Abstract
This study aimed to investigate the clinical feasibility and effects of percutaneous renal sympathetic nerve radiofrequency ablation in patients with heart failure. A total of 20 patients with heart failure were enrolled, aged from 47 to 75 years (63±10 years). They were divided into the standard therapy (n = 10), and renal nerve radiofrequency ablation groups (n = 10). There were 15 males and 5 female patients, including 8 ischemic cardiomyopathy, 8 dilated cardiomyopathy, and 8 hypertensive cardiopathy. All of the patients met the criteria of New York Heart Association classes III-IV cardiac function. Patients with diabetes and renal failure were excluded. Percutaneous renal sympathetic nerve radiofrequency ablation was performed on the renal artery wall under X-ray guidance. Serum electrolytes, neurohormones, and 24 h urine volume were recorded 24 h before and after the operation. Echocardiograms were performed to obtain left ventricular ejection fraction at baseline and 6 months. Heart rate, blood pressure, symptoms of dyspnea and edema were also monitored. After renal nerve ablation, 24 h urine volume was increased, while neurohormone levels were decreased compared with those of pre-operation and standard therapy. No obvious change in heart rate or blood pressure was recorded. Symptoms of heart failure were improved in patients after the operation. No complications were recorded in the study. Percutaneous renal sympathetic nerve radiofrequency ablation may be a feasible, safe, and effective treatment for the patients with severe congestive heart failure.
Keywords: Heart failure, renal denervation, radiofrequency ablation, sympathetic activity
Introduction
Heart failure, which is also called cardiac insufficiency or congestive heart failure, is a complicated clinical syndrome. This syndrome results from continuous injury acting on the heart, such as hypertension, ischemia, and atherosclerosis. Heart failure is the ultimate outcome of all types of heart diseases and is the main cause of death. After the first damage occurs, it aggravates the course of myocardial remodeling gradually by increasing neuroendocrine factors and cytokines [1]. At the same time, the injured myocardium releases more neuroendocrine factors and cytokines, which harm normal myocardium [2,3]. This process can become a vicious circle. The renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system play an important part in the course of myocardial remodeling [4]. The sympathetic nervous system, as well as the RAAS, becomes over-activated because of development of heart failure. Angiotensin-converting enzyme inhibitors, β-blockers, and aldosterone receptor antagonists are basic drugs in guidelines for chronic heart failure [5]. Most patients with heart failure present with signs and symptoms of acute fluid retention. Diuretics are one of the most common drugs prescribed in heart failure treatment, and they can remove overlord fluid volume in congestive heart-failure patients. These patients are initially sensitive to diuretic therapy. An increase in renal sympathetic nerve activity subsequently results in an increase in renal vascular resistance and renin levels, as well as retention of sodium and water [6]. Progression of congestive heart failure (CHF) and chronic use of diuretics could lead to diuretic resistance. A previous study showed that renal sympathetic nerve activity is related to diuretic resistance [7]. Recent experiments have been performed to treat heart failure by decreasing renal sympathetic nerve activity [8].
In the current study, we designed a therapy to treat CHF, which has been used for treatment of hypertension in the past few years. In this study, we aimed to investigate the effect of blocking renal sympathetic nerve activity in patients with chronic refractory heart failure by radiofrequency ablation in local sympathetic nerves, and the result was encouraging.
Methods
Patient selection
Twenty hospitalized patients were enrolled between June and November 2013 with chronic refractory CHF (Eight patients had dilated cardiomyopathy, eight had ischemic cardiomyopathy, and four had hypertensive cardiopathy). All of them had symptoms and signs of dyspnea and edema, with cardiac function between classes III-IV in the New York Heart Association cardiac function grade. The patients were confirmed as having cardiac enlargement by echocardiography and a left ventricular ejection fraction (LVEF) < 40%. Diuretics, angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), or β-blockers were taken by these patients for at least one month. Patients with diabetes and chronic renal insufficiency were excluded. The 20 patients were divided into two groups: the standard therapy group (n = 10) and the renal radiofrequency ablation group (n = 10). The two groups had the same drug treatment during the hospitalization period, such as loop diuretics, digoxin, and nitrates. The renal radiofrequency ablation group had renal radio frequency ablation as well as drug treatment. There was no difference for the baseline clinical characteristics in the two groups (Table 1). The Southeast University committee approved the study. Oral and written informed consent were obtained from all patients. The mean follow-up period was 6 months. The endpoints of this study were death and rehospitalization because of worsening heart failure. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Southeast University. Written informed consent was obtained from all participants.
Table 1.
Baseline clinical characteristics in the two groups
| Clinical characteristics | Standard therapy group (n = 10) | Renal nerve ablation group (n = 10) | P value |
|---|---|---|---|
| Mean age (mean ± SD, years) | 64±5 | 63±10 | 0.909 |
| Gender (male/female, n) | 7/3 | 8/2 | 0.343 |
| Heart rate (mean ± SD, bpm) | 79±4 | 80±7 | 0.759 |
| MAP (mmHg) | 95.1±10.1 | 100.3±12.1 | 0.309 |
| 24 h urine volume (mean ± SD, ml) | 1235±217 | 1145±153 | 0.244 |
| Hemoglobin (mean ± SD, Hb/L) | 127±8.34 | 125.2±10.6 | 0.578 |
| Creatinine(mean ± SD, µmol/L) | 106.5±13.1 | 105.2±12.2 | 0.809 |
| BNP(mean ± SD, pg/L) | 577.5±92.2 | 628.9±131.4 | 0.373 |
| Medication (%) | |||
| Loop diuretic | 100 | 100 | 1.00 |
| Spirolactone | 100 | 90 | 0.343 |
| ACEI/ARB | 70 | 80 | 0.678 |
| β-blocker | 20 | 40 | 0.168 |
Note: P < 0.05 is considered statistically significant.
Renal nerve radiofrequency ablation procedure
The experiment was performed in a cardiology catheter room. After local anesthesia, femoral artery puncture was performed in the patients. A radiofrequency ablation electrode was then placed into the renal artery through the femoral artery. Local radiofrequency ablation was performed on the renal artery wall under X-ray guidance. Renal artery angiography was required before and after ablation to investigate contraindications and complications (Figure 1). Ablation was performed at four to six points in different positions of the renal artery wall, and the treatment time lasted for 2 min at each point. The ablation power was 8-12J. After ablation was finished, the ablation electrode was pulled out and the puncture point was bandaged.
Figure 1.
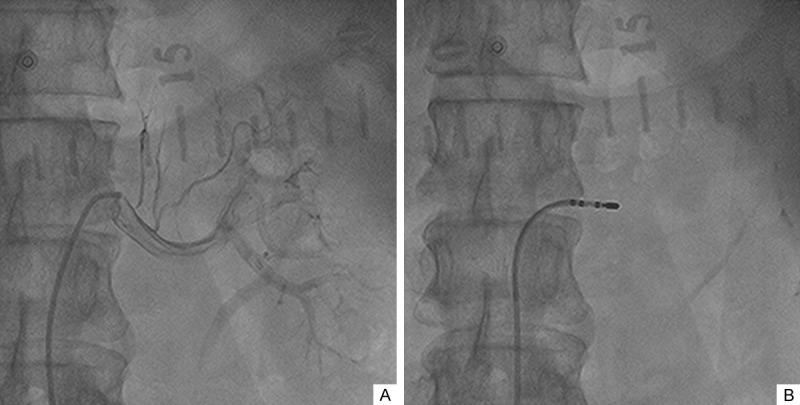
Renal artery angiography and renal denervation by radiofrequency ablation. A: Renal artery angiography in the left kidney; B: Radiofrequency ablation on the left renal artery wall under X-ray guidance.
Laboratory analyses
Twenty-four-hour total urine volume, blood pressure, and heart rate were measured before and 24 h after the renal nerve ablation. Additionally, neuroendocrine hormones including renin, aldosterone, angiotensin II, B-type natriuretic peptides (BNP), adrenalin, dopamine and noradrenalin were measured in each patient’s blood sample. Plasma electrolytes and creatinine levels were measured by standard laboratory methods. Plasma neuroendocrine hormones were detected by radioimmunoassay. Changes in symptoms of heart failure such as dyspnea and edema were also observed.
Echocardiography
Transthoracic echocardiographic examinations including two dimensional color flow and pulsed Doppler using a 2.5-3.5 MHz transducer were underwent in all of the patients. LVEF, fractional shortening (FS) and the diameter of left ventricle were under measured by echocardiography before and 24 hours after ablation and 6 months later.
Major adverse cardiovascular events (MACE)
The average follow-up period for each patient was 6 months. Information on cardiac function and MACE were recorded from the outpatient service or telephone interview after ablation. LVEF, FS, and the diameter of the left ventricle were measured by echocardiography at 6th months after ablation. Echocardiographic evaluation was performed by the same specialist before and after renal nerve ablation. BNP levels and renal artery color Doppler were also needed during the interview. MACE were defined as death or rehospitalization owing to aggravated heart failure.
Statistical analysis
Statistical analysis was performed with the SPSS v 13.0 (SPSS lnc. USA). All of the data are expressed as mean ± standard deviation (SD). Continuous variables between the two groups and between baseline and post-procedure were compared by unpaired or paired Student’s t test as appropriate. P < 0.05 was considered statistically significant.
Results
No complications, such as arrhythmia, oliguria, or renal artery dissection, occurred in the patients who received renal nerve ablation.
Changes in urine volume, plasma neuroendocrine hormones and cardiac function
Compared with standard therapy group, the 24-hour urine volume was significantly higher in radiofrequency ablation group 24 hours after radiofrequency ablation therapy (P < 0.05). Levels of plasma renin, aldosterone, angiotensin II, BNP, dopamine, noradrenalin, and adrenalin were significantly lower in the renal radiofrequency ablation therapy group when compared with the standard therapy group. These neurohormones were also significantly lower after radiofrequency ablation therapy when compared to baseline values. There was no difference in heart rate or mean arterial pressure between the two groups at baseline and 24 hours after surgery. The symptoms of heart failure such as dyspnea and edema were significantly improved. After operation, LVEF was significantly increased in renal nerve ablation group (Table 2).
Table 2.
Biochemical and physiological parameters in renal nerve ablation group and Standard therapy group
| Variables | Renal nerve ablation group | Standard therapy group |
|---|---|---|
| HR (beat/min) | ||
| Baseline | 80±7 | 79±5 |
| 24 hours | 78±6 | 79±4 |
| MAP(mmHg) | ||
| Baseline | 100.3±12.1 | 95.1±10.1 |
| 24 hours | 98.1±14.1 | 97.3±13.7 |
| 24-hour urine volume (ml) | ||
| Baseline | 1235±217 | 1145±155 |
| 24 hours | 1505±220#,* | 1135±142 |
| Creatinine (µmol/L) | ||
| Baseline | 105.2±12.2 | 106.5±13.1 |
| 24 hours | 106.3±14.5* | 105.3±12.0 |
| NYHA class | ||
| Baseline | 2.8±0.5 | 2.7±0.4 |
| 24 hours | 2.0±0.4#,* | 2.7±0.5 |
| LVEF% | ||
| Baseline | 38±3 | 38±2 |
| 24 hours | 43±3#,* | 38±3 |
| Plasma renin (µg/(L×h)) | ||
| Baseline | 4.19±1.14 | 4.21±0.85 |
| 24 hours | 2.59±1.18#,* | 4.17±0.87 |
| Plasma ATII (µg/L) | ||
| Baseline | 141.19±17.78 | 139.27±4.58 |
| 24 hours | 87.75±15.79#,* | 137.27±20.38 |
| Plasma ALD (ηg/L) | ||
| Baseline | 150.03±26.54 | 164.20±11.17 |
| 24 hours | 107.36±15.19#,* | 162.15±21.33 |
| Plasma BNP(pg/mL) | ||
| Baseline | 629±131 | 577±92 |
| 24 hours | 460±69#,* | 561±78 |
| Plasma DA (pg/ml) | ||
| Baseline | 72.23±15.80 | 66.93±14.32 |
| 24 hours | 51.51±11.69#,* | 64.91±14.67 |
| Plasma adrenaline (pg/ml) | ||
| Baseline | 76.84±13.94 | 44.96±7.53 |
| 24 hours | 44.96±7.53#,* | |
| Plasma NE (pg/ml) | ||
| Baseline | 238.91±52.06 | 236.96±40.01 |
| 24 hours | 162.80±33.68#,* | 231.89±40.73 |
P < 0.01 vs. baseline;
P < 0.01 vs. standard therapy.
HR: heart rate; MAP: mean arterial blood pressure; LVEF: left ventricular ejection fraction; ATII: angiotensin II; ALD: aldosterone; BNP: brain natriuretic peptide; DA: dopamine; NE: noradrenalin; Data are means ± SD.
Changes in cardiac function, BNP and MACE at 6 month
At the 6-months’ follow-up, LVEF, FS, and the diameter of the left ventricle in the radiofrequency ablation therapy group were significantly higher than those in the standard therapy group. Plasma BNP levels were significantly lower in the radiofrequency ablation therapy group than those in the standard therapy group. There was no significant difference in LVEF or BNP in the standard therapy group in 6 months. No renal arterial stenosis found by B-type ultrasonic in the radiofrequency ablation therapy group. In the radiofrequency ablation therapy group, two patients were readmitted for worsening heart failure, and the mean time for the hospital stay was 5±3 days. However, in the standard therapy group, the number of readmission for worsening heart failure was eight, and the average time for hospital stay was 9±5 days, which was longer compared with radiofrequency ablation therapy group (Table 3).
Table 3.
Changes in cardiac function, BNP and MACE at 6 month follow-up
| Clinical characteristics | Renal nerve blockade | Standard therapy | P values |
|---|---|---|---|
| LVEF (mean ± SD, %) | 45±3 | 38±4 | 0.001 |
| FS (mean ± SD, %) | 24±2 | 19±2 | 0.001 |
| LV (mean ± SD, cm) | 6.0±0.3 | 6.7±0.4 | 0.007 |
| BNP (mean ± SD, pg/ml) | 424±141 | 604±185 | 0.001 |
| MACE (%) | 20% | 80% | 0.024 |
Note: LVEF: left ventricular ejection fraction; BNP: brain natriuretic peptide; MACE: major adverse cardiovascular event; FS: fraction shortening; LV: left ventricular diameter.
Discussion
Our study showed that percutaneous renal sympathetic nerve radiofrequency ablation was safe and effective for a small sample of patients with chronic refractory heart failure. The major findings of the present study were that 24 hours after renal nerve radiofrequency ablation, daily urine output was significantly higher, and plasma renin, angiotensin II, aldosterone, BNP, dopamine, adrenaline, and noradrenaline levels, as well as symptoms of dyspnea and edema, were significantly lower in the radiofrequency ablation group compared with baseline values and the standard therapy group. Blood pressure and heart rate were not significantly different after ablation between the two groups. No complications, such as renal arterial stenosis, renal arterial dissection, or femoral artery pseudoaneurysm, were found in the ablation group. Moreover, the rate of MACE and plasma BNP levels were significantly lower, while LVEF was significantly higher, in the radiofrequency ablation group than in the standard therapy group during follow-up (mean 180 days).
CHF is a complicated clinical syndrome, which results from many types of heart diseases. The mechanism of CHF is related to neurohormonal activation, including increased sympathetic tone and activation of the RAAS [9], which leads to worsening of left ventricular function by increasing ventricular pre- and after-load. Excessively activated sympathetic nerves, especially an increase in activity of renal sympathetic nerves, may aggravate renal vascular resistance, activate the RAAS, increase renin levels and retention of sodium and water, and ultimately lead to diuretic resistance [10]. Diuretic resistance is independently related to the rate of death and fatal heart failure. The risk of diuretic insensitivity increases with the development of heart failure and use of long term diuretics. The superfluous liquid volume within the body cannot be effectively removed from the body because of diuretic resistance, which consequently makes heart failure difficult to control [11]. As a result, activation of the sympathetic nervous system plays an important in the progress and long-term prognosis of CHF. Therefore, lowering sympathetic nerve activity is important for the therapy of advanced CHF [12]. In 2004, a large clinical trial (MOXCON), which lowered activity of sympathetic nerves in the whole body in patients with CHF, failed because of increasing mortality in patients [13]. In this trial, plasma noradrenalin levels were reduced by more than 50%. Therefore, scientists have focused on lowering local sympathetic activity to treat CHF.
Renal sympathetic nerves play an important part in increasing sympathetic activity of the whole body in CHF [14]. Impulses are transmitted through the afferent nerves to the hypothalamus, and this causes a reflex through the afferent nerves to increase sympathetic activity [15]. This increase in sympathetic activity contributes to increased peripheral vascular resistance and vascular remodeling, as well as left ventricular remodeling and dysfunction. Therefore, treating CHF by blocking renal sympathetic nerve may be effective [16]. Recently, our study group tested the effects of blocking renal sympathetic nerve activity in patients with CHF by a single percutaneous injection of local anesthetic under guidance by computed tomography [17]. This technique has successfully been used to block drug resistant flank pain in patients with loin pain and hematuria syndrome. In the studies, after renal nerve blockade, 24 h urine volume was significantly increased, while plasma aldosterone, BNP, renin, angiotensin II, and atrial natriuretic peptide levels, as well as dyspnea and edema, were significantly reduced in the renal nerve blockade group compared with baseline and the standard therapy group. Additionally, the rate of MACE and plasma BNP levels was significantly lower during 3 to 12 months of follow-up However, anesthetic may act only temporarily, and the effects in this study could not last for a long time. Therefore, researchers need to find a new method which is safer, more effective, and can last for a long time, even permanently.
Catheter-based renal denervation may be a useful method, because it could reduce sympathetic outflow and blood pressure [18]. Since the first nerve radiofrequency ablation operation by Krum on hypertension-resistant patients [19], this technique has cured thousands of patients in the past few years. Many patients with hypertension have benefited from this operation [20,21]. Though a blind trial with sham control didn’t show a significant reduction of blood pressure in patients with resistant hypertension by renal nerve radiofrequency ablation [22], it cannot deny the effect of renal denervation by renal-artery radiofrequency ablation. Sympathetic nerve activity was significantly reduced and left ventricular function was improved in some reports on nerve radiofrequency ablation [23]. However, there are few reports on renal denervation in CHF patients. Only a few research institutes are devoted to studying this issue. Therefore, we designed the current experiment to investigate the effects of renal sympathetic nerve radiofrequency ablation in severe heart failure.
Conclusion
In our study with a small sample of patients, we found that diuretic resistance in patients with refractory heart failure was relieved after renal nerve radiofrequency ablation. No complications were found during and after the operation. In conclusion, renal nerve radiofrequency ablation is a safe and effective treatment strategy for patients with refractory heart failure and diuretic resistance. Larger studies are needed to confirm the effects of this strategy.
Acknowledgements
The study and publication cost has been funded by Health Department of Jiangsu Province.
Disclosure of conflict of interest
None.
Abbreviations
- CHF
congestive heart failure
- RAAS
renin-angiotensin-aldosterone system
- LVEF
left ventricular ejection fraction
- ACEIs
angiotensin-converting enzyme inhibitors
- ARBs
angiotensin II receptor blockers
- BNP
B-type natriuretic peptides
- FS
fractional shortening
- MACE
major adverse cardiovascular events
- HR
heart rate
- MAP
mean arterial blood pressure
- ATII
angiotensin II
- ALD
aldosterone
- DA
dopamine
- NE
noradrenalin
References
- 1.Ye S, Gamburd M, Mozayeni P, Koss M, Campese VM. A limited renal injury may cause a permanent form of neurogenic hypertension. Am J Hypertens. 1998;11:723–728. doi: 10.1016/s0895-7061(98)00030-2. [DOI] [PubMed] [Google Scholar]
- 2.Packer M. Neurohormonal interactions and adaptations in congestive heart failure. Circulation. 1988;77:721–730. doi: 10.1161/01.cir.77.4.721. [DOI] [PubMed] [Google Scholar]
- 3.Kon V. Neural control of renal circulation. Miner Electrolyte Metab. 1989;15:33–43. [PubMed] [Google Scholar]
- 4.Asano K, Dutcher DL, Port JD, Minobe WA, Tremmel KD, Roden RL, Bohlmeyer TJ, Bush EW, Jenkin MJ, Abraham WT, Raynolds MV, Zisman LS, Perryman MB, Bristow MR. Selective downregulation of the angiotensin II AT1-receptor subtype in failing human ventricular myocardium. Circulation. 1997;95:1193–1200. doi: 10.1161/01.cir.95.5.1193. [DOI] [PubMed] [Google Scholar]
- 5.Taylor J. The 2012 ESC Guidelines on Heart Failure. Eur Heart J. 2012;33:1703–1704. doi: 10.1093/eurheartj/ehs138. [DOI] [PubMed] [Google Scholar]
- 6.Kirchheim H, Ehmke H, Persson P. Sympathetic modulation of renal hemodynamics, renin release and sodium excretion. Klin Wochenschr. 1989;67:858–864. doi: 10.1007/BF01717340. [DOI] [PubMed] [Google Scholar]
- 7.Id D, Bertog SC, Wunderlich N, Sievert H. Catheter-based renal sympathectomy. J CardiovaseSurg (Tofino) 2010;51:721–739. [PubMed] [Google Scholar]
- 8.Dai Z, Yu S, Zhao Q, Meng Y, He H, Tang Y, Wang X, Xiao J, Wang X, Huang C. Renal sympathetic denervation suppresses ventricular substrate remodelling in a canine high-rate pacing model. EuroIntervention. 2014;10:392–399. doi: 10.4244/EIJV10I3A65. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, Tkacs NC. Current concepts of neurohormonal activation in heart failure: mediators and mechanisms. AACN Adv Crit Care. 2008;19:364–385. doi: 10.1097/01.AACN.0000340718.93742.c4. quiz 386-387. [DOI] [PubMed] [Google Scholar]
- 10.Ye S, Zhong H, Yanamadala V, Campese VM. Renal injury caused by intrarenal injection of phenol increases afferent and efferent renal sympathetic nerve activity. Am J Hypertens. 2002;15:717–724. doi: 10.1016/s0895-7061(02)02959-x. [DOI] [PubMed] [Google Scholar]
- 11.Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-tern outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J. 2005;26:906–913. doi: 10.1093/eurheartj/ehi184. [DOI] [PubMed] [Google Scholar]
- 12.Souza DR, Mill JG, Cabral AM. Chronic experimental myocardial infarction produces antinatriuresis by a renal nerve-dependent mechanism. Braz J Med Biol Res. 2004;37:285–293. doi: 10.1590/s0100-879x2004000200017. [DOI] [PubMed] [Google Scholar]
- 13.Pocock S, Wilhelmsen L, Dickstein K, Francis G, Wittes J. The data monitoring experience in the MOXCON trial. Eur Heart J. 2004;25:1974–1978. doi: 10.1016/j.ehj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Parati G, Esler M. The human sympathetic nervous system: its relevance in hypertension and heart failure. Eur Heart J. 2012;33:1058–1066. doi: 10.1093/eurheartj/ehs041. [DOI] [PubMed] [Google Scholar]
- 15.Mulder J, Hökfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol. 2013;304:R675–682. doi: 10.1152/ajpregu.00599.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raphael CE. Renal denervation: potential indications and review of trial data. Clin Med. 2014;6:s38–40. doi: 10.7861/clinmedicine.14-6-s38. [DOI] [PubMed] [Google Scholar]
- 17.Dai QM, Fen Y, Lu J, Ma GS. Efficacy of regional renal nerve blockade in patients with chronic refractory heart failure. Chin Med J (Engl) 2013;126:1076–1080. [PubMed] [Google Scholar]
- 18.Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension. 2014;64:118–124. doi: 10.1161/HYPERTENSIONAHA.113.03098. [DOI] [PubMed] [Google Scholar]
- 19.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension. A multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 20.Pancholy SB, Shantha GP, Patel TM, Sobotka PA, Kandzari DE. Meta-analysis of the effect of renal denervation on blood pressure and pulse pressure in patients with resistant systemic hypertension. Am J Cardiol. 2014;114:856–861. doi: 10.1016/j.amjcard.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Hamza M, Khamis H. Renal sympathetic denervation for treatment of resistant hypertension: Egyptian experience. J Interv Cardiol. 2014;27:423–427. doi: 10.1111/joic.12135. [DOI] [PubMed] [Google Scholar]
- 22.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 23.Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, Hamady M, Hughes AD, Sever PS, Sobotka PA, Francis DP. First-in-man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH-Pilot study. Int J Cardiol. 2013;162:189–192. doi: 10.1016/j.ijcard.2012.09.019. [DOI] [PubMed] [Google Scholar]


