or *dialysis:ti,ab,kw (Word variations have been searched)
#5. MeSH descriptor: [Dialysis] explode all trees
#6. MeSH descriptor: [Renal Dialysis] explode all trees
#7. MeSH descriptor: [Peritoneal Dialysis] explode all trees
#8. MeSH descriptor: [Peritoneal Dialysis, Continuous Ambulatory] explode all trees
#9. MeSH descriptor: [Hemodialysis, Home] explode all trees
#10. MeSH descriptor: [Hemodialysis Units, Hospital] explode all trees
#11. #4 or #5 or #6 or #7 or #8 or #9 or #10
#12. statin:ti,ab,kw or *statin:ti,ab,kw (Word variations have been searched)
#13. MeSH descriptor: [Hydroxymethylglutaryl-CoA Reductase Inhibitors] explode all trees
#14. #12 or #13
#15. #3 and #11 and #14
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Passman R. Prevention of sudden cardiac death in dialysis patients: drugs, defibrillators or what else. Blood Purif. 2013;35:49–54. doi: 10.1159/000345178. [DOI] [PubMed] [Google Scholar]
- 2.Minder CM, Blumenthal RS, Blaha MJ. Statins for primary prevention of cardiovascular disease: the benefits outweigh the risks. Curr Opin Cardiol. 2013;28:554–60. doi: 10.1097/HCO.0b013e32836429e6. [DOI] [PubMed] [Google Scholar]
- 3.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 4.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 5.National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60:850–86. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Marz W, Genser B, Drechsler C, Krane V, Grammer TB, Ritz E, Stojakovic T, Scharnagl H, Winkler K, Holme I, Holdaas H, Wanner C. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin J Am Soc Nephrol. 2011;6:1316–25. doi: 10.2215/CJN.09121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winkler K, Hoffmann MM, Krane V, Drechsler C, Wanner C. Lipoprotein-associated phospholipase A2 and outcome in patients with type 2 diabetes on haemodialysis. Eur J Clin Invest. 2012;42:693–701. doi: 10.1111/j.1365-2362.2011.02634.x. [DOI] [PubMed] [Google Scholar]
- 8.Holdaas H, Holme I, Schmieder RE, Jardine AG, Zannad F, Norby GE, Fellstrom BC. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22:1335–41. doi: 10.1681/ASN.2010090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan KE, Thadhani R, Lazarus JM, Hakim RM. Modeling the 4D Study: statins and cardiovascular outcomes in long-term hemodialysis patients with diabetes. Clin J Am Soc Nephrol. 2010;5:856–66. doi: 10.2215/CJN.07161009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JE, Oh KH, Choi KH, Kim SB, Kim YS, Do JY, Kim YL, Kim DJ. Statin therapy is associated with improved survival in incident peritoneal dialysis patients: propensity-matched comparison. Nephrol Dial Transplant. 2011;26:4090–4. doi: 10.1093/ndt/gfr229. [DOI] [PubMed] [Google Scholar]
- 11.Zimarino M, Corazzini A, Ricci F, Di Nicola M, De Caterina R. Late thrombosis after double versus single drug-eluting stent in the treatment of coronary bifurcations: a meta-analysis of randomized and observational Studies. JACC Cardiovasc Interv. 2013;6:687–95. doi: 10.1016/j.jcin.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. doi: 10.1136/bmj.g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med. 2013;11:48. doi: 10.1186/1741-7015-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huedo-Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 16.Gotz AK, Boger CA, Hirschmann C, Schmitz G, Riegger GA, Kramer BK. Effect of HMG-CoA-reductase inhibitors on survival in type 2 diabetes patients with end stage diabetic nephropathy. Eur J Med Res. 2005;10:155–60. [PubMed] [Google Scholar]
- 17.Shrier I, Boivin JF, Steele RJ, Platt RW, Furlan A, Kakuma R, Brophy J, Rossignol M. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. Am J Epidemiol. 2007;166:1203–9. doi: 10.1093/aje/kwm189. [DOI] [PubMed] [Google Scholar]
- 18.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 19.Chang YH, Hsieh MC, Wang CY, Lin KC, Lee YJ. Reassessing the benefits of statins in the prevention of cardiovascular disease in diabetic patients--a systematic review and meta-analysis. Rev Diabet Stud. 2013;10:157–70. doi: 10.1900/RDS.2013.10.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 21.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889–934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Nigwekar SU, Hegbrant J, Strippoli GF. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev. 2013;9:CD004289. doi: 10.1002/14651858.CD004289.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power A. Stroke in dialysis and chronic kidney disease. Blood Purif. 2013;36:179–83. doi: 10.1159/000356086. [DOI] [PubMed] [Google Scholar]
- 24.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Perkovic V, Hegbrant J, Strippoli GF. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;5:CD007784. doi: 10.1002/14651858.CD007784.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Castellon MA, Marshall RS. Statin use and brain hemorrhage: real risk or unfounded fear? JAMA Neurol. 2014;71:1353–4. doi: 10.1001/jamaneurol.2014.2463. [DOI] [PubMed] [Google Scholar]
- 26.Kwan BC, Beddhu S, Kronenberg F, Cheung AK. Does statin therapy improve cardiovascular outcomes in patients with type 2 diabetes receiving hemodialysis? Nat Clin Pract Nephrol. 2006;2:76–7. doi: 10.1038/ncpneph0101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


