Abstract
Background: Accumulated studies have revealed that vascular endothelial growth factor (VEGF) plays an essential role in the progression of glioma, but the prognostic significance of VEGF expression for patients with glioma remains unknown. Method and material: A literature search of public databases (PubMed, ISI Web of Science, Science Direct, Cochrane Central Register of Controlled Trials, Wiley Online Library, China National Knowledge Infrastructure, China Biology Medicine disc, Chongqing VIP and Wan Fang Data) was conducted. A meta-analysis was performed to evaluate the association between the overexpression of VEGF and the survival for the glioma patients. Subsequently we evaluated the impact of VEGF expression on the pathological grade of glioma. Results: A total of 32 articles with 2307 cases contributed to this analysis, of which 31 reported overall survival (OS) and 5 reported progression-free survival (PFS). In this meta-analysis, VEGF overexpression significantly identified the unfavorable outcome on OS (HR = 1.647, 95% CI: 1.324~2.048, P < 0.001, Z = 4.48) but not on PFS (HR = 1.021, 95% CI: 0.974~1.070, P = 0.393). Subgroup analyses also revealed that high level of VEGF was associated with the poor OS for the patients with glioma according to region, case number, specimen type, method to detect VEGF and statistical method. Furthermore, the significant correlation was achieved between VEGF expression and the pathological grade of glioma (r = 0.307, P < 0.001). Conclusion: This study suggests that VEGF expression is significantly correlated with the glioma progression and may be a valuable prognostic factor on OS for the glioma patients.
Keywords: Vascular endothelial growth factor, glioma, prognosis, meta-analysis
Introduction
Glioma, the most prevalent intracranial neoplasia in adults, is classified as grade I to grade IV for its differentiation, based on the World Health Organization (WHO) criteria. Studies have pointed to an increasing incidence of gliomas over the past few decades. Though diagnostic and therapeutic techniques have been improved tremendously in last ten years, the survival of patients with malignant glioma remains still poor. Several prognostic factors are well established for glioma patients, such as isocitrate dehydrogenase 1 (IDH1), P53, epidermal growth factor receptor (EGFR), and Ki-67 [1-4]. Nevertheless, there is a compelling demand to explore more prognostic markers to prolong the survival of glioma patients.
Vascular endothelial growth factor (VEGF) is an endothelial, cell-specific mitogen, which acts as a prime mediator in angiogenesis. As a critical pro-angiogenic factor, VEGF is also involved in carcinogenesis and metastasis in cancers [5,6]. Increasing evidence suggested that VEGF is a prognostic factor of many cancers, such as lung cancer, hepatocellular carcinoma, gastric cancer, colon cancer and osteosarcoma [7-11], but the predictive value of VEGF on the survival for glioma patients has not been clarified. Recent studies have focused on the interaction between glioblastoma cells and blood vessels. Bevacizumab, an angiogenesis inhibitor, has been observed to increase PFS in patients with glioblastoma multiforme, by inhibiting both VEGF and vascular permeability [12]. Evidence is accumulating that VEGF may play a vital role in the progression of glioma, but the potential of VEGF as a prognostic marker for glioma remains dismal. In the light of the previous studies, we performed a meta-analysis to explore the prognostic value of VEGF for glioma patients.
Materials and methods
Search strategy
An electronic literature search was conducted in PubMed, ISI Web of Science, Science Direct, Cochrane Central Register of Controlled Trials and Wiley Online Library, which are English Databases up to 28th March 2015. For Chinese Databases, the search identified the eligible studies in China National Knowledge Infrastructure (CNKI), China Biology Medicine disc (CBM), Chongqing VIP and Wan Fang Data. The search was based on the keywords as follows: (“vascular endothelial growth factor” or “VEGF” or “VEGFA” or “VEGFB” or “VEGFC” or “VEGFD” or “angiogenesis” or “bevacizumab” or “endostatin”) and (“glioma” or “astrocytoma” or “oligodendroglioma” or “oligoastrocytoma” or “ependymoma” or “glioblastoma” or “gliomatosis cerebri” or “brain cancer” or “brain neoplasm” or “brain tumor” or “GBM” or “AA” or “AO” or “DIPG” ) and (“prognos*” or “surviv*” or “follow-up studies” or “mortality” or “incidence” or “predict” or “outcome”).
Selection criteria
All eligible studies were included by the following criteria: (1) glioma patients should be affirmed pathologically; (2) the association between VEGF expression and OS or PFS should be evaluated for glioma patients; (3) a hazard ratio (HR) should be provided or the sufficient data should be available to calculate a HR for OS or PFS; (4) it should be the most recent or complete study if the same patient cohort were reported more than once by the same authors or research group; (5) it should be written in either Chinese or English in full text.
The studies were considered ineligible by the following exclusion criteria: (1) review, experimental studies, conference abstracts, expert opinion or case report; or (2) no sufficient data for calculating the HR.
Data extraction and assessment of study quality
Two authors (WJ Chen and Xin Zhang) reviewed all of the included studies independently and extracted the following data: first author’s name, publish year, region, case number, WHO grade, test method, specimen type, survival, HR and statistical method. We labeled the data without reporting the above contents as “not applicable”. Each discrepancy was resolved by discussion and consensus among the authors. Newcastle-Ottawa quality assessment scale was used to assess the quality of each study.
Statistical analysis
The relationship of VEGF expression with the pathological grade of glioma was analyzed by using a two-sided Chi-square test and spearman’s rank correlation. HRs and 95% CIs were used to estimate overall effects for survival outcomes. Study region, case number, specimen type, VEGF test method, statistical method were analyzed for subgroup analyses. HR greater than 1 with 95% CI not overlapping 1 indicates a poor prognosis for the VEGF-positive group. The Z test was used to determine the significance of the combined HR (P < 0.05 was considered as statistically significant).We used the methods described by Parmar et al. and the software Engauge Digitizer Version4.1 (http://digitizer.sourceforge.net/) when the studies provided Kaplan-Meier survival curves but no HRs with 95% CIs [13]. Moreover, the multivariate HRs and 95% CIs were combined when multivariate and univariate analyses of OS and/or PFS were available in the same study, which could better reveal the influence of multiple factors on the survival response. The Q-statistic was selected to test the statistical heterogeneity. The random-effects model was used when the Q-test reported a P value < 0.05 by using the method described previously [14]. Otherwise, the fixed-effects model (Mantel-Haenszel method) [15] was selected. We also used the I2-statistic to calculate heterogeneity (I2 less than 25%, no heterogeneity; I2 = 25-50%, moderate heterogeneity; and I2 greater than 50%, large or extreme heterogeneity). Publication bias was estimated by a funnel plot and Egger’ test [16,17]. All two-sided P values less than 0.05 were considered to be significant. SPSS20 and STATA version 12.0 software were used for the statistical calculation.
Results
Literature search and characteristics of included studies
The flow diagram for the study selection process was depicted in Figure 1. In total, 32 studies were included in the analysis, of which 31 reported the OS and 5 reported the PFS for glioma patients [18-49]. These 32 studies published between 2000 and 2015 include 2307 cases, among which 5 studies [19,22,25,36,49] were in Chinese. In the included studies, ten studies [19,25,29,30,36,40,41,45,46,49] with 713 cases reported the of VEGF overexpression with the pathological grade of gliomas. In total, 17 Asian studies, 8 European studies, 5 American studies and 1 African study were included in the current meta-analysis. The characteristics of included studies were presented in Table 1.
Figure 1.
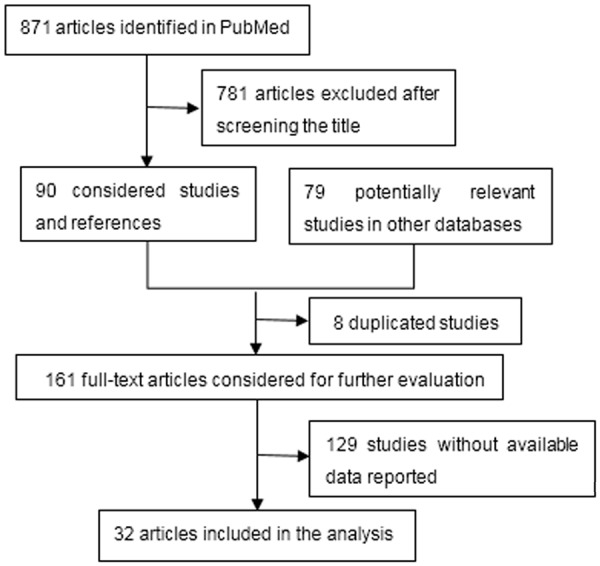
A Flow Diagram for the Study Selection Process.
Table 1.
The characteristics of included studies in the meta-analysis
| Author and year | Region | Case | Grade | Specimen type | Assay | HR (95% CI) | Outcome | Survival | Method | quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Bian 2000 | China | 48 | I-IV | Tumor tissues | IHC | 6.625 (0.875, 50.230) | NS* | OS | Survival Curve | 6 |
| Zhong 2001 | China | 94 | I-IV | Tumor tissues | IHC | 3.876 (1.408, 10.750) | Poor | OS | HR (multivariate) | 7 |
| Hara 2004 | Japan | 100 | II-IV | Tumor tissues | IHC | 0.904 (0.463, 1.765) | NS | OS | HR (multivariate) | 7 |
| Liu 2004 | China | 50 | I-IV | Tumor tissues | IHC | 4.275 (0.816, 22.390) | NS | OS | HR (univariate) | 6 |
| Nam 2004 | Korea | 26 | IV | Tumor tissues | RT-PCR | 3.175 (0.858, 11.740) | NS | OS | Original Data | 7 |
| Zhou 2005 | China | 87 | III-IV | Tumor tissues | qRT-PCR | 1.226 (0.390, 3.595) | NS | OS | HR (multivariate) | 3 |
| Buccoliero 2006 | Italy | 43 | IV | Tumor tissues | IHC | 1.562 (0.717, 3.405) | NS | OS | Original Data | 7 |
| Cheng 2006 | China | 60 | I-IV | Tumor tissues | IHC | 2.114 (1.054, 4.255) | Poor | OS | HR (univariate) | 6 |
| Carlson 2007 | USA | 71 | III-IV | Tumor tissues | RT-PCR | 4.340 (2.240, 8.430) | Poor | OS | HR (univariate) | 6 |
| Sathornsumetee 2008 | USA | 68 | III-IV | Tumor tissues | IHC | 1.180 (0.500, 2.830) | NS | OS | HR (multivariate) | 7 |
| Flynn 2008 | USA | 62 | IV | Tumor tissues | IHC | 1.840 (1.060, 3.210) | Poor | OS | HR (multivariate) | 7 |
| Zeng 2009 | China | 56 | I-IV | Tumor tissues | IHC | 1.070 (0.540, 1.770 | NS | OS | Survival Curve | 6 |
| Yoo 2010 | Korea | 76 | I-IV | Tumor tissues | IHC | 1.021 (0.574, 1.815) | NS | OS | HR (multivariate) | 6 |
| Piperi 2011 | Greece | 97 | II-IV | Tumor tissues | IHC | 0.974 (0.543, 1.749) | NS | OS | HR (multivariate) | 7 |
| Saetta 2011 | Greece | 60 | II-IV | Tumor tissues | IHC | 1.007 (0.991, 1.023) | NS | OS | HR (multivariate) | 5 |
| El-Sayed 2011 | Egypt | 26 | I-IV | Tumor tissues | IHC | 17.074 (3.491, 83.520) | Poor | OS | Original Data | 7 |
| BeriNAan-Neagoe 2012 | Romania | 14 | IV | Tumor tissues | RT-PCR | 0.910 (0.180, 4.640) | NS | OS | HR (NA*) | 6 |
| Castells 2012 | Spain | 71 | IV | Tumor tissues | RT-PCR | 1.631 (0.955, 1.663) | NS | OS | HR (multivariate) | 6 |
| Fan 2012 | China | 62 | II-IV | Tumor tissues | IHC | 1.710 (0.770, 3.783) | NS | OS | Survival Curve | 6 |
| Smith 2012 | UK | 79 | III-IV | Tumor tissues | IHC | 0.559 (0.291, 1.077) | NS | OS | HR (multivariate) | 7 |
| Cao 2013 | Japan | 22 | I-IV | Tumor tissues | IHC | 2.748 (0.321, 23.560) | NS | OS | Original Data | 7 |
| Shin 2013 | Korea | 67 | IV | Tumor tissues | IHC | 1.010 (0.500, 2.040) | NS | OS | HR (multivariate) | 6 |
| Xu 2013 | China | 88 | I-IV | Tumor tissues | IHC | 0.560 (0.191, 1.641) | NS | OS | HR (multivariate) | 6 |
| Xu 2013 | China | 80 | NA | Tumor tissues | IHC | 1.830 (0.903, 3.713) | NS | OS | Survival Curve | 6 |
| Xu 2013 | China | 36 | NA | Tumor tissues | IHC | 3.310 (0.560, 19.500) | NS | OS | Survival Curve | 6 |
| Jensen 2013 | USA | 18 | III-IV | Tumor tissues | ELISA | 8.727 (1.375,55.350) | Poor | OS | HR (univariate) | 6 |
| Tabouret 2013 | France | 26 | II-IV | Blood | ELISA | 3.170 (1.193, 8.422) | Poor | OS | HR (multivariate) | 7 |
| Jensen 2013 | USA | 18 | III-IV | Tumor tissues | ELISA | 0.460 (0.160, 1.373) | NS | PFS | HR (univariate) | 6 |
| Krauze 2013 | USA | 202 | IV | Urine | ELISA | 1.001 (0.998, 1.005) | NS | PFS | HR (multivariate) | 3 |
| Shin 2013 | Korea | 67 | IV | Tumor tissues | IHC | 1.550 (0.790, 3.020) | NS | PFS | HR (multivariate) | 5 |
| Tabouret 2013 | France | 26 | II-IV | Blood | ELISA | 2.822 (1.088, 7.321) | Poor | PFS | HR (multivariate) | 5 |
| Chinorean 2014 | Romania | 14 | IV | Blood | ELISA | 2.340 (0.580, 9.440) | NS | OS | HR (NA) | 6 |
| Nambirajan 2014 | India | 126 | I-III | Tumor tissues | IHC | 1.200 (0.300, 4.200) | NS | OS | HR (multivariate) | 6 |
| Clara 2014 | Brazil | 208 | IV | Tumor tissues | IHC | 1.940 (1.223, 3.078) | Poor | OS | HR (multivariate) | 7 |
| Takano 2014 | Japan | 37 | III-IV | Blood | ELISA | 3.480 (1.546, 7.840) | Poor | OS | Survival Curve | 6 |
| McLeNAon 2015 | USA | 22 | I-III | Tumor tissues | IHC | 1.038 (1.010, 1.068) | Poor | PFS | HR (univariate) | 4 |
NA for not applicable, NS for not significant.
Meta-analysis
Thirty-one studies provided the sufficient data evaluable for OS in this meta-analysis. VEGF positive expression conferred the poor OS for glioma patients with HR = 1.647 (Z=4.48; Figure 2). The heterogeneity was found for the pooled HR for OS (I2 = 72.3%, P < 0.001). However, the prognostic effect of VEGF positive expression for glioma patients was not significant in PFS analysis group with the pooled HR of 1.021 (95% CI: 0.974~1.070, P = 0.393, Z = 0.860; I2 = 72.6%, P=0.006; Figure 3) with 5 studies included. Additionally, we divided patients into different subgroups for OS classified by region, case number, specimen type, VEGF test method and statistical method. The combined HR was 1.492 (95% CI: 1.206~1.844, P < 0.001) and 2.191 (95% CI: 1.638~2.929, P < 0.001) in the Asian studies and American studies, respectively. Next, we divided the studies into two groups depending on the case number less or more than sixty. VEGF positive expression was a valuable prognostic marker in both small sample sizes (N ≤ 60, HR = 2.398, 95% CI: 1.547~3.716, P < 0.001) and large sample sizes (N > 60, HR = 1.395, 95% CI: 1.081~1.799, P = 0.011). The subgroup analyses were proceeded among the specimen types. In the tumor tissues and blood sample group, the combined HRs were 1.547 (95% CI: 1.242~1.928, P < 0.001) and 3.155 (95% CI: 1.784~5.579, P < 0.001), respectively. VEGF expression was also considered as a prognostic factor according to different VEGF test methods. Significant impacts of VEGF expression were observed in IHC group (HR = 1.394, 95% CI: 1.112~1.747, P = 0.004), RT-PCR group (HR = 2.286, 95% CI: 1.175~4.446, P = 0.015) and ELISA group (HR = 3.447, 95% CI, 1.999~5.942, P < 0.001), respectively. Finally, we also found an inverse effect of VEGF expression on OS according to the statistical methods. The pooled HRs were 1.797 (95% CI: 1.275~2.533, P = 0.001), 2.633 (95% CI: 1.455~4.763, P = 0.001) and 3.359 (95% CI: 2.147~5.256, P < 0.001) in the group providing survival curve, original data and univariate HR, respectively (Table 2).
Figure 2.

A Forest Plot of the Combined Relative HR form Random-effect OS.
Figure 3.
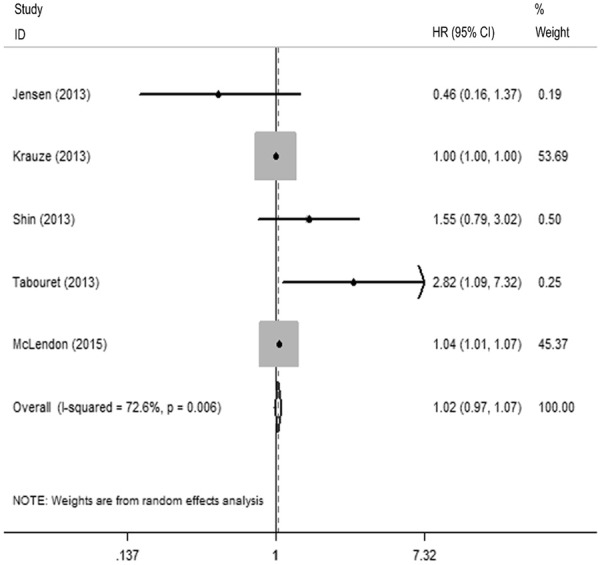
A Forest Plot of the Combined Relative HR form Random-effect PFS.
Table 2.
Summarized HRs of subgroup analyses for OS in the meta-analysis
| Stratified analysis | Study (N) | HR (95% CI) | P | Z | Heterogeneity | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| I2 | P | Statistical model | |||||
| Region | |||||||
| Asia | 17 | 1.492 (1.206, 1.844) | 0.000 | 3.690 | 34.9% | 0.077 | Fixed-effects model |
| Europe | 8 | 1.218 (0.893, 1.663) | 0.213 | 1.250 | 69.0% | 0.002 | Random-effects model |
| America | 5 | 2.191 (1.638, 2.929) | 0.000 | 5.290 | 54.8% | 0.065 | Fixed-effects model |
| Case number | |||||||
| Small | 15 | 2.398 (1.547, 3.716) | 0.000 | 3.910 | 72.2% | 0.000 | Random-effects model |
| Large | 16 | 1.395 (1.081, 1.799) | 0.011 | 2.560 | 57.5% | 0.002 | Random-effects model |
| Specimen type | |||||||
| Tumor tissues | 28 | 1.547 (1.242, 1.928) | 0.000 | 3.890 | 70.9% | 0.000 | Random-effects model |
| Blood | 3 | 3.155 (1.784, 5.579) | 0.000 | 3.950 | 0.0% | 0.890 | Fixed-effects model |
| Assay | |||||||
| IHC | 22 | 1.394 (1.112, 1.747) | 2.880 | 0.004 | 61.5% | 0.000 | Random-effects model |
| RT-PCR | 4 | 2.286 (1.175, 4.446) | 2.440 | 0.015 | 64.8% | 0.037 | Random-effects model |
| ELISA | 4 | 3.447 (1.999, 5.942) | 4.450 | 0.000 | 0.0% | 0.730 | Fixed-effects model |
| Method | |||||||
| Survival curve | 6 | 1.797 (1.275, 2.533) | 0.001 | 3.350 | 33.7% | 0.183 | Fixed-effects model |
| Original data | 4 | 2.633 (1.455, 4.763) | 0.001 | 3.200 | 57.9% | 0.068 | Fixed-effects model |
| HR (multivariate) | 15 | 1.246 (0.994, 1.562) | 0.057 | 1.910 | 65.4% | 0.000 | Random-effects model |
| HR (univariate) | 4 | 3.359 (2.147, 5.256) | 0.000 | 5.300 | 11.1% | 0.338 | Fixed-effects model |
Heterogeneity analysis results
Significant heterogeneities were found in the analysis between VEGF and OS and PFS (I2 = 72.30%, P < 0.001 and I2 = 72.60%, P = 0.006, respectively). In the subgroup analyses for OS, the heterogeneities were reduced in the Asian and American studies (I2 = 34.9%, P = 0.077 and I2 = 54.8%, P = 0.065). The pooled HR in blood tissue group did not show obvious heterogeneity (I2 = 0.0%, P = 0.890). The pooled HR also reached low heterogeneity in ELISA group (I2 = 0.0%, P = 0.730). Moreover, heterogeneity was not noticeable in the group of survival curve group, original data and univariate HR (I2 = 33.7%, P = 0.183; I2 = 57.9%, P = 0.068 and I2 = 11.1%, P = 0.338) (Table 2).
Publication bias
The Funnel plot and Begg’s test did not show any evidence of publication bias (P = 0.507 for OS, P = 1 for PFS; Figures 4 and 5).
Figure 4.

A Funnel Blot for the publication bias test of OS studies.
Figure 5.

A Funnel Blot for the publication bias test of PFS studies.
Correlation of VEGF overexpression with the pathological grade of gliomas
The Chi-square test was applied to analyze the relationship between VEGF expression and glioma pathological grade. The results revealed that VEGF positive rate was higher in high-grade glioma (70.27%) than that in low-grade glioma (39.54%, Z = -8.199, P < 0.005). VEGF expression was shown to be positively relevant with pathological grade of gliomas by spearman’s rank correlation (r = 0.307, P < 0.005) (Table 3).
Table 3.
Correlation of VEGF expression with the pathological grade of gliomas from the available included studies
| Study | Positive | Negative | Chi-square test | Spearman | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Low-grade | High-grade | Low-grade | High-grade | |||
| Zhong 2001 | 24 | 15 | 11 | 0 | ||
| Cheng 2006 | 8 | 37 | 8 | 7 | ||
| Zeng 2009 | 2 | 38 | 6 | 10 | ||
| Yoo 2010 | 2 | 31 | 12 | 31 | ||
| El-Sayed 2010 | 8 | 14 | 3 | 1 | ||
| Fan 2012 | 2 | 27 | 7 | 16 | ||
| Cao 2013 | 3 | 18 | 2 | 4 | ||
| Xu 2013 | 36 | 56 | 20 | 4 | ||
| Nambirajan 2014 | 18 | 25 | 58 | 25 | ||
| Tabouret 2014 | 18 | 25 | 58 | 23 | ||
| Total | 121 | 286 | 185 | 121 | Z = -8.199, P < 0.001 | r = 0.307, P < 0.001 |
Discussion
VEGF, with a molecular weight of 38.2 kDa, is involved in triggering the process of angiogenesis in neoplasia. VEGF also stimulates capillary permeability, angiogenesis and endothelial cell growth [50]. Glioma, a highly vascularized tumor, develops with progressive angiogenesis. VEGF has been indicated to be a potential biomarker in serum/plasma and cerebrospinal fluid of glioma patients [51,52]. VEGF upregulation also increases cell density, leading to the tumor hypoxia [53]. VEGF, induced by hypoxia via HIF-1a, acts as a central proangiogenic factor in blood vessel formation by stimulating VEGFR-2/KDR in glioma [54]. Additionally, the prognostic value of VEGF upregulation for survival in glioma patients was described previously [55,56]. However, other researchers argued that VEGF could not be an independent prognostic factor for the survival of glioma patients [57,58]. Hence, well-designed studies with large sample size are still needed to provide strong evidence to explore the prognostic value of VFGF for the glioma patients.
Our study suggested that higher positive rate of VEGF expression was found in the group of high-grade glioma compared to the low-grade glioma. Previous studies also reported that VEGF upregulation was involved in the angiogenesis and progression of glioma, suggesting the participation of VEGF in positive regulation of neoangiogenesis and proliferation during gliomagenesis [59,60]. Consistent with the previous researches, our study revealed that VEGF expression was significantly related to glioma pathological grade by analyzing the 713 cases available in the included studies.
In this meta-analysis, our result implied that VEGF overexpression was notably associated with OS of glioma patients. Of note, the subgroup analyses also proved the strong prognostic relevance of VEGF overexpression on OS of glioma patients. Contrary to our expectation, no significance was found between VEGF overexpression and PFS of glioma patients. The main reason might probably be that too few studies were available reporting the relation between VEGF expression and PFS of glioma patients. Hence, larger cohort would be required to investigate the impact of VEGF on PFS of gliomas.
Nevertheless, several limitations did exist in the present meta-analysis. Firstly, we only included English and Chinese studies in this meta-analysis while the coincident studies in other languages were not included. Secondly, another key bias might be extrapolating the HRs and the 95% CI by different methods. When the studies did not provide the HRs, we extrapolated HRs from the survival curves or estimated them from the sufficient data, which might be less reliable than the ones obtained from published statistics. Thirdly, journals often favor studies with positive results, whereas the negative results might not be showed in publications. Fourthly, we did not exclude the studies with limited numbers of cases, which might be an important bias since the small scale probably did not provide reliable evidences for this analysis.
In conclusion, this meta-analysis was the first one to yield the association between VEGF expression and the survival of glioma patients. The VEGF overexpression is significantly associated with the OS of the glioma patients but not with PFS. Moreover, we infer the significant association between VEGF expression and glioma pathological grade in a large number of cases included. Our study suggests that VEGF shows the significant relevance to glioma pathological grade and might be a valuable prognostic factor for the OS of the patients with glioma. Nevertheless, well-designed studies with larger cohort are needed to explore the valuable role of VEGF in glioma.
Acknowledgements
The study was supported by the fund of Guangxi Zhuang Autonomous Region University Student Innovative Plan (No. 201410598026), China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Disclosure of conflict of interest
None.
References
- 1.Zou P, Xu H, Chen P, Yan Q, Zhao L, Zhao P, Gu A. IDH1/IDH2 mutations define the prognosis and molecular profiles of patients with gliomas: a meta-analysis. PLoS One. 2013;8:e68782. doi: 10.1371/journal.pone.0068782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huncharek M, Kupelnick B. Epidermal growth factor receptor gene amplification as a prognostic marker in glioblastoma multiforme: results of a meta-analysis. Oncol Res. 2000;12:107–112. doi: 10.3727/096504001108747576. [DOI] [PubMed] [Google Scholar]
- 3.Levidou G, El-Habr E, Saetta AA, Bamias C, Katsouyanni K, Patsouris E, Korkolopoulou P. P53 immunoexpression as a prognostic marker for human astrocytomas: a meta-analysis and review of the literature. J Neurooncol. 2010;100:363–371. doi: 10.1007/s11060-010-0204-y. [DOI] [PubMed] [Google Scholar]
- 4.Chen WJ, He DS, Tang RX, Ren FH, Chen G. Ki-67 is a Valuable Prognostic Factor in Gliomas: Evidence from a Systematic Review and Meta-analysis. Asian Pac J Cancer Prev. 2015;16:411–420. doi: 10.7314/apjcp.2015.16.2.411. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 6.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 7.Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin XQ, Yu LK, Song Y. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4:1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 8.Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–1832. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Tang D, Wang S, Li QG, Zhang JR, Li P, Lu Q, Niu G, Gao J, Ye NY, Wang DR. High expressions of galectin-1 and VEGF are associated with poor prognosis in gastric cancer patients. Tumour Biol. 2014;35:2513–2519. doi: 10.1007/s13277-013-1332-8. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Kim R, Quintini C, Hashimoto K, Fujiki M, Diago T, Eghtesad B, Miller C, Fung J, Tan A, Menon KV, Aucejo F. Prognostic role of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma undergoing liver transplantation. Liver Transpl. 2015;21:101–111. doi: 10.1002/lt.24013. [DOI] [PubMed] [Google Scholar]
- 11.Yu XW, Wu TY, Yi X, Ren WP, Zhou ZB, Sun YQ, Zhang CQ. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol. 2014;35:155–160. doi: 10.1007/s13277-013-1019-1. [DOI] [PubMed] [Google Scholar]
- 12.Das S, Marsden PA. Angiogenesis in glioblastoma. N Engl J Med. 2013;369:1561–1563. doi: 10.1056/NEJMcibr1309402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian XW, Du LL, Shi JQ, Cheng YS, Liu FX. Correlation of bFGF, FGFR-1 and VEGF expression with vascularity and malignancy of human astrocytomas. Analytical and Quantitative Cytology and Histology. 2000;22:267–274. [PubMed] [Google Scholar]
- 19.Zhong D, Li X, Zhang G. [Multivariate analysis of the parameters related to prognosis of astrocytoma] . Zhonghua Bing Li Xue Za Zhi. 2001;30:345–349. [PubMed] [Google Scholar]
- 20.Hara A, Okayasu I. Cyclooxygenase-2 and inducible nitric oxide synthase expression in human astrocytic gliomas: correlation with angiogenesis and prognostic significance. Acta Neuropathol. 2004;108:43–48. doi: 10.1007/s00401-004-0860-0. [DOI] [PubMed] [Google Scholar]
- 21.Nam DH, Park K, Suh YL, Kim JH. Expression of VEGF and brain specific angiogenesis inhibitor-1 in glioblastoma: prognostic significance. Oncol Rep. 2004;11:863–869. [PubMed] [Google Scholar]
- 22.Liu XQ, Zhang K, Wang XF, Gao C. The relevance of VEGF, bFGF and PTEN with glioma malignant degree and prognosis. Chinese Journal of Neurosurgery. 2004;20:14–17. [Google Scholar]
- 23.Zhou YH, Hess KR, Liu L, Linskey ME, Yung WK. Modeling prognosis for patients with malignant astrocytic gliomas: quantifying the expression of multiple genetic markers and clinical variables. Neuro Oncol. 2005;7:485–494. doi: 10.1215/S1152851704000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buccoliero AM, Caldarella A, Gheri CF, Taddei A, Paglierani M, Pepi M, Mennonna P, Taddei GL. Inducible cyclooxygenase (COX-2) in glioblastoma--clinical and immunohistochemical (COX-2-VEGF) correlations. Clin Neuropathol. 2006;25:59–66. [PubMed] [Google Scholar]
- 25.Cheng BC, Wang JH, Feng CG, LI CY. Expressions of osteopontin and vascular endothelial growth factor in brain glioma and their association with the prognosis. Chinese Journal of Neuromedicine. 2006:22–26. [Google Scholar]
- 26.Carlson MR, Pope WB, Horvath S, Braunstein JG, Nghiemphu P, Tso CL, Mellinghoff I, Lai A, Liau LM, Mischel PS, Dong J, Nelson SF, Cloughesy TF. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13:2592–2598. doi: 10.1158/1078-0432.CCR-06-2772. [DOI] [PubMed] [Google Scholar]
- 27.Flynn JR, Wang L, Gillespie DL, Stoddard GJ, Reid JK, Owens J, Ellsworth GB, Salzman KL, Kinney AY, Jensen RL. Hypoxia-regulated protein expression, patient characteristics, and preoperative imaging as predictors of survival in adults with glioblastoma multiforme. Cancer. 2008;113:1032–1042. doi: 10.1002/cncr.23678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sathornsumetee S, Cao Y, Marcello JE, Herndon JE 2nd, McLendon RE, Desjardins A, Friedman HS, Dewhirst MW, Vredenburgh JJ, Rich JN. Tumor angiogenic and hypoxic profiles predict radiographic response and survival in malignant astrocytoma patients treated with bevacizumab and irinotecan. J. Clin. Oncol. 2008;26:271–278. doi: 10.1200/JCO.2007.13.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoo H, Sohn S, Nam BH, Min HS, Jung E, Shin SH, Gwak HS, Lee SH. The expressions of carbonic anhydrase 9 and vascular endothelial growth factor in astrocytic tumors predict a poor prognosis. Int J Mol Med. 2010;26:3–9. doi: 10.3892/ijmm_00000427. [DOI] [PubMed] [Google Scholar]
- 30.El-Sayed M, Taha MM. Immunohistochemical expression of cycloxygenase-2 in astrocytoma: correlation with angiogenesis, tumor progression and survival. Turk Neurosurg. 2011;21:27–35. [PubMed] [Google Scholar]
- 31.Piperi C, Samaras V, Levidou G, Kavantzas N, Boviatsis E, Petraki K, Grivas A, Barbatis C, Varsos V, Patsouris E, Korkolopoulou P. Prognostic significance of IL-8-STAT-3 pathway in astrocytomas: correlation with IL-6, VEGF and microvessel morphometry. Cytokine. 2011;55:387–395. doi: 10.1016/j.cyto.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Saetta AA, Levidou G, El-Habr EA, Panayotidis I, Samaras V, Thymara I, Sakellariou S, Boviatsis E, Patsouris E, Korkolopoulou P. Expression of pERK and pAKT in human astrocytomas: correlation with IDH1-R132H presence, vascular endothelial growth factor, microvascular characteristics and clinical outcome. Virchows Arch. 2011;458:749–759. doi: 10.1007/s00428-011-1074-1. [DOI] [PubMed] [Google Scholar]
- 33.Berindan-Neagoe I, Chiorean R, Braicu C, Florian IS, Leucuta D, Crisan D, Cocis A, Balacescu O, Irimie A. Quantitative mRNA expression of genes involved in angiogenesis, coagulation and inflammation in multiforme glioblastoma tumoral tissue versus peritumoral brain tissue: lack of correlation with clinical data. Eur Cytokine Netw. 2012;23:45–55. doi: 10.1684/ecn.2012.0302. [DOI] [PubMed] [Google Scholar]
- 34.Castells X, Acebes JJ, Majos C, Boluda S, Julia-Sape M, Candiota AP, Arino J, Barcelo A, Arus C. Development of robust discriminant equations for assessing subtypes of glioblastoma biopsies. Br J Cancer. 2012;106:1816–1825. doi: 10.1038/bjc.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith SJ, Tilly H, Ward JH, Macarthur DC, Lowe J, Coyle B, Grundy RG. CD105 (Endoglin) exerts prognostic effects via its role in the microvascular niche of paediatric high grade glioma. Acta Neuropathol. 2012;124:99–110. doi: 10.1007/s00401-012-0952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoo H, Sohn S, Nam BH, Min HS, Jung E, Shin SH, Gwak HS, Lee SH. Expression of CA9 and VEGF in glioma and their relation with prognosis. Int J Mol Med. 2012:916–919. [Google Scholar]
- 37.Krauze AV, Won M, Graves C, Corn BW, Muanza TM, Howard SP, Mahadevan A, Schultz CJ, Haas ML, Mehta MP, Camphausen KA. Predictive value of tumor recurrence using urinary vascular endothelial factor levels in patients receiving radiation therapy for Glioblastoma Multiforme (GBM) Biomark Res. 2013;1:29. doi: 10.1186/2050-7771-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin JH, Lee YS, Hong YK, Kang CS. Correlation between the prognostic value and the expression of the stem cell marker CD133 and isocitrate dehydrogenase1 in glioblastomas. J Neurooncol. 2013;115:333–341. doi: 10.1007/s11060-013-1234-z. [DOI] [PubMed] [Google Scholar]
- 39.Xu HW, Huang YJ, Xie ZY, Lin L, Guo YC, Zhuang ZR, Lin XP, Zhou W, Li M, Huang HH, Wei XL, Man K, Zhang GJ. The expression of cytoglobin as a prognostic factor in gliomas: a retrospective analysis of 88 patients. BMC Cancer. 2013;13:247. doi: 10.1186/1471-2407-13-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Zhong Z, Yuan J, Zhang Z, Wei Q, Song W, Chen H. Collaborative overexpression of matrix metalloproteinase-1 and vascular endothelial growth factor-C predicts adverse prognosis in patients with gliomas. Cancer Epidemiol. 2013;37:697–702. doi: 10.1016/j.canep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Cao WD, Kawai N, Miyake K, Zhang X, Fei Z, Tamiya T. Relationship of 14-3-3zeta (zeta), HIF-1alpha, and VEGF expression in human brain gliomas. Brain Tumor Pathol. 2014;31:1–10. doi: 10.1007/s10014-013-0135-3. [DOI] [PubMed] [Google Scholar]
- 42.Chiorean R, Berindan-Neagoe I, Braicu C, Florian IS, Leucuta D, Crisan D, Cernea V. Quantitative expression of serum biomarkers involved in angiogenesis and inflammation, in patients with glioblastoma multiforme: Correlations with clinical data. Cancer Biomark. 2014;14:185–194. doi: 10.3233/CBM-130310. [DOI] [PubMed] [Google Scholar]
- 43.Clara CA, Marie SK, de Almeida JRW, Wakamatsu A, Oba-Shinjo SM, Uno M, Neville M, Rosemberg S. Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1 alpha in human glioblastoma. Neuropathology. 2014;34:343–352. doi: 10.1111/neup.12111. [DOI] [PubMed] [Google Scholar]
- 44.Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol. 2014;16:280–291. doi: 10.1093/neuonc/not148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nambirajan A, Sharma MC, Gupta RK, Suri V, Singh M, Sarkar C. Study of stem cell marker nestin and its correlation with vascular endothelial growth factor and microvascular density in ependymomas. Neuropathol Appl Neurobiol. 2014;40:714–725. doi: 10.1111/nan.12097. [DOI] [PubMed] [Google Scholar]
- 46.Tabouret E, Boudouresque F, Barrie M, Matta M, Boucard C, Loundou A, Carpentier A, Sanson M, Metellus P, Figarella-Branger D, Ouafik L, Chinot O. Association of matrix metalloproteinase 2 plasma level with response and survival in patients treated with bevacizumab for recurrent high-grade glioma. Neuro Oncol. 2014;16:392–399. doi: 10.1093/neuonc/not226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takano S, Ishikawa E, Nakai K, Matsuda M, Masumoto T, Yamamoto T, Matsumura A. Bevacizumab in Japanese patients with malignant glioma: from basic research to clinical trial. Onco Targets Ther. 2014;7:1551–1562. doi: 10.2147/OTT.S67621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLendon RE, Lipp E, Satterfield D, Ehinger M, Austin A, Fleming D, Perkinson K, Lefaivre M, Zagzag D, Wiener B, Gururangan S, Fuchs H, Friedman HS, Herndon JE 2nd, Healy P. Prognostic marker analysis in pediatric intracranial ependymomas. J Neurooncol. 2015;122:255–261. doi: 10.1007/s11060-014-1711-z. [DOI] [PubMed] [Google Scholar]
- 49.Zeng FY, Tan WY, Hu DS. Expression of Epidermal Growth Factor Receptor (EGFR) and Vascular Endothelial Growth Factor (VEGF) in the Treatment of Glioma and Its Significance. China Cancer. 2009:920–923. [Google Scholar]
- 50.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 51.Peles E, Lidar Z, Simon AJ, Grossman R, Nass D, Ram Z. Angiogenic factors in the cerebrospinal fluid of patients with astrocytic brain tumors. Neurosurgery. 2004;55:562–567. doi: 10.1227/01.neu.0000134383.27713.9a. discussion 567-568. [DOI] [PubMed] [Google Scholar]
- 52.Sampath P, Weaver CE, Sungarian A, Cortez S, Alderson L, Stopa EG. Cerebrospinal fluid (vascular endothelial growth factor) and serologic (recoverin) tumor markers for malignant glioma. Cancer Control. 2004;11:174–180. doi: 10.1177/107327480401100305. [DOI] [PubMed] [Google Scholar]
- 53.Rijken PF, Bernsen HJ, Peters JP, Hodgkiss RJ, Raleigh JA, van der Kogel AJ. Spatial relationship between hypoxia and the (perfused) vascular network in a human glioma xenograft: a quantitative multi-parameter analysis. Int J Radiat Oncol Biol Phys. 2000;48:571–582. doi: 10.1016/s0360-3016(00)00686-6. [DOI] [PubMed] [Google Scholar]
- 54.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chan AS, Leung SY, Wong MP, Yuen ST, Cheung N, Fan YW, Chung LP. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol. 1998;22:816–826. doi: 10.1097/00000478-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Abdulrauf SI, Edvardsen K, Ho KL, Yang XY, Rock JP, Rosenblum ML. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosurg. 1998;88:513–520. doi: 10.3171/jns.1998.88.3.0513. [DOI] [PubMed] [Google Scholar]
- 57.Korshunov A, Golanov A. The prognostic significance of vascular endothelial growth factor (VEGF C-1) immunoexpression in oligodendroglioma. An analysis of 91 cases. J Neurooncol. 2000;48:13–19. doi: 10.1023/a:1006475312401. [DOI] [PubMed] [Google Scholar]
- 58.Korshunov A, Golanov A, Timirgaz V. Immunohistochemical markers for prognosis of ependymal neoplasms. J Neurooncol. 2002;58:255–270. doi: 10.1023/a:1016222202230. [DOI] [PubMed] [Google Scholar]
- 59.Hlobilkova A, Ehrmann J, Knizetova P, Krejci V, Kalita O, Kolar Z. Analysis of VEGF, Flt-1, Flk-1, nestin and MMP-9 in relation to astrocytoma pathogenesis and progression. Neoplasma. 2009;56:284–290. doi: 10.4149/neo_2009_04_284. [DOI] [PubMed] [Google Scholar]
- 60.Ma C, Li Y, Zhang X, Zhao G, Xu H. Levels of vascular endothelial growth factor and matrix metalloproteinase-9 proteins in patients with glioma. J Int Med Res. 2014;42:198–204. doi: 10.1177/0300060513481924. [DOI] [PubMed] [Google Scholar]


