Abstract
Aims: To investigate the effect of miR-195 overexpression on radiosensitivity and the relevant molecular mechanisms in breast cancer. Methods: miR-195 mimics were transfected to breast cancer cell line MCF-7 by gene transfection; miR-195 expression level in cells was determined by real-time quantitative PCR. Cellular viability was observed by colony formation assay after the cells were irradiated with X-ray. Apoptosis was detected by annexin V/propidium iodide (PI) staining. Protein expression was evaluated by Western blot assay. Results: After miR-195 mimics were transfected, miR-195 expression in breast cancer cells was significantly upregulated (P < 0.05). miR-195 overexpression significantly enhanced the response to radiation of MCF-7 cells with a decrease in colony survival at individual doses of X-ray (P < 0.05). After the cells were irradiated with 5 Gy, apoptotic rate was significantly increased in miR-195-overexpressed group with a rate of 18.13% ± 1.57%; moreover, BCL-2 protein expression was significantly downregulated (P < 0.05). Conclusion: Exogenetic miR-195 expression could significantly enhance the radiosensitivity of human breast cancer cells; this finding may be attributed to BCL-2 downregulation to promote radiation-induced apoptosis.
Keywords: Breast cancer, miR-195, radiosensitivity, apoptosis
Introduction
Breast cancer is one of the most common causes of death in women and considered as a significant global health problem [1]. Breast cancer is mainly treated by radiation therapy. With radiation therapy, the survival benefit of patients who have undergone breast-conserving surgery is significantly promoted; furthermore, local control is effectively improved and risk of recurrence in chestwall and regional lymph node is reduced [2]. However, the heterogeneity of X-ray responses among breast cancer patients limits clinical applications of radiotherapy. Therefore, studies have been conducted to improve the radiosensitivity of patients with breast cancer.
MicroRNA (miRNA) is a newly discovered non-coding single-strand RNA with approximately 21 to 24 nucleotides. miRNA also complementarily combines with the 3’-untranslated region (UTR) of target gene mRNA, either completely or incompletely; thus, target mRNA is degraded or translation is inhibited, thereby negatively regulating target gene expression [3,4]. When disrupted, miRNA expression is involved in cancer development and progression. miRNA is also an important biomarker of early diagnosis, efficacy assessment, and prediction of prognosis [5]. As such, studies have extensively investigated the roles of downregulated miR-195 expression in breast cancer formation [6]. Studies have also revealed that miR-195 overexpression can inhibit proliferation and invasion of breast cancer cells [7]; in addition, miR-195 overexpression can promote Adriamycin-induced apoptosis of MCF-7 cells. Therefore, the sensitivity of breast cancer to Adriamycin is significantly increased [8]. However, whether miR-195 upregulation can increase the response of breast cancer cells to radiation remains unknown. Thus far, no reports have clarified this matter.
In this study, the effects of miR-195 and irradiation on breast cancer cell line were analyzed; molecular mechanisms relevant to this process were also investigated.
Materials and methods
Cell culture and irradiation
Human breast cancer cell line MCF-7 was purchased from the Cell Bank of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences; MCF-7 cells were conventionally cultured in RPMI-1640 medium (GIBCO company) with 10% fetal bovine serum (Hyclone Company) at 37°C in a 5% CO2-humidified incubator. Afterward, the cells were irradiated with 6 MeV X-ray by using a linear accelerator (Varian Medical Systems, USA) at a dose rate of 200 cGy/min with a source-to-skin distance (SSD) of 100 cm.
Gene transfection and grouping
miR-195 mimics and negative control (NC) were purchased from Shanghai GenePharma Co., Ltd. MCF-7 cells in logarithmic phase were inoculated in six-well plates at 2 × 105 cells/well. Cell transfection was performed at 80% confluence by using Lipofectamine 2000 (Invitrogen Corporation) in accordance with the manufacturer’s instructions. At 6 h after transfection, the medium was replaced with a fresh medium containing serum; the cells were routinely sub-cultured.
Real-time reverse transcription-PCR
Total RNA was extracted using TRIZOL method and reverse-transcribed to obtain the corresponding cDNA by using a Taqman miRNA reverse transcription kit. The reaction conditions of reverse transcription were set as follows: 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. miR-195 expression was detected by Taqman probe-based real-time PCR assay in accordance with the manufacturer’s instructions. U6 transcript was amplified as control. Real-time PCR was carried out under the following conditions: 95°C for 10 min, 40 cycles of 95°C for 15 s, and 60°C for 15 s in an ABI Prism 7500 (Applied Biosystems, USA). The results were calculated using ∆Ct method, as previously described [9].
Cell colony formation assay
The cells in logarithmic phase were inoculated in six-well plates (0 Gy group, 2 × 102 cells/well; 2 Gy group, 5 × 102 cells/well; 4 Gy group, 1 × 103 cells/well; 6 Gy group, 2.5 × 103 cells/well; and 8 Gy group, 5 × 103 cells/well). Adherent cells were irradiated with X-ray at corresponding doses and then cultured for 10 d to 14 d until a colony was formed. The cells were fixed with 75% methanol and then stained with Giemsa. Cell colonies (with ≥ 50 cells) were counted under a microscope by using the following equations: cloning efficiency (%) = number of colonies/number of inoculated cells × 100%; survival fraction (SF) = cloning efficiency in irradiated group/cloning efficiency in control group × 100%.
Annexin V-FITC/PI staining
Cells were inoculated in six-well plates at 3 × 105 cells/well. Adherent cells were subsequently irradiated with 5 Gy X-ray and continuously incubated for 24 h. Afterward, the cells were collected, washed twice with cold PBS, and resuspended in 100 µl of binding buffer. Annexin V-FITC (5 μl; BD company) and PI (5 μl; BD Company) were added to the cell suspension, incubated for 15 min in the dark, and immediately detected by flow cytometry.
Western blot assay
Cell lysate was added to subconfluent cells and placed in an ice bath for 15 min. The proteins were quantified using a BCA protein assay kit (Pierce Co., Ltd.), separated by denaturing electrophoresis of 15% SDS-PAGE, and transferred to a membrane; the membrane was then blocked with 5% milk. Mouse anti-human BCL-2 monoclonal antibody (1:1000, Abcam Company) was added and incubated overnight at 4°C. Goat anti-rabbit IgG (1:3000, Zhongshan Golden Bridge Company) was added and incubated at room temperature for 1 h. Afterward, ECL reagent (Pierce Company) was added and sealed with film. After a tablet was pressed and exposed in the darkroom, the membrane was developed and fixed. The films were scanned and gray values of various samples were analyzed with Quantity One 4.5 software. GAPDH (rabbit anti-human monoclonal antibody, 1:2000; Cell Signaling Technology Company) was used as reference.
Statistical analysis
Experimental data were expressed as x̅ ± SD and analyzed in SPSS16.0. The significances between experimental groups and control groups were determined by one-way ANOVA; Student’s t-test was used to compare the quantitative ratios of different groups. All P values are two-sided and values less than 0.05 were considered statistically significant
Results
miR-195 expression in breast cancer cells
miR-195 mimics were transfected and expression was detected by real-time PCR at 48 h after transfection to investigate the role of miR-195 in MCF-7 cells. miR-195 expression in untransfected MCF-7 cells was defined as 1; miR-195 mimics significantly increased miR-195 expression to 9.15-fold (P < 0.05; Figure 1). By contrast, miR-195 expression did not change significantly in the NC-transfected group compared with the untransfected group (P > 0.05). These results demonstrated that miR-195 mimics could upregulate miR-195 expression in breast cancer MCF-7 cells.
Figure 1.
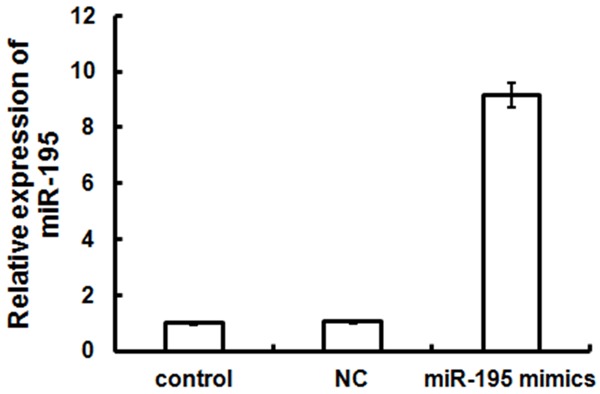
miR-195 expression level in MCF-7 cells after these cells were transfected with miR-195 mimics. Compared with untransfected or NC-transfected cells, transfected cells showed a significant increase in miR-195 expression level (P < 0.05).
Effects of miR-195 on radiosensitivity of breast cancer cells
Colony forming assay was performed to determine whether miR-195 enhances the sensitivity of breast cancer cell lines to radiation. The cells were transfected with miR-195 mimics and NC; afterward, the cells were irradiated with 0 Gy to 8 Gy and plated to evaluate colony forming efficiency. The results showed that a high dose of radiation elicited a significant dose-dependent killing effect on cells. At various doses of X-ray, miR-195 overexpression caused a significant increase in radiation-induced cell death and stimulated the formation of few small colonies compared with that of the NC-transfected group (P < 0.05; Figure 2). A cell survival curve was fitted using a multi-target single-hit model; the result indicated that SF2 value of miR-195 mimic-transfected group was 0.42, which was significantly lower (P < 0.05) than that of untransfected group (0.51) and NC group (0.52). However, SF2 values of control and NC groups were not significantly different (P > 0.05). SER values in miR-195-overexpressed and NC groups were 1.24 and 1.02, respectively; this result further suggested that miR-195 overexpression could enhance the sensitivity of breast cancer MCF-7 cells to irradiation.
Figure 2.
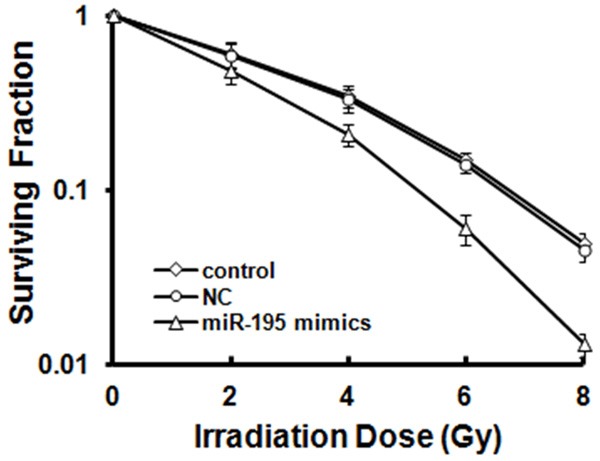
MCF-7 cells were transfected with miR-195 mimics or NC for 24 h and then irradiated with 0, 2, 4, 6, and 8 Gy. After 14 d, the cells were fixed with acetone and stained with crystal violet. Colonies with ≥ 50 cells were counted. Surviving fractions were calculated after the plating efficiency of appropriate control groups was normalized and plotted as mean ± S.E. in a log scale.
Mechanisms of miR-195 mimic-induced radiosensitivity
Apoptosis was evaluated by FACS with annexin V/PI staining to explore the mechanisms associated with the upregulated miR-195-induced radiosensitivity. Cell apoptotic rates of the untransfected group (5.67% ± 1.01%) and the NC group (6.13% ± 0.87%) were similar at 24 h after irradiation with 5 Gy (Figure 3). By contrast, the transfection of miR-195 mimics caused an increase in the number of annexin V-positive MCF-7 cells; the apoptotic rate of the cells in the miR-195-overexpressed group increased to 18.13% ± 1.57%, which was significantly different from that in the control group (P < 0.05).
Figure 3.
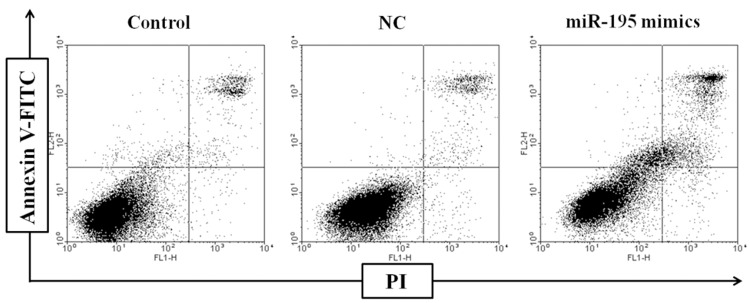
Effects of miR-195 on MCF-7 cell apoptosis. MCF-7 cells were transfected with miR-195 mimics or NC, apoptosis was quantified by annexin V/PI staining at 24 h after irradiation with 5 Gy.
Effects of miR-195 upregulation on BCL-2 protein
BCL-2 protein expression in MCF-7 cells at 72 h after transfection was detected by Western blot assay. The results showed that BCL-2 protein level in miR-195 mimic transfection group was decreased to 24.7%, which was significantly lower than that in the untransfected group and the NC group (P < 0.05). Conversely, no significant difference was observed between untransfected and NC groups (P > 0.05). Therefore, miR-195 overexpression effectively inhibited BCL-2 protein expression (Figure 4).
Figure 4.
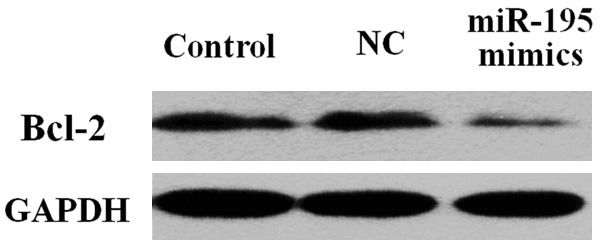
BCL-2 protein expression in MCF-7 cells after transfection. Compared with untransfected and NC-transfected cells, miR-195 mimic-transfected cells showed a significant decrease in BCL-2 protein expression.
Discussion
Differential miRNA expression is closely related to tumor radiosensitivity; as such, the response of tumor to radiotherapy can be regulated by altering miRNA expression level. This strategy may be potentially applied to increase the inherent radiosensitivity of tumor cells [10,11]. Weidhaas et al. used miRNA chips to analyze differential miRNA expression in lung cancer cells before and after irradiation, and found that.expressions of 81 miRNAs significantly varied;especially the downregulation of let-7. Furthermore, lung cancer cells exhibit significantly increased radiosensitivity by the upregulation of let-7 expression; by contrast, these cells show resistance to radiation by the inhibition of let-7 expression [12].
miR-195 belonging to the miR-15 gene family is located on chromosome 17p13.1 [13]. miR-195 functions as a tumor suppressor gene, which is downregulated in various tumors, including esophageal squamous cell cancer, liver cancer, bladder cancer, and adrenal cancer [14-16]. The present study mainly discussed the effect of miR-195 on the radiosensitivity of breast cancer cells. Exogenous miR-195 mimics were transfected to MCF-7 cells, which were subsequently irradiated with X-ray. The colony forming efficiency of the transfected cells was decreased significantly compared with that of the control cells. Colony formation assay is an effective technique to determine the proliferation potential of a cell; thus, this assay is considered as gold standard of evaluation of cellular radiosensitivity in vitro. Based on the cloning efficiency of cells in different groups, the multi-target single-hit model was used to fit the cell survival curve. The result showed that miR-195 overexpression in the cells caused a decrease in SF2 value. SF2 value can indicate the radiosensitivity of tumor cells; furthermore, this value can be used to calculate sensitization enhancement ratio (SER). The SER of cells in the mimic-transfected group was > 1, suggesting that miR-195 overexpression significantly increased the radiosensitivity of breast cancer cells.
Apoptosis induction is the main mechanism of eradicating tumor cells by radiation therapy; the strength of apoptotic response is closely related to the sensitivity of cells to radiation therapy [17]. miR-195 overexpression significantly reduces mitochondrial membrane potential, induces the release of cytochrome c into the cytoplasm, and activates caspase-9; as a result, apoptosis is promoted [18,19]. Our study also found that miR-195 overexpression significantly increased X-ray-induced apoptosis of breast cancer cells by reduction of the level of BCL-2 protein. BCL-2, as an important anti-apoptotic protein, is involved in the mitochondrial apoptotic pathway, and exerts its function through inhibiting the release of cytochrome c from the mitochondrial inner membrane into the cytoplasm [20]. Recently, BCL-2 has been identified to be a direct target of miR-195 that can inhibit BCL-2 protein expression by binding the 3’-UTR of BCL-2 [18]; which is consistent with our study. In addation to targeting BCL-2, miR-195 can induce apoptosis by regulating other target molecules. For example, overexpressed miR-195 can promote apoptosis by negative regulation of SIRT1, which encodes the NAD-dependent deacetylase sirtuin-1 [21]. miR-195 has also reported to regulate the cell cycle by targeting the molecules associated with G1–S checkpoints and promote apoptosis. Our results confirmed that miR-195 overexpression increased the sensitivity of breast cancer cells to X-ray by inhibiting BCL-2 protein expression in MCF-7 cells.
When compared with the conventional radiotherapeutic sensitizer, Gene-targeting sensitizer can not only change the intrinsic radiosensitivity of tumor cells, but also has the function of inducing apoptosis and directly activation of the immune response [22], which may be one of the most efficient strategies to enhance radiosensitization. Our study demonstrated that miR-195 can reverse the resistance of breast cancer cells to radiation therapy, which provided experimental evidence as basis of individualized breast cancer treatment and clinical applications. In subsequent studies, the effects of miR-195-regulated BCL-2 expression on radiation sensitization of breast cancer cells should be investigated by in vivo experiments and clinical trials. Furthermore, screening of downstream target molecules of miR-195 should be performed to establish a regulatory network with pro-apoptotic effects and to clarify the mechanisms of miR-195 induced radiosensitization on breast cancer.
Acknowledgements
This study was supported by the grants from National Natural Science Foundation of China (Nos. 30960439 and 81101693), National Key Clinical Specialty (Oncology), Yunnan Health Science Fund (2012WS0039).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Warren LE, Punglia RS, Wong JS, Bellon JR. Management of the regional lymph nodes following breast-conservation therapy for early-stage breast cancer: an evolving paradigm. Int J Radiat Oncol Biol Phys. 2014;90:772–777. doi: 10.1016/j.ijrobp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 3.De Santa F, Iosue I, Del Rio A, Fazi F. microRNA biogenesis pathway as a therapeutic target for human disease and cancer. Curr Pharm Des. 2013;19:745–764. [PubMed] [Google Scholar]
- 4.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang W, Liu J, Wang G. The role of microRNAs in human breast cancer progression. Tumour Biol. 2014;35:6235–6244. doi: 10.1007/s13277-014-2202-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhao FL, Dou YC, Wang XF, Han DC, Lv ZG, Ge SL, Zhang YK. Serum microRNA-195 is down-regulated in breast cancer: a potential marker for the diagnosis of breast cancer. Mol Biol Rep. 2014;41:5913–5922. doi: 10.1007/s11033-014-3466-1. [DOI] [PubMed] [Google Scholar]
- 7.Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang Y, Zou C, Zhang X, Liu S, Wang X, Zhao D, Sun Q, Zeng Z, Dress A, Lin MC, Kung HF, Rui H, Liu LZ, Mao F, Jiang BH, Lai L. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin Cancer Res. 2011;17:1722–1730. doi: 10.1158/1078-0432.CCR-10-1800. [DOI] [PubMed] [Google Scholar]
- 8.Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, Weng Y. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep. 2013;30:877–889. doi: 10.3892/or.2013.2532. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Meng G, Guo QN. Changes in genomic imprinting and gene expression associated with transformation in a model of human osteosarcoma. Exp Mol Pathol. 2008;84:234–239. doi: 10.1016/j.yexmp.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Cellini F, Morganti AG, Genovesi D, Silvestris N, Valentini V. Role of microRNA in response to ionizing radiations: evidences and potential impact on clinical practice for radiotherapy. Molecules. 2014;19:5379–5401. doi: 10.3390/molecules19045379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metheetrairut C, Slack FJ. MicroRNAs in the ionizing radiation response and in radiotherapy. Curr Opin Genet Dev. 2013;23:12–19. doi: 10.1016/j.gde.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weidhaas JB, Babar I, Nallur SM, Trang P, Roush S, Boehm M, Gillespie E, Slack FJ. MicroRNAs as potential agents to alter resistance to cytotoxic anticancer therapy. Cancer Res. 2007;67:11111–11116. doi: 10.1158/0008-5472.CAN-07-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flavin RJ, Smyth PC, Laios A, O’Toole SA, Barrett C, Finn SP, Russell S, Ring M, Denning KM, Li J, Aherne ST, Sammarae DA, Aziz NA, Alhadi A, Sheppard BL, Lao K, Sheils OM, O’Leary JJ. Potentially important microRNA cluster on chromosome 17p13.1 in primary peritoneal carcinoma. Mod Pathol. 2009;22:197–205. doi: 10.1038/modpathol.2008.135. [DOI] [PubMed] [Google Scholar]
- 14.Fei X, Qi M, Wu B, Song Y, Wang Y, Li T. MicroRNA-195-5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012;586:392–397. doi: 10.1016/j.febslet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Zheng C, Li J, Wang Q, Liu W, Zhou J, Liu R, Zeng Q, Peng X, Huang C, Cao P, Cao K. microRNA-195 functions as a tumor suppressor by inhibiting CBX4 in hepatocellular carcinoma. Oncol Rep. 2015;33:1115–22. doi: 10.3892/or.2015.3734. [DOI] [PubMed] [Google Scholar]
- 16.Sun N, Ye L, Chang T, Li X. microRNA-195-Cdc42 axis acts as a prognostic factor of esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:6871–6879. [PMC free article] [PubMed] [Google Scholar]
- 17.Balcer-Kubiczek EK. Apoptosis in radiation therapy: a double-edged sword. Exp Oncol. 2012;34:277–285. [PubMed] [Google Scholar]
- 18.Singh R, Saini N. Downregulation of BCL2 by miRNAs augments drug-induced apoptosis--a combined computational and experimental approach. J Cell Sci. 2012;125:1568–1578. doi: 10.1242/jcs.095976. [DOI] [PubMed] [Google Scholar]
- 19.Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, Wang NS. MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol. 2011;34:549–559. doi: 10.1159/000333809. [DOI] [PubMed] [Google Scholar]
- 20.Kvansakul M, Hinds MG. The Bcl-2 family: structures, interactions and targets for drug discovery. Apoptosis. 2015;20:136–150. doi: 10.1007/s10495-014-1051-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Yang Y, Wang Y, Li J, Schiller PW, Peng T. MicroRNA-195 promotes palmitate-induced apoptosis in cardiomyocytes by down-regulating Sirt1. Cardiovasc Res. 2011;92:75–84. doi: 10.1093/cvr/cvr145. [DOI] [PubMed] [Google Scholar]
- 22.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–253. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]


