Abstract
Objective: To explore the clinical effects of ultra-early microsurgery (< 24 hours) combined with extraventricular drainage for the treatment of poor-grade aneurysms. Methods: A total of 60 patients with poor-grade aneurysms were randomly divided into a microsurgery combined with extraventricular drainage (MED) group and conventional microsurgery (M) group. The prognosis was comparatively studied for these 2 groups. Results: All patients underwent a Glasgow Outcome Scale (GOS) assessment during a 6-month to 2-year follow-up. The excellent recovery (GOS, 4-5 points) rate for the MED group was 30% higher than that of the M group, while the poor recovery (GOS, 1-2 points) rate was 26.7% lower than that of the M group (P = 0.016). The incidence of acute brain swelling (26.7% vs 53.3%; P = 0.035), cerebral infarction (20% vs 46.7%; P = 0.025), and vasospasm (16.7% vs 40%; P = 0.045) for the MED group was significantly lower than that of the M group. Conclusions: For microsurgery combined with extraventricular drainage, the risk of cerebral infarction and vasospasm were significantly reduced for patients with poor-grade aneurysms, and the prognosis was better.
Keywords: Microsurgery, poor-grade aneurysm, extraventricular drainage, cerebral infarction, vasospasm
Introduction
A poor-grade aneurysm is a serious disease with high morbidity and mortality of 60%-90% [1-3]. Conservative treatment was normally suggested because of poor medical practice for this uncommon disease. However, for the first treatment, early microsurgery or endovascular treatment is preferable with a good prognosis [4-6]. However, diffuse brain swelling (DBS), vasospasm, and cerebral infarction still seriously affect prognosis postoperatively. The incidence of vasospasm following aneurysm rupture is as high as 70%, of which 17%-40% could lead to delayed neurologic dysfunction [7,8]. Sasaki et al. found that the occurrence rate of cerebral infarction was 4.4% for patients with aneurysms who underwent microsurgery [9]. The rate was even higher for patients with poor-grade aneurysms. A reduction in DBS would be beneficial to prevent vasospasms and cerebral infarction, while improving cerebral blood flow and perfusion. Thus, methods to reduce DBS are now critical for the prognosis of poor-grade aneurysms. In this study, we examined ultra-early microsurgery combined with extraventricular drainage for the treatment of poor-grade aneurysms. Patients who received treatment with ultra-early microsurgery and extraventricular drainage had a better prognosis than those who received a single microsurgical treatment.
Methods
Inclusion and exclusion criteria
The diagnostic criteria for poor-grade aneurysms were based on the Hunt and Hess scale; specifically, a scale from 4 to 5 indicated a poor grade. Diagnosis of ruptured aneurysms was completed using head computed tomography angiography (CTA) or digital subtraction angiography (DSA). The inclusion criteria included: (1) Hunt and Hess scale of IV and V; (2) no cerebral hernia was found preoperatively; (3) DBS or local brain swelling; and (4) combined with intracranial hematoma or ventricle hematocele. The exclusion criteria included: (1) traumatic subarachnoid hemorrhage or subarachnoid hemorrhage with unknown causes; (2) onset time of > 24 hours; (3) medical history included severe heart or lung disease and other risk factors affecting prognosis after aneurysm-clipping surgery; (3) age of > 90 years; (4) recurrent aneurysm or residual aneurysm necessitating secondary surgery; (5) multiple aneurysms; and (6) patients who abandoned treatment.
Ethical review
Our study was approved by the institutional review board (IRB) of Wuxi 101 Hospital of Chinese People’s Liberation Army (PLA). Written consent was obtained from each patient.
Study method
Sixty patients were randomly assigned to undergo ultra-early microsurgery combined with extraventricular drainage or a single microsurgery. All operations were completed by the same experienced microsurgical surgeon.
Clinical data
The microsurgery with extraventricular drainage (MED) group comprised the following: the patients (male/female: 13/17) were 44-73 years of age (63 ± 13.2 years). There were 14 cases with an anterior communicating artery, 10 cases with a middle cerebral artery, 4 cases with posterior circulation, and 2 cases with a distal anterior cerebral artery. According to the Hunt and Hess score, the number of cases with grades IV and V was 16 and 14, respectively.
The microsurgery (M) group comprised the following: the patients (male/female: 16/14) were 31-78 years of age (63 ± 14.5 years). There were 14 cases with an anterior communicating artery, 12 cases with a middle cerebral artery, and 4 cases with a posterior communicating artery. According to the Hunt and Hess score, the number of cases with grades IV and V was 12 and 18, respectively (Table 1).
Table 1.
Basic and clinical characteristics of the patients
| Characteristics | MED group (n = 30) | M group (n = 30) |
|---|---|---|
| Sex | ||
| Female | 17 | 14 |
| Male | 13 | 16 |
| Age | 63 ± 13.2 | 63 ± 14.5 |
| Hunt and Hess | ||
| IV | 16 | 12 |
| V | 14 | 18 |
| Location | ||
| ACoA | 14 | 14 |
| MCA | 10 | 12 |
| Posterior circulation | 4 | 4 |
| DACA | 2 | 0 |
ACoA, anterior communicating artery; MCA, middle cerebral artery; DACA: distal anterior cerebral artery aneurysms; Posterior circulation, contained the posterior communicating artery.
Pre-surgical procedure
All patients underwent tracheal intubation to preserve a normal oxygen concentration, and intravenous access was established to ensure sufficient blood supply. CTA or DSA examination was conducted. CT perfusion (CTP) was also used to assess cerebral blood flow (CBF) and cerebral blood vessels (CBV), if possible. Hemostatic and anti-vasospasm agents were helpful for patients before surgery. To reduce intracranial pressure, 20% mannitol (125 mL) was used for patients who had a sudden unilateral (bilateral) pupil dilation or acute DBS. There was no obvious difference in perioperative treatment between the 2 groups.
Surgical procedure
All 60 patients with poor-grade aneurysms had their surgery within 24 hours, depending on their CTA examination scores (aneurysm size and location, neck width of aneurysm, etc.). Forty-six cases underwent a trans-sylvian fissure approach, while 6 cases underwent an interhemispheric approach for an anterior interhemispheric hematoma and 8 cases underwent a posterior fossa craniotomy. For decompression, all patients underwent routine craniotomy with a bone flap area of more than 5 × 5 cm. After aneurysm occlusion, the MED group underwent an extraventricular drainage, while the M group did not.
Postoperative treatment and monitoring
Both groups had almost the same postoperative treatments. Briefly, all patients were administered nimodipine to reduce cerebral vasospasm and to improve brain blood circulation. Some medicines were routinely used such as hemostatic, free radical scavengers, acid inhibitors, and dehydrants. Thirty cases in the M group underwent a lumbar puncture or continuous external lumbar drainage of cerebral spinal fluid after the epidural drainage tube was removed. The extraventricular drainage tube should be removed within 2 weeks after surgery, or it could lead to intracranial infection. Transcranial Doppler (TCD) was performed to detect cerebral vasospasms, and head CT was routinely performed to detect brain or cerebral infarction after surgery.
Follow-up, outcomes, and postoperative complication assessment
Outcomes were evaluated for at least 6 months (range, 6 months to 2 years) after surgery according to GOS score with an in-person interview. The same neurosurgical surgeon completed all the outcome evaluations.
GOS was rated and classified into the following 5 levels: level 5 indicated good recovery (patients had a normal life and lived independently, such as able to return to work or school); level 4 indicated moderate disability (patients could take care of themselves and live independently but were unable to return to work or school); level 3 indicated severe disability (patients could not take care of themselves, were unable to live independently, required help, and remained conscious); level 2 indicated a vegetative state (patients were in a long-term coma and unable to interact with others); and level 1 indicated death. In our study, favorable outcomes were associated with 4 and 5; a poor functional outcome was associated with 1 and 2, while severe disability was associated with a 3 based on the GOS score.
Postoperative complications were assessed by comparing the occurrences of vasospasm, acute brain swelling, intracranial infection, and cerebral infarction. Vasospasm was defined as a slowing of blood flow velocity and reduction in blood volume detected by TCD, or vascular stenosis was detected using DSA or CTA examination. Acute brain swelling was defined if an encephalocele or a hardening of brain tissue was bulging from the bone defect area. Intracranial infection was indicated if the cerebrospinal fluid cultures were positive after surgery. Cerebral infarction, diagnosed by CT or MRI, was defined as an ischemic brain lesion. Using CT scans, cerebral infraction was indicated by a low-density irregular lamellar with clear margins.
Statistical analysis
Data analysis was performed using SPSS 14.0 (SPSS, Inc; Anhui Medical University, China). Quantitative data are expressed as mean ± standard deviation (SD). Categorical variables are expressed as frequency and percentage. To compare data between 2 treatment groups in a univariate analysis, independent 2-sample t test or rank sum test was used for continuous variables, and χ2 test or Fisher’s exact test was used for categorical variables. In statistical assessments, a P value of < 0.05 was considered significant.
Results
Relevant prognosis bias factors
Different Hunt and Hess scores and ages may lead to bias in prognosis. To analyze that possibility, a t test was used to analyze differences in age, and the rank sum test was adopted to analyze the Hunt and Hess scores between the 2 groups. After the statistical analysis, there were no significant differences between the 2 groups regarding age and Hunt and Hess scores (P > 0.05) (Table 2).
Table 2.
Prognosis-related bias factors for the 2 groups
| Variable | MED group (n = 30) | M group (n = 30) | P value |
|---|---|---|---|
| Age | 63 ± 13.2 | 63 ± 14.5 | 1.000 |
| Hunt and Hess | |||
| IV | 16 | 12 | 0.305 |
| V | 14 | 18 |
Quantitative data are expressed as mean ± standard deviation with an independent 2-sample t test; The rank sum test was used for ranked data.
Clinical outcomes
Aneurysms were completely occluded as confirmed by reexamination CTA or DSA. No cases in either group experienced rebleeding or relapses. According to the GOS scales, a higher percentage of cases in the MED group (50%; 15/30) had an excellent recovery (GOS, 4-5), as compared with cases in the M group (20%; 6/30). However, a lower percentage of cases in the MED group (33.3%; 10/30) had a poor recovery (GOS, 1-2), as compared with cases in the M group (60%; 18/30) (a typical case is presented in Figure 1).
Figure 1.
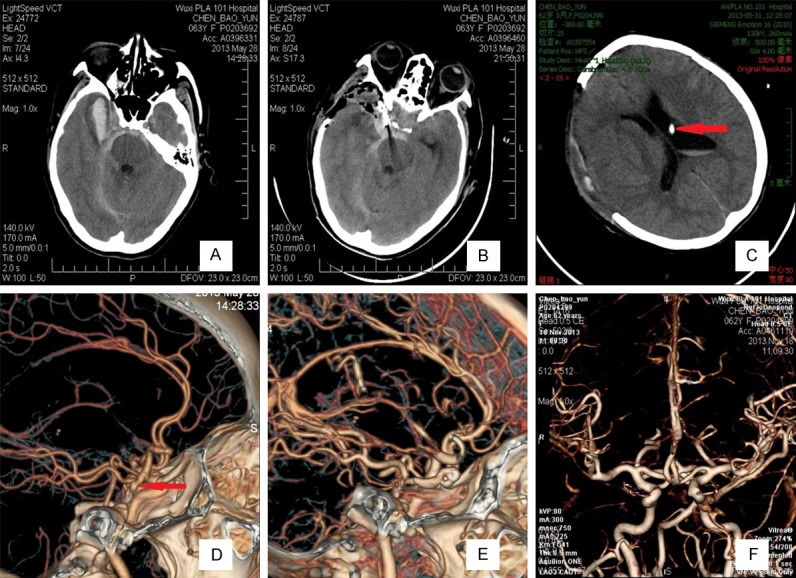
Images obtained from the 60 patients who were assessed at a Hunt-Hess grade V and Fisher grade 3 before undergoing ultra-early microsurgery combined with extraventricular drainage. After a 14-month follow-up, their Glasgow Outcome Scale was 5. A. A CT scan showed extensive SAH (especially at the basal cistern) and right temporal frontal lobe intracerebral hematoma. B. A postoperative CT scan showed that the aneurysm was occluded and that the hematoma was sufficiently removed. C. Three days postoperatively, a CT scan showed that the ventricle was normal. Red arrows indicate that the extraventricular drainage tube in the ventricle. D. Computed tomography angiography (CTA) showed right posterior communicating aneurysms, and red arrows indicate the aneurysm. E. Computed tomography angiography (CTA) after ultra-early microsurgical treatment showing that the aneurysm was perfectly occluded. F. Six months postoperatively, CTA showed no obvious intracranial vascular stenosis and normal vascular morphology.
Acute brain swelling
Although all patients underwent a decompressive craniectomy, the incidence of acute brain swelling was still high in both groups. However, the M group had a higher rate of acute brain swelling than the MED group (53.3% vs 26.7%; P = 0.035) (Table 3). Both groups underwent similar treatment plans such as using human blood albumin as a dehydration therapy; the acute brain swelling in many of the patients was gradually reduced.
Table 3.
Postoperative complications
| Complications | MED group (n = 30) | M group (n = 30) | P value |
|---|---|---|---|
| Acute brain swelling | 8 (26.7%) | 16 (53.3%) | 0.035 |
| Vasospasm | 6 (20%) | 14 (46.7%) | 0.028 |
| Intracranial infection | 6 (20%) | 5 (16.7%) | 0.739 |
| Cerebral infarction | 5 (16.7%) | 12 (40%) | 0.045 |
Pearson’s χ2 test was used in the statistical analysis.
Vasospasm
TCD was used to monitor vasospasms daily. Six of the 30 patients (20%) experienced different degrees of cerebral vasospasms in the MED group, while the M group had 14 (46.7%) patients who experienced vasospasms. The 2 groups underwent similar treatments to reduce cerebral vasospasms, and there were no surgical complications. Differences between the 2 groups were significant (P = 0.028; Table 3).
Intracranial infection
Postoperatively, 6 and 5 patients were diagnosed with an intracranial infection in the MED and M groups, respectively (Table 3). There were no differences between the 2 groups. These 11 patients were cured with anti-infective therapy.
Cerebral infarction
A cerebral infarction occurred in 5 patients (16.7%) in the MED group and 12 patients (40%) in the M group. The value in the MED group was significantly lower than that of the M group (P = 0.045; Table 3). Cerebral infarctions mainly occurred in the basal ganglia region and surgical areas.
Discussion
Although ultra-early microsurgery could improve the prognosis of patients with poor-grade aneurysms to some extent, high mortality and morbidity still remain challenges with surgical treatment [10-12]. Extraventricular drainage, especially combined with hydrocephalus, was a useful and common treatment for intraventricular blood for patients with Hunt and Hess grade IV or V [13]. However, there was no consensus regarding whether it would be helpful to perform an extraventricular drainage after aneurysm removal. Preliminarily, our department suggested that postoperative extraventricular drainage would be helpful for patients with poor-grade aneurysms during the recovery period. To this end, we designed this prospective, randomized, and comparative clinical study to compare the effects of extraventricular drainage in aneurysm surgery. Through this clinical trial, we found that the incidence of acute brain swelling, vasospasms, and cerebral infarction were significantly reduced in the MED group, as compared with the M group. However, the incidence of intracranial infection was not different between the 2 groups, indicating that extraventricular drainage did not increase the infection risk. Importantly, 50% of patients in the MED group experienced a good recovery, while only 20% of patients in the M group had a good outcome; 33.3% of patients in the MED group had a poor outcome, as compared with 60% of patients in the M group (P = 0.016; Table 4).
Table 4.
Post treatment outcomes for the 2 groups
| Variable | N | Favorable | Disability | Poor | P value |
|---|---|---|---|---|---|
| MED | 30 | 15 (50%) | 5 (16.7%) | 10 (33.3%) | P = 0.016 |
| M | 30 | 6 (20%) | 6 (20%) | 18 (60%) |
Rank sum test was used in the statistical analysis.
DBS might have occurred preoperatively because of intracerebral hematoma or hydrocephalus. With an increase in cerebral edema, cerebral contusion, and aggravation of hydrocephalus postoperatively, brain swelling would become more serious [14,15]. Kienin et al. considered that because of a damaged blood brain barrier (BBB) [16], vasospasm and endogenous toxic substances contributed to brain swelling after aneurysm rupture. In our present study, the incidence of acute brain swelling postoperatively was higher (53.3.3%) in the M group, as compared with the MED group (26.7%). Because of extraventricular drainage, we could reduce the ICP and brain tissue damage preferably by releasing bloody CSF.
The incidence of vasospasm was only 20% in the MED group and as high as 46.7% in the M group. The possible reasons might include: (1) extraventricular drainage could release a subarachnoid hematocele, which could lead to cerebral vasospasm [17-20]; And [2] promotion of cerebrospinal fluid circulation to reduce inflammatory cytokines such as IL-1, IL-6, FR, and TNF-α that could cause cerebral vasospasm [21,22]. Patients with poor-grade aneurysms had a high incidence of vasospasms, and 17%-40% of patients experienced delayed neurologic sequelae [3,8,9]. Extraventricular drainage could reduce the incidence of vasospasm and could improve outcomes for these patients.
The incidence of local or large cerebral infarctions was 16.7% in the MED group, as compared with 40% in the M group. There are a variety of reasons these patients might experience a cerebral infarction, such as intra-operative perforating vessel injury [9,23]. Subarachnoid hematocele and cerebral vasospasms could also lead to cerebral infarction [9,24]. Extraventricular drainage after surgery not only eliminated the subarachnoid hematocele and ventricle hematocele quickly, but also could alleviate cerebral vasospasms. Therefore, it could reduce the incidence of cerebral infarction after surgery.
The timing of extraventricular drainage is critical as many studies reported that extraventricular drainage could increase the risk of rebleeding for patients with poor-grade aneurysms [25,26]. Before occlusion of an aneurysm, an aneurysm is susceptible to rupture. Rapid intracranial pressure reduction using extraventricular drainage would lead to aneurysm rebleeding. Therefore, it would be safe and effective to perform extraventricular drainage after aneurysm occlusion. In our study, there was no incidence of rebleeding for the 30 patients who underwent extraventricular drainage. Fifty-two of the 60 patients were selected for puncture of the lateral ventricle frontal horns, while 8 of the 60 patients were selected for puncture of the lateral ventricle occipital horns; the puncture depth was about 5-6 cm, and the drainage tube could be kept in place for < 2 weeks.
Conclusion
In summary, we found that the incidence of acute brain swelling, vasospasm, and cerebral infarction were significantly reduced for patients with poor-grade aneurysms who underwent ultra-early microsurgery combined with extraventricular drainage. However, our study was limited by the small number of patients in each group. A larger, multicenter, randomized controlled trial should be conducted in the future.
Disclosure of conflict of interest
None.
References
- 1.Rordorf G, Ogilvy CS, Gress DR, Crowell RM, Choi IS. Patients in poor neurological condition after subarachnoid hemorrhage: early management and long-term outcome. Acta Neurochir (Wien) 1997;139:1143–1151. doi: 10.1007/BF01410974. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald RL, Rosengart AJ, Schutheiss KE, Tolentino J. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 3.Komotar RJ, Schmidt JM, Starke RM, Claassen J, Wartenberg KE, Lee K, Badjatia N, Connolly ES Jr, Mayer SA. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397–411. doi: 10.1227/01.NEU.0000338946.42939.C7. [DOI] [PubMed] [Google Scholar]
- 4.Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group: International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with raptured intracranial aneurysms: a randomised comparison of effects on survival, dependency. seizures, rebleeding, subgroups and aneurysm occlusion. Lancet. 2005;366:809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwkamp DJ, de Gans K, Algra A, Albrecht KW, Boomstra S, Brouwers PJ, Groen RJ, Metzemaekers JD, Nijssen PC, Roos YB, Tulleken CA, Vandertop WP, van Gijn J, Vos PE, Rinkel GJ. Timing of aneurysm surgery in subarachnoid haemorrhage- an observational study in The Netherlands. Acta Neurochir (Wien) 2005;147:815–821. doi: 10.1007/s00701-005-0536-0. [DOI] [PubMed] [Google Scholar]
- 7.Otani N, Nawashiro H, Wada K, Nagatani K, Takeuchi S, Kobayashi H, Osada H, Suzuki T, Shima K. Surgical results after primary decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013.;118:269–272. doi: 10.1007/978-3-7091-1434-6_51. [DOI] [PubMed] [Google Scholar]
- 8.Janjua N, Mayer SA. Cerebral vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care. 2003;9:113–119. doi: 10.1097/00075198-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki T, Kodama N, Matsumoto M, Suzuki K, Konno Y, Sakuma J, Endo Y, Oinuma M. Blood flow disturbance in perforating arteries attributable to aneurysm surgery. J Neurosurg. 2007;107:60–67. doi: 10.3171/JNS-07/07/0060. [DOI] [PubMed] [Google Scholar]
- 10.Otani N, Nawashiro H, Wada K, Nagatani K, Takeuchi S, Kobayashi H, Osada H, Suzuki T, Shima K. Surgical results after primary decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;118:269–272. doi: 10.1007/978-3-7091-1434-6_51. [DOI] [PubMed] [Google Scholar]
- 11.Pan JW, Tong Y, Wen L, Wan S, Zhan RY, Zhan YY. Ultra-early surgery for poor-grade intracranial aneurysmal subarachnoid hemorrhage: a preliminary study. Yonsei Med J. 2009;50:521–524. doi: 10.3349/ymj.2009.50.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowling R, Laidlaw J, Mitchell P, Sandström N, Yan B. Comparison of microsurgery and endovascular treatment on clinical outcome following poor-grade subarachnoid hemorrhage. J Clin Neurosci. 2013;20:1213–1218. doi: 10.1016/j.jocn.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Shin YS, Kim SY, Kim SH, Ahn YH, Yoon SH, Cho KH, Cho KG. One-stage embolization in patients with acutely ruptured poor-grade aneurysm. Surg Neurol. 2005;63:149–155. doi: 10.1016/j.surneu.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Chhabra R, Gupta R, Gupta SK, Khosla VK, Mohindra S, Mukherjee KK. Decompressive surgery for acute subdural haematoma leading to contralateral extradural haematoma: A report of two cases and review of literature. Br J Neurosurg. 2005;19:490–494. doi: 10.1080/02688690500495216. [DOI] [PubMed] [Google Scholar]
- 15.Xi G, Wagner KR, Keep RF, Hua Y, de Courten-Myers GM, Broderick JP, Brott TG, Hoff JT. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–2586. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 16.Kiening KL, Härtl R, Unterberg AW, Schneider GH, Bardt T, Lanksch WR. Brain tissue PO2-monitoring in comatose patients: implications for therapy. Neurol Res. 1997;19:233–240. doi: 10.1080/01616412.1997.11740805. [DOI] [PubMed] [Google Scholar]
- 17.Vollrath BA, Weir BK, Macdonald RL, Cook DA. Intracellular mechanisms involved in the responses of cerebrovascular smooth muscle cell to hemoglobin. J Neurosurg. 1994;80:261–268. doi: 10.3171/jns.1994.80.2.0261. [DOI] [PubMed] [Google Scholar]
- 18.Endo S, Hirashima Y, Nishijima M, Otsuji T, Takaku A. An experimental model of symptomatic vasospasm induced by oxyhemoglobin in rabbits. Stroke. 1994;25:657–662. doi: 10.1161/01.str.25.3.657. [DOI] [PubMed] [Google Scholar]
- 19.Ishiguro M, Murakami K, Link T, Zvarova K, Tranmer BI, Morielli AD Wellman GC. Acute and chronic effects of oxyhemoglobin on voltage-dependent ion channels in cerebral arteries. Acta Neurochir Suppl. 2008;104:99–102. doi: 10.1007/978-3-211-75718-5_19. [DOI] [PubMed] [Google Scholar]
- 20.Windmüller O, Lindauer U, Foddis M, Einhäupl KM, Dirnagl U, Heinemann U Dreier JP. Ion changes in spreading ischaemia induce rat middle cerebral artery constriction in the absence of no. Brain. 2005;128:2042–2051. doi: 10.1093/brain/awh545. [DOI] [PubMed] [Google Scholar]
- 21.Gu YX, Li PL, Leng B, Mao Y, Ni W, Song DL. The relationship between IL-6 in CSF and occurrence of vasospasm after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:203–208. doi: 10.1007/978-3-7091-0353-1_35. [DOI] [PubMed] [Google Scholar]
- 22.Fu X, Hazen SL, Rasmussen PA, Ransohoff RM, Provencio JJ, Siu A. CSF neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit Care. 2010;12:244–251. doi: 10.1007/s12028-009-9308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fridriksson S, Säveland H, Jakobsson KE, Edner G, Zygmunt S, Brandt L, Hillman J. Intraoperatire complications in aneurysm surgery: a prospective national study. J Neurosurg. 2002;96:515–522. doi: 10.3171/jns.2002.96.3.0515. [DOI] [PubMed] [Google Scholar]
- 24.Browne KD, Chen XH, Graham DI, Stein SC, Smith DH. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery. 2006;59:781–787. doi: 10.1227/01.NEU.0000227519.27569.45. [DOI] [PubMed] [Google Scholar]
- 25.Fujita S, Hosoda K, Hamano S, Kawaguchi T, Iwakura M, Shose Y. Effect of clot removal and surgical manipulation on regional cerebral blood flow and delayed vasospasm in early aneurysm surgery for subarachnoid hemorrhage. Surg Neurol. 1999;51:81–88. doi: 10.1016/s0090-3019(97)00508-9. [DOI] [PubMed] [Google Scholar]
- 26.Delfino R, Leblance R, Paré L. The relationship of ventricular drainage to aneurysmal releeding. J Neurosurg. 1992;76:422–427. doi: 10.3171/jns.1992.76.3.0422. [DOI] [PubMed] [Google Scholar]


