Abstract
Background: Normal saline is commonly used for rinsing the abdominal cavity and many surgeons claim that it is not harmful to peritoneum. We found in patients treated with laparoscopic surgery, that mean 25% of the instilled fluid is not drained and dwells in the abdominal cavity. Therefore we evaluated changes of the saline biocompatibility during its dwell in the rats abdominal cavity. Methods: In 10 anesthetized rats normal saline were instilled into the abdominal cavity and samples of the dwelling solution were collected every 30 minutes, for 4 hours. Inflammatory parameters and effect of the collected samples on in vitro cultured rats mesothelial cells were studied. Results: Low pH of the saline was normalized, but number of cells and % of macrophages and eosinophils, as well as elastase activity and MCP-1 and TGF-β concentration increased, proportionally to the dwell time. Fluid samples tested ex-vivo suppressed proliferation of the mesothelial cells and induced biphasic (stimulation/inhibition) effect on synthesis of MCP-1 in these cells. Similar pattern of release was observed for TF, whereas synthesis of t-PA in the mesothelial cells was strongly suppressed. Conclusions: Mesothelial cells exposed in vivo to normal saline dwelling in the abdominal cavity acquire properties which may accelerate formation of the peritoneal adhesions.
Keywords: Normal saline, mesothelium, inflammation, coagulation, fibrinolysis
Introduction
One of the potential complication of the surgical procedures is formation of the peritoneal adhesions. Therefore during surgery all precautions are taken to reduce damage to the tissues and to wash-out residual blood, clots and tissue debris. There is no standard solution used for that purpose and often 0.9% NaCl solution, called normal saline is used for that purpose. We found in our previous in vitro studies, that normal saline induces oxidative stress in the mesothelial cells with subsequent release of tissue factor [1]. Buffered solutions such as Earles or Hanks solutions are more biocompatible for mesothelial cells than normal saline [2].
The main criticism of the in vitro experiments on the mesothelial cells is that contrary to in vivo conditions exposure of the cells to the tested fluid is long and its composition is steady. Critics of the in vitro experiments claim that during rinsing of the abdominal cavity fluid stays in the peritoneal cavity only for a short period of time and afterwards is removed and additionally, instantly after infusion its composition is changing. However due to anatomy of the abdominal cavity it is not probable that all fluid instilled into the peritoneal cavity can be drained out and one can assume that some fluid is left between the intestinal loops, specially it can drain down by gravity to the lowest areas of the abdomen. Changes of the fluid composition during its dwell in the abdominal cavity not necessarily make it more biocompatible. Normal saline solution has nonphysiological concentrations and ratio of sodium and chloride, its osmolality is slightly higher than osmoality of the body fluids and its pH is strongly acidic. Despite the fact that cytotoxic effect of 0.9% NaCl solution was described already in the very beginning of the 20th century [3], it is still considered as the physiological solution. However even in conditions of the peritoneal dialysis, when the infused dialysis fluids dwell in the peritoneal cavity for at least 4 hours, use of 0.9% NaCl as the dialysis fluid does not prevent peritoneal injury and it is even stronger than in presence of hypertonic fluids [4,5].
The goal of the present study was description how composition of the 0.9% NaCl solution is changing during its dwell within the abdominal cavity and how these changes affect biocompatibility of that fluid.
Material and methods
The experimental protocol was approved by the Animal Ethics Committee of the author’s institution. Study was performed on 10 male rats with a mean body weight 388 ± 22 g. Anesthesia was induced with ether and maintained with neuroleptoanalgesia fentany l0,004 mg/100 gr. and droperidol 0.2 mg/100 gr. After shaving the abdomen, which afterwards was covered with sterile adhesive 3M TegadermTM Medical-Surgical Division, (St. Paul, USA), abdominal cavity was opended with 2 cm midline incision. Then 30 mL of the sterile 0.9% NaCl solution, preheated to 37°C was instilled into the peritoneal cavity. The opening of the abdominal cavity was temporarily closed with sterile clamp. Every 30 minutes the abdomen was reopened and 2 ml of the fluid sample were collected for further analysis.
Studied fluid parameters
Cell count and differential count were evaluated in fluid samples. Also pH of the fluid was measured. Protein concentration in the fluid was measured with Lowry method [6] and elastase activity with a colorimetric method using substrate N-t-BOC-L-alanine-p-nitrophenyl esther (Sigma Chemical Co) [7]. Additionally concentrations of MCP-1, VEGF and TGF-β were measured in the fluid samples with commercially available ELISA kits from Biosource Europe S.A. (Nivelles, Belgium).
In vitro testing of the fluid samples
Fluid samples obtained 30’, 60’, 120’ and 240’ after intraperitoneal infusion of the 0.9% NaCl solution were tested in vitro on primary cultures of the rat’s peritoneal mesothelium. Cultures of the mesothelial cells were obtained from rats in which, under ether anesthesia, abdominal cavity was treated during 30 minutes with trypsin 0.05%-EDTA 0.02% solution. Detached cells, floating in the trypsin solution were spun, washed twice in culture medium M199 supplemented with 10% fetal calf serum- FCS (GIBCO, Invitrogen Life Technologies, Paisley UK). Afterwards the cells were cultured at 37°C in 5% CO2 atmosphere, in 25 cm2 culture flasks (Nunc A/S, Denmark) until their monolayer was established. Then mesothelial cells were harvested with trypsin 0.05%-EDTA 0.02% solution, washed with medium M199 + 10% FCS and reseeded into 48-wells culture clusters (Nunc A/S, Denmark). Effect of the fluids samples on the in vitro growth of the cells and on synthesis of MCP-1, t-PA and Tissue Factor (TF) by mesothelial cells treated with the fluids samples were studied. Concentration of t-PA was measured with ELISA kit from Oxford Biomedical Research (Rochester Hill, MI, USA). Concentration of TF was measured with ELISA kit from American Diagnostic Inc. (Stamford, CT, USA).
For growth experiment cells were seeded into 48-wells cluster at density 2.5 × 104/well and after 18 hours culture medium was removed from the wells and replaced with culture medium or culture medium mixed 1:1 (v/v) with 0.9% NaCl or 0.9% NaCl samples collected after their abdominal dwell ( pH of all samples was adjusted to 7.4). Additionally 3H-methyl-thymidine was added to each well to get its final concentration 1 µ Ci/mL. After 24 hours incubation cell were harvested from the wells with trypsin 0.05%-EDTA 0.02% solution, precipitated with 20% trichloroacetic acid (TCA). The cells precipitate was washed twice with TCA and afterwards lysed in 0.1 NaOH. Radioactivity of the cells lysate was measured in a β-scintillation counter. Incorporation of the radiolabelled 3H-methyl-thymidine into DNA of the growing cells was used as an index of their proliferation.
Synthesis of MCP-1, t-PA and TF in mesothelial cells was studied in the cells monolayer, which were exposed to culture medium or to culture medium mixed 1:1 (v/v) with 0.9% NaCl or with the studied 0.9% NaCl samples collected after their abdominal dwell ( pH of all samples was adjusted to 7.4). After 24 hours incubation supernatant was collected from the wells and stored at -80°C, for measurement of MCP-1, t-PA or TF. Cells were lysed with 0.1N NaOH and total protein concentration in the cells lysate was measured with Lowry method. Release of MCP-1, t-PA or TF from the cells was expressed per amount of cell protein.
Results are presented as mean ± SD. Analysis of the results was performed with Friedman test or with Kruskal-Wallis test with post hoc analysis with Dunn’s test. A p value 0.05 was considered statistically significant.
Results
Composition of the intraperitoneally instilled 0.9% NaCl solution was gradually changing during its dwell in the abdominal cavity, although these changes were not very rapid. Value of the fluid’s pH was still acidic until the 2nd hour of its dwell in the abdominal cavity (Figure 1). At the same time fluids protein concentration, elastase activity and cell counts were gradually increasing (Figure 1). Concentrations of sodium and chloride were gradually decreasing reaching after 4 hours mean concentration of 142.1 ± 5.0 mmol/L and 120.9 ± 6.2 mmol/L, respectively. Concentration of potassium reached after 4 hours mean value of 4.1 ± 0.4 mmol/L. There was not only increase in cell count but also differential count was changed: as compared to the empty abdominal cavity amount of macrophages and eosinophils was increased, whereas percentage of neutrophils and lymphocytes was reduced (Figure 2). Concentration of MCP-1 and TGF-β in the dwelling fluid was gradually increasing over the time, but there was no significant change in VEGF level (Figure 3).
Figure 1.
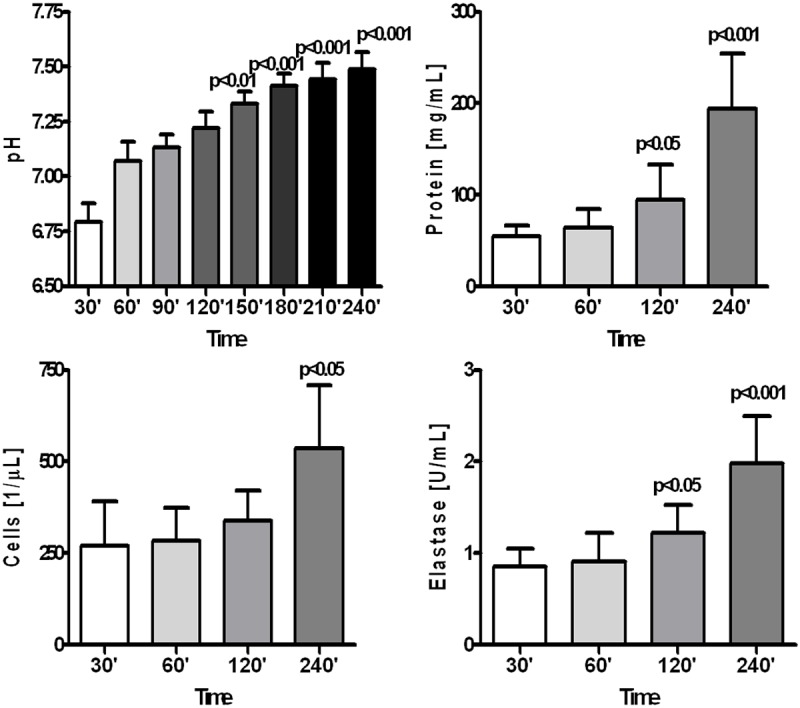
Changes in pH, total protein concentration, cell count and elastase activity in 0.9% NaCl solution dwelling in the peritoneal cavity of rats during 240 minutes.
Figure 2.
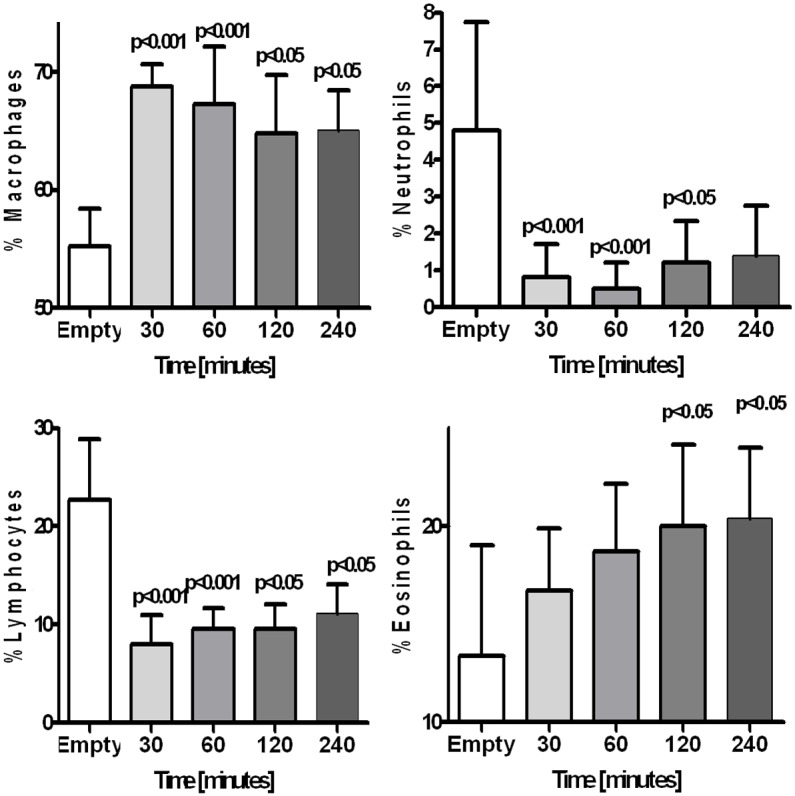
Changes in percentage of macrophages, neutrophils, lymphocytes and eosinophils in 0.9% NaCl dwelling in the rats peritoneal cavity up to 240 minutes, compared to empty peritoneal cavity (Time 0 minutes).
Figure 3.
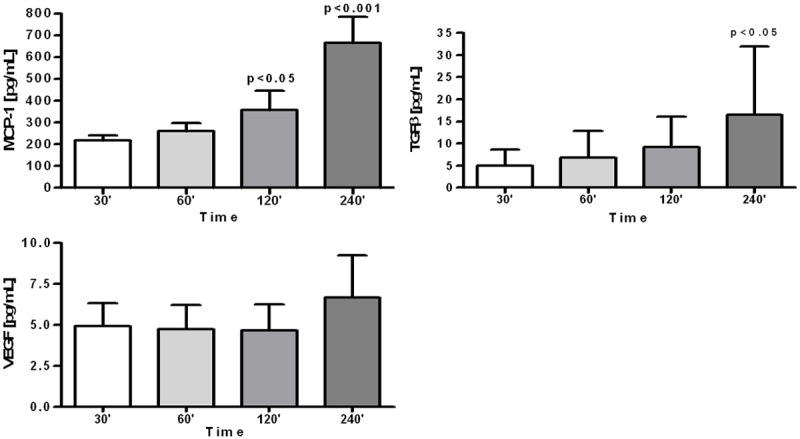
Concentration of monocyte themoattractant protein-1 (MCP-1), transforming growth factor β (TGF-β) and vascular endothelial growth factor (VEGF) in the 0.9% NaCl solution dwelling in the rats peritoneal cavity up to 240 minutes.
Fluid samples obtained after various time of dwell in the peritoneal cavity were tested on the rats mesothelial cells maintained in in vitro culture. They suppressed proliferation of the mesothelial cells and that effect was proportional to the dwell time (Figure 4A). Synthesis of MCP-1 was initially stimulated by collected fluid samples, with the maximal effect observed with samples obtained after 60 minutes dwell, but that effect was decreasing when samples obtained after longer dwell were tested (Figure 4B). Collected fluid samples stimulated release of TF from the mesothelial cells and that effect was the strongest with unused 0.9% NaCl fluid, but was still increased (+ 38%, P<0.05 vs. medium) with saline samples collected from the abdominal rats cavity after 120 minutes dwell (Figure 5A). Exposure of the mesothelial cells to 0.9% NaCl strongly suppressed release of t-PA (-58%, P<0.001, vs medium) and that effect was even stronger with the fluids samples collected after intraabdominal dwell of saline up to 240 minutes (Figure 5B).
Figure 4.
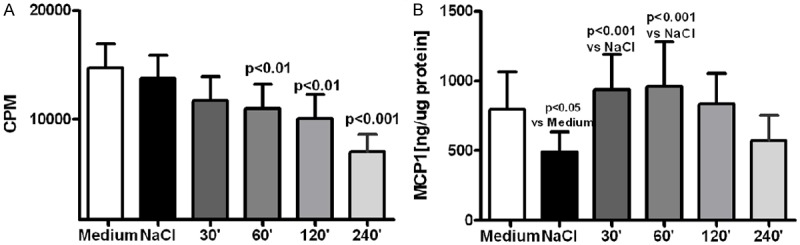
Proliferation of in vitro cultured rat mesothelial cells (A) and synthesis of MCP-1 in these cells (B) when exposed to standard culture medium, medium mixed with 0.9% NaCl (1:1, v/v) or with samples of 0.9% NaCl solution obtained after their dwell in the rats abdominal cavity up to 240 minutes.
Figure 5.
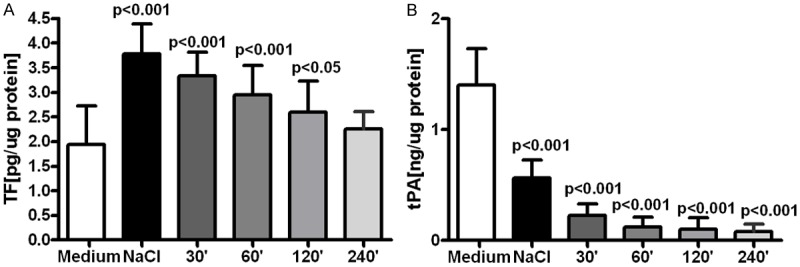
Release of TF (A) and t-PA (B) from the mesothelial cells in in vitro culture exposed to standard culture medium, medium mixed with 0.9% NaCl (1:1 v/v) or with samples of 0.9% NaCl solution obtained after their dwell in the rats abdominal cavity up to 240 minutes.
Discussion
Normal saline is routinely used for washing the abdominal cavity. Despite its unphysiological composition potential side effect of its use are neglected because it is often assumed that instilled solution is removed from the peritoneal cavity within minutes since its infusion. However total drainage of the instilled fluid is not always possible and one can assume that some volume of that solution dwells longer in the abdominal cavity. We estimated, based on observations done in 45 patients undergoing laparoscopic surgery, that mean 25% of the infused solution is not drained from the abdominal cavity and probably undergoes gradual absorption. Results of our study show, that composition of the 0.9% NaCl fluid is changing during its dwell in the abdominal cavity, however these changes are not rapid and its pH is acidic even 2 hours since its infusion. Additionally activity of elastase increased in the dwelling fluid, what reflects intraperitoneal inflammatory reaction. Solution dwelling within the peritoneal cavity is enriched with cells migrating from the blood stream. Observed increased in percentage of eosinophils is suggesting peritoneal allergic reaction, observed also in some patients at the beginning of treatment with peritoneal dialysis [8]. In previous studies in animals it was shown that saline lavage of the abdominal cavity induces eosinophilia in animals [9]. Hellman et al. found that recruited eosinophils in peritoneal dialysis fluid demonstrate altered phenotype, showing signs of activation [10]. Edelstam et al. suggested that increased number of eosinophils in the peritoneal cavity is a potential factor to formation of the peritoneal adhesions [11].
In the 0.9% NaCl fluid dwelling in the peritoneal cavity during 4 hours significant increase of MCP-1 and TGF-β was observed (Figure 3). In women with endometriosis there was a significant correlation between peritoneal MCP-1 level and adhesions scores [12]. Treatment of mice with anti-MCP-1 antibody reduced formation of adhesions and surgery within the abdominal cavity [13]. Also TGF-β is considered as a factor promoting formation of the peritoneal adhesions [14] and its effect may be due reduction of the mesothelial cells fibrinolytic activity [15]. We can conclude that peritoneal lavage with normal saline does not prevent appearance within the abdominal of cytokines promoting formation of the peritoneal adhesions.
Results from the ex-vivo experiments prove that despite gradual normalization of the normal saline pH and electrolytes concentration that solution is not becoming more biocompatible towards the mesothelial cells. Proliferation of the mesothelial cells was inhibited by the fluid samples collected from the abdominal cavity, and that effect was proportional to the length of the dwell time (Figure 4). Also synthesis of MCP-1 was only initially stimulated, when compared with the unused 0.9% NaCl solution, but that effect was seen only with fluid samples which dwelled in the abdominal cavity no longer than 60 minutes (Figure 4). Lack of significant stimulation of MCP-1 synthesis in mesothelial cells seems to contradict observed increased with time concentration of that chemokine in the dwelling saline solution (Figure 3). However MCP-1 may be produced in these rats by peritoneal macrophages stimulated with elastase [16]. Similar to transient increase of MCP-1 release from the mesothelial cells was enhanced release by these cells of TF (Figure 5A). Bottled KD et al. described that inflammation stimulates increased release of TF from the mesothelium what may predispose to formation of adhesions [17]. Our results showing strongly suppressed release of t-PA from the mesothelial cells exposed not only to the unused 0.9% NaCl solution, but even stronger effect in presence of fluid samples collected after 240 minutes of the intraabdominal dwell (Figure 5B) suggest that impaired fibrinolytic activity may further predispose in such conditions to formation of adhesions. Such effect was probably due increasing TGFβ concentration in the fluid samples (Figure 3), which suppresses expression of t-PA in the mesothelial cells [18].
One can assume therefore that functional properties of the mesothelial cells become suppressed due to their exposure to normal saline solution dwelling in the abdominal cavity, what may promote formation of the peritoneal adhesions.
In conclusion, we demonstrated that 0.9% NaCl solution, despite changes in its composition during dwelling in the abdominal cavity, is becoming less biocompatible towards the mesothelial cells. Therefore we think that normal saline solution should not be used for peritoneal lavage, because induced by that fluid dysfunction of the mesothelial cells may accelerate formation of the peritoneal adhesions.
Disclosure of conflict of interest
None.
References
- 1.Połubinska A, Breborowicz A, Staniszewski R, Oreopoulos DG. Normal saline induces oxidative stress in peritoneal mesothelial cells. J Pediatr Surg. 2008;43:1821–1826. doi: 10.1016/j.jpedsurg.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Połubinska A, Winckiewicz M, Staniszewski R, Breborowicz A, Oreopoulos DG. Time to reconsider saline as the ideal rinsing solution during abdominal surgery. Am J Surg. 2006;192:281–285. doi: 10.1016/j.amjsurg.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 3.Cushing H. Concerning the poisonous effect of poisonous effect of pure sodium chloride solutions upon the nerve-muscle preparation. Am J Physiol. 1901;6:77–90. [Google Scholar]
- 4.Wang T, Heimburger O, Quereshi AR, Waniewski J, Bergström J, Lindholm B. Physiological saline is not a biocompatible peritoneal dialysis solution. Int J Artif Organs. 1999;22:88–93. [PubMed] [Google Scholar]
- 5.Styszyński A, Podkowka R, Wieczorowska-Tobis K, Kwiatkowska B, Ksiazek K, Breborowicz A, Oreopoulos DG. Glucose suppresses peritoneal inflammatory reactions and mesothelial hyperplasia caused by intraperitoneal saline infusion. Adv Perit Dial. 2002;18:21–25. [PubMed] [Google Scholar]
- 6.Lowry OH, Rosenbrough NJ, Farr AL. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 7.Akatsuka M, Yamamoto Y, Tobetto K, Yasui T, Ando T. Suppressive effects of hyaluronic acid on elastase release from rat peritoneal leukocytes. J Pharm Pharmacol. 1993;45:110–114. doi: 10.1111/j.2042-7158.1993.tb03693.x. [DOI] [PubMed] [Google Scholar]
- 8.Ejaz AA, Fitzpatrick PM, Durkin AJ, Wasiluk A, Haley WE, Goalen MJ, Ing TS, Zachariah PK. Pathophysiology of peritoneal fluid eosinopilia in peritoneal dialysis patients. Nephron. 1998;81:125–130. doi: 10.1159/000045266. [DOI] [PubMed] [Google Scholar]
- 9.Lindor LJ, Loegering DA, Wassom DL, Gleich GJ. Recovery of eosinophils from the peritoneal cavity of the guineas pig. J Immunol Methods. 1981;41:125–134. doi: 10.1016/0022-1759(81)90280-5. [DOI] [PubMed] [Google Scholar]
- 10.Hellman C, Lundahl J, Hylander B, Hallden G. Phenotypic alterations of recruited eosinophils in peritoneal fluid eosinophilia. Perit Dial Int. 2000;20:295–300. [PubMed] [Google Scholar]
- 11.Edelstam G, Fredens K, Venge P. Role of eosinophilic granulocytes in women with infertility and pelvic adhesions. Inflammation. 1994;18:361–370. doi: 10.1007/BF01534434. [DOI] [PubMed] [Google Scholar]
- 12.Zeyneloglu HB, Senturk LM, Seli E, Oral E, Olive DL, Arici A. The role of monocyte chemotactic protein-1 in intraperitoneal adhesion formation. Hum Reprod. 1998;13:1194–1199. doi: 10.1093/humrep/13.5.1194. [DOI] [PubMed] [Google Scholar]
- 13.Zeyneloglu HB, Seli E, Senturk LM, Gutierrez LS, Olive DL, Arici A. The effect of monocyte chemotactic protein 1 in intraperitoneal adhesion formation in a mouse model. Am J Osbtet Gynecol. 1998;179:438–443. doi: 10.1016/s0002-9378(98)70376-x. [DOI] [PubMed] [Google Scholar]
- 14.Chegini N. TGF-beta system: the principal profibrotic mediator of peritoneal adhesion formation. Semin Reprod Med. 2008;26:298–312. doi: 10.1055/s-0028-1082388. [DOI] [PubMed] [Google Scholar]
- 15.Falk P, Ma C, Chegini N, Holmdahl L. Differential regulation of mesothelial cell fibrinolysis by transforming growth factor beta 1. Scand J Clin Lab Invest. 2000;60:439–447. doi: 10.1080/003655100448419. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara K, Yamaguchi Y, Okabe K, Ogawa M. Neutrophil elastase enhances macrophage production of chemokines in receptor-mediated reaction. Res Commun Mol Pathol Pharmacol. 1999;103:139–147. [PubMed] [Google Scholar]
- 17.Bottels KD, Laszik Z, Morissey JH, Kinasewitz GT. Rissue factor expression in mesothelial cells: induction both in vivo and in vitro. Am J Respir Cell Moll Biol. 1997;17:164–172. doi: 10.1165/ajrcmb.17.2.2438. [DOI] [PubMed] [Google Scholar]
- 18.Falk P, Ma C, Chegini N, Holmdahl L. Differential regulation of mesothelial cel fibrinolysis by transforming growth factor beta 1. Scand J Clin Lab Invest. 2000;60:439–447. doi: 10.1080/003655100448419. [DOI] [PubMed] [Google Scholar]


