Abstract
Purpose/Aim: This study aims to investigate the impact of lovastatin on neuroinflammation in 6-OHDA-treated microglia cells. Materials and methods: 6-Hydroxydopamine (6-OHDA)-treated microglia cells were used to investigate the neuroprotective nature of lovastatin. After incubation with 6-OHDA and/or lovastatin for 24 h, test kits were used to detect the levels of LDH and glutamate, which were released from PC12 cells exposed to different culture media. The mRNA levels of TNF-α, IL-6 and IL-1β were determined by RT-PCR and the protein levels were analyzed by Western blot. Results: LDH and glutamate levels in 6-OHDA-incubated PC12 cells increased, when compared with those in the controls, while incubation with lovastatin inhibited this elevation. The expression levels of TNF-α IL-6 and IL-1β were significantly upregulated after treatment with 6-OHDA. The 6-OHDA-stimulated mRNA and protein levels of TNF-α IL-6 and IL-1β were reduced by lovastatin. Conclusions: Our results suggest that Lovastatin is able to induce neuroprotection by inhibiting inflammatory cytokines. The data provide direct evidence of the potential application of lovastatin for the treatment of neuroinflammatory diseases.
Keywords: Parkinson’s disease, lovastatin, microglia, inflammatory cytokines
Introduction
Statins are cholesterol-lowering agents, but more and more effects of statins have been found, including neuroprotection, immunomodulation, and anti-inflammation. Statins can induce neuroprotective effects by various mechanisms such as lowering cholesterol; inhibiting intracellular adhesion molecule-1 (ICAM-1); decreasing b-amyloid production and serum apolipoprotein E (APOE) levels, antithrombotic effects, and anti-inflammatory responses; modifying cognition related receptors; and augmenting endothelial nitric oxide synthase [1-5]. It had been demonstrated that lipophilic statin can decrease the incidence of PD [6,7]; however, the exact mechanisms are still unclear. If statins are potentially beneficial for PD, they may present an interesting challenge.
Microglial activation plays an important role in neuroinflammation, which contributes to neuronal damage. Activated microglia can promote neuronal injury through the release of proinflammatory and cytotoxic factors, including tumor necrosis factor (TNF)-a, interleukin (IL)-1β, IL-6, NO and reactive oxygen species (ROS) [8]. The inhibition of pro-inflammatory mediators in microglia can attenuate the severity of Parkinson’s disease (PD) [9-12]. Progressive inflammation and neuronal cell death which can be also caused by this activation of microglia may result in further reactive microglia activation, which may lead to a vicious cycle of inflammation and neuronal injury that fuels progressive DA neurodegeneration in Parkinson’s disease. [13,14]. Excessive microglial activation was confirmed in the SNpc of patients with PD [15]. Because substantia nigra has the highest density of microglia [16] and more susceptibility to inflammatory attack, microglial activation can damage dopaminergic neurons in substantia nigra, Moreover, level of intracellular glutathione of dopaminergic neurons is lower than other area in substantia nigra, which degrade antioxidant ability and increase the sensitivity to microglia dy mediated oxidative stress [17]. The study had showed that the number of activated microglia had a conspicuous increase in the SNpc of 6-OHDA-lesioned rats at 10 and 28 days post-lesion [18]. All these findings indicated that microglial cells involves in pathogenesis by different ways.
Thus, anti-inflammatory treatment via inhibition of microglial activation is regarded as a promising strategy for the prevention of neurodegenerative diseases [19-22].
Accordingly, in vitro study was applied to investigate the effect of Lovastatin on 6-OHDA-stimulated microglia cells.
Materials & methods
Primary microglial cell culture
Sprague-Dawley rats were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). Experiments were carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Bioethics Committee of He Nan University of science and technology. Primary microglia was cultured from postnatal day 1 Sprague-Dawley rats as previously described [23]. Briefly, cerebral cortical fragments were dissociated by soft trituration in ice-cold DMEM containing 10% FBS, 2 mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin. The cortical fragments were resuspended in culture medium and made into a single cell suspension by repeated pipetting. Single cells were plated on 100-mm culture dishes and incubated for 2 weeks. The microglia were detached from the flasks by mild shaking and applied to a nylon mesh to remove astrocytes and cell clumps. Isolated microglia were plated on 24-well plates at a density of 2×105 cells/well, and cultures with > 90% purity of microglia (identified by an OX-42-specific antibody) were used. The cells were cultured for 48h before drug treatment.
MTT cell viability assay
MTT assay was performed to evaluate the MICROGLIA cell viability after 6-OHDA and lovastatin treatment. Briefly, the MICROGLIA cells were grown in 96-well plates (100 ul/well) at a density of 1×105 cells/ml. After incubation, the cells were washed once with PBS before adding 100 µl of free serum medium containing MTT (final concentration, 0.5 mg/ml) to each well. After incubation for 4 h, the supernatant was removed, and the cells were washed twice with PBS and the formazan product was dissolved in 100 ml of dimethyl sulfoxide (DMSO). The absorbance was read at a wavelength of 570 nm in a microplate reader. The results were expressed as percentage of the control group.
LDH assay and glutamate measurement
LDH assay
The cell viability was also measured by determining the activity of LDH released into the medium when cellular membranes were damaged [24]. The total LDH activity was measured following specifications of In vitro Toxicology Assay Kit LDH based Tox-7 (Sigma-Aldrich, USA). The absorbance was measured in an automatic microplate reader at 490 nm. The data were presented as the percentage of LDH in 6-OHDA group, which was designated as 100%.
Glutamate assay
The concentration of glutamate was measured according to Glutamate Assay Protocol (BioVision, USA). Briefly, 10 µl of the 0.1 M glutamate standard solution was diluted using 990 µl of the assay buffer to generate 1 mM standard glutamate. Subsequently, 0, 2, 4, 6, 8, and 10 µl of the diluted glutamate standard solution were added to a 96-well plate in duplicate to generate 0, 2, 4, 6, 8, and 10 nmol/well standard. The volume was brought to 50 µl with assay buffer. The cells (1×106) were homogenized in 100 µl of assay buffer and centrifuged at 13,000 g to remove the insoluble material. Then, 50 µl of the sample was added to well of the 96-well plate. The optical density was measured at 450 nm using a microplate reader. The background was corrected by subtracting the value derived from the 0 glutamate control from all sample readings, and then the concentration of glutamate was calculated according to the instruction.
Quantitative real-time RT-PCR
For quantitative real-time RT-PCR, the total RNA was extracted using TRIzol (Invitrogen), according to the manufacturer’s protocol, and the cDNA was synthesized using primers with the Advantage RT for PCR kit (BD Biosciences). We quantified the PCR amplifications using SYBR Green PCR Master Mix (Applied Biosystems), and the results were normalized to GAPDH gene expression. All the experiments were performed in triplicate and repeated at least three times.
Western blotting analysis
The cells were pretreated with simvastatin and/or 6-OHDA/SiRNA and were rinsed thrice with ice-cold PBS, and incubated in ice-cold lysis buffers for 20 min on ice. Cells were sonicated and centrifuged at 14000 g for 15 minutes. The supernatants were collected for protein determination by BCA assay. The samples were mixed with 5× Laemmli buffer and heated for 5 min at 100°C. Then, samples of equivalent total protein (30 μg) were run in NuPage Bis-Tris 10% gels (Invitrogen) and transferred to PVDF membrane. The PVDF membranes were incubated with the following primary antibodies: rabbit anti-TNF-α (1:1000), rabbit anti-NMDAR1 (1:800), and rabbit anti-β-actin (1:2000) overnight at 4°C. The next day, the PVDF membranes were washed thrice and horseradish peroxidase-conjugated secondary antibodies were added. Negative controls were prepared by excluding the primary antibodies. The images were analyzed using the alphaview software. The results were expressed as folds of the control group.
Statistical analysis
The data were expressed as mean ± SEM. MTT, LDH, and different protein quantifications with Western blot were analyzed using one-way ANOVA, followed by Tukey’s post hoc analysis (SPSS 15.0 program, Chicago, IL). P values of less than 0.05 were regarded as statistically significant.
Results
Effects of 6-OHDA and lovastatin on microglia cell viability
The cytotoxic effects of Lovastatin were evaluated with the MTT assay by measuring the viability of microglia cells that were incubated with Lovastatin (0, 1, 2, 3, 4 μM) for 24 h in the absence of 6-OHDA (Figure 1A). We also examined the viability of microglia cells treated with 2 μM Lovastatin for 6, 12, 24 or 48 h in absence of 6-OHDA. Interestingly, no significant differences in cell viability were found between normal microglia cells and microglia cells treated with 2 μM Lovastatin for 24 h (Figure 1B), which indicates that the inhibitory effect that we observed was not due to cytotoxicity.
Figure 1.
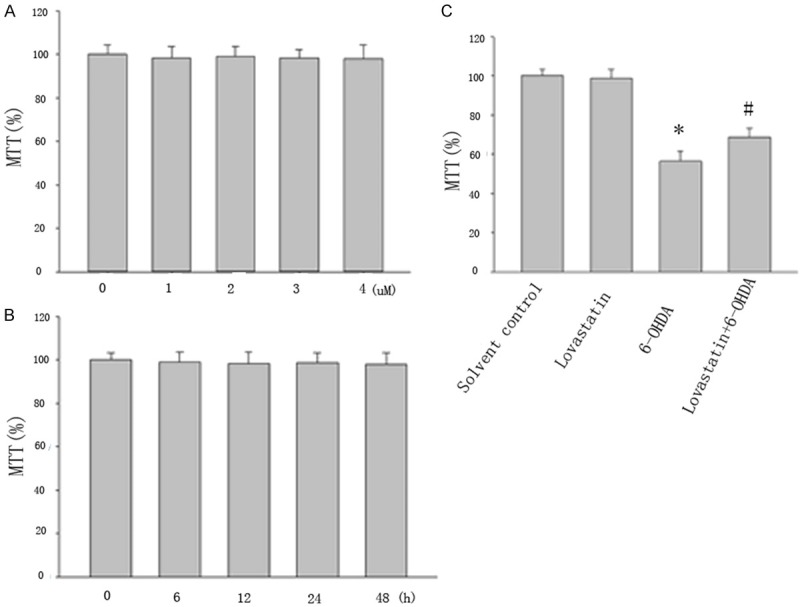
Viability in lovastatin-treated cells was evaluated using the MTT assay. Cells were incubated with lovastatin (1 to 4 μM) (A) and with 2 μM lovastatin for 6 to 48 h (B) and 6-OHDA (100 μM) for 24 h (C). Lovastatin protected MICROGLIA cells against 6-OHDA neurotoxicity. MTT value in 6-OHDA treated group significantly reduced compared with controls (*P<0.001, 6-OHDA vs solvent controls), but lovastatin partially reversed this reduction (#P<0.001, 6-OHDA vs 6-OHDA + lovastatin). The results are displayed as the percentages of the control samples. The data are presented as the means ± S.E.M. (n=9) for three independent experiments.
To investigate the effect of 6-OHDA and lovastatin on microglia cells, we exposed the cells to 6-OHDA (100 uM) and lovastatin (2 μM) for 24 h period of time, and examined a variety of markers of cell death. MTT value in 6-OHDA treated group significantly reduced compared with controls, but lovastatin upregulated this reduction (Figure 1C).
Effects of 6-OHDA and lovastatin on LDH and glutamate
LDH is released from the cells following membrane collapse, and the released LDH is usually considered as a sign of late cell death [24]. Our result showed that LDH increased in 6-OHDA-incubated microglia cell, when compared with that in controls (Figure 2A), whereas lovastatin incubation prevented this elevation (Figure 2A). Glutamate is the most abundant excitatory neurotransmitter and recognized as another important sign of cell death. Our result indicated that glutamate increased in 6-OHDA-incubated microglia cells, when compared with that in controls (Figure 2B), whereas lovastatin incubation prevented this elevation (Figure 2B), demonstrating a significant neuroprotective effect of lovastatin in vitro PD model.
Figure 2.
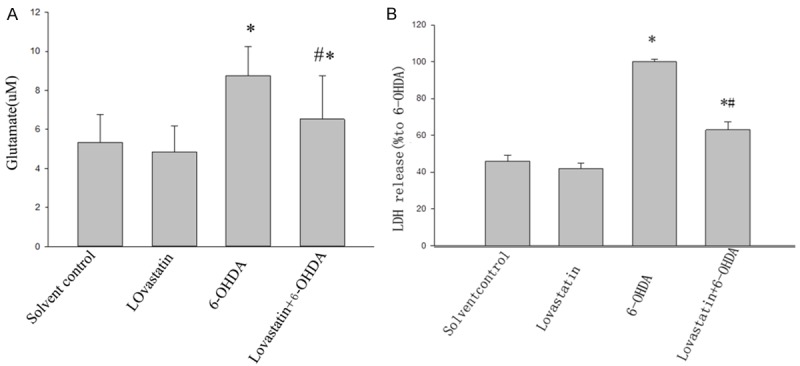
The effects of lovastatin on 6-OHDA-induced LDH and glutamate. Lovastatin reduced 6-OHDA-induced LDH and glutamate (*P<0.001, 6-OHDA vs solvent controls, n=9; A), while lovastatin incubation abolished this elevation (#P< 0.001, 6-OHDA vs 6-OHDA + lovastatin, n=9; A). In 6-OHDA incubated MICROGLIA, glutamate was increased compared with controls (6-OHDA vs controls, *P<0.001; B), while lovastatin treatment abolished this elevation (6-OHDA vs 6-OHDA + Lovastatin, #P<0.001, B). All the results are expressed as mean ± S.E.M. (n=9).
Analysis of TNF-α, IL-6 and IL-1β mRNA by real-time PCR
Using quantitative RT-PCR, we were able to detect that the expression of TNF-α, IL-6 and IL-1β mRNA was significantly elevated in 6-OHDA mediated microglia compared with the solvent control, while lovastatin incubation abolished this elevation. The primers were designed with the “Primer3Output” program (Table 1). The number of copies of the gene of interest in each sample was extrapolated from the corresponding standard curve by the indicated software. For each sample, duplicate determinations were made, and the gene copy number was normalized by the amount of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same sample. The primer selection was performed with the aid of the Light Cycler Probe Design Software, version 1.0 (Idaho Technology Inc., Alameda, CA, USA). The mRNA level for the control group was set as 100% (Figure 3A-C).
Table 1.
Primers used for real-time RT-PCR
| Target gene | Primer sequences | Amplicon (bp) | |
|---|---|---|---|
| TNF-a | FP | 5’-ATGAGCACGGAAAGCATG-3’ | 276 |
| RP | 5’-TACGGGCTTGTCACTCGAGTT-3’ | ||
| IL-6 | FP | 5’-GACAAAGCCAGAGTCATTCA-3’ | 229 |
| RP | 5’-ACTAGGTTTGCCGAGTAGAC-3 | ||
| IL-1β | FP | 5’-TTGTGGCTGTGGAGAAGCTG-3’ | 377 |
| RP | 5’-GCCGTCTTTCATACACAGG-3’ | ||
| GAPDH | FP | 5’-AAC CTG CCA AGT ATG ATG-3’ | 119 |
| RP | 5’-GGA GTT GCT GTT GAA GTC-3’ |
Figure 3.
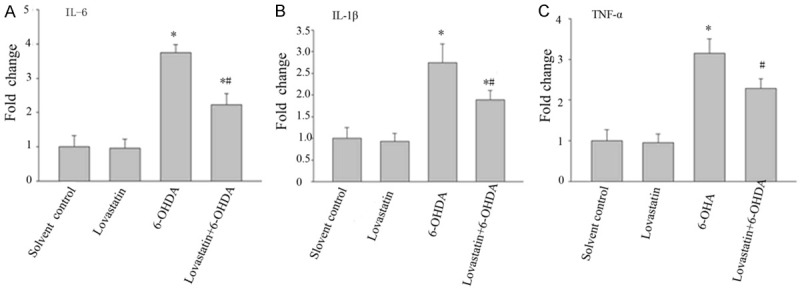
The effects of lovastatin on 6-OHDA-induced IL-6, IL- IL-1β and TNF-α mRNA expression in MICROGLIA cells. The cells were treated with the indicated doses of lovastatin and 6-OHDA. After incubation for 24 h, the mRNA levels of IL-6, IL- IL-1β and TNF-α were determined by RT-PCR. The data are presented as the mean ± S.E.M. (n=5 wells for each condition) for three independent experiments (*P<0.001 vs. solvent controls; #P<0.001 vs. 6-OHDA).
Lovastatin regulates the levels of TNF-α, IL-6 and IL-1β in 6-OHDA-treated microglia cells using western blot analysis
To confirm that the neuroprotection mediated is associated with inflammatory cytokines, TNF-α, IL-6 and IL-1β were measured western blot. After 24 h, 6-OHDA incubation pronouncedly increased TNF-α, IL-6 and IL-1β by expression compared with controls (Figure 4A-C); while this elevation was significantly abolished following lovastatin treatment.
Figure 4.
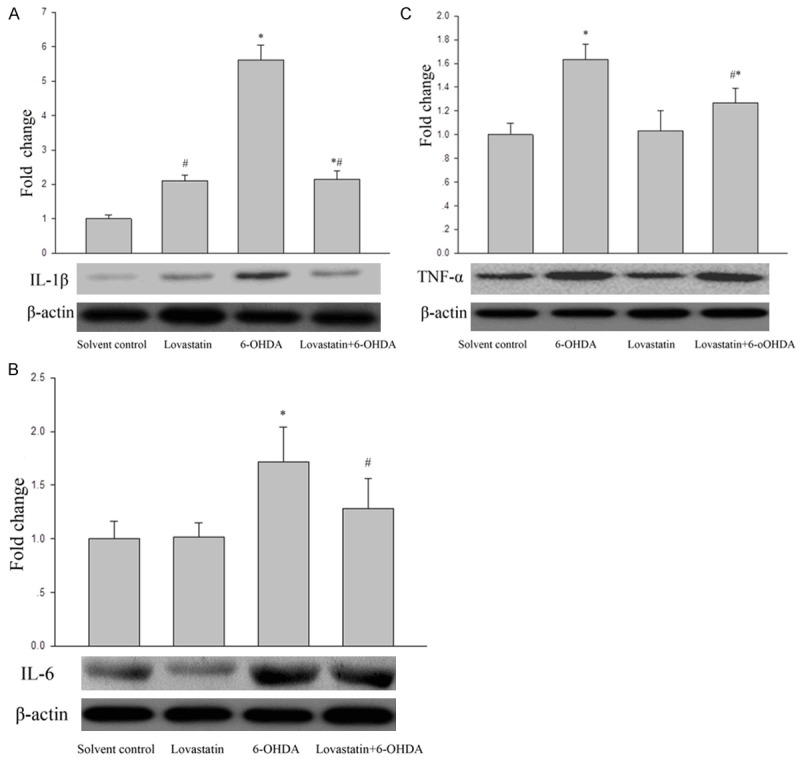
The effects of lovastatin on 6-OHDA-induced TNF-α(C), IL-6 (B) and IL-1β (A) protein expression in MICROGLIA cells. The cells were treated with the indicated doses of lovastatin and 6-OHDA. After incubation for 24 h, the protein levels of TNF-α (C), IL-6 (B) and IL-1β (A) were analyzed by Western blot. The data are presented as the mean ± S.E.M. (n=5 wells for each condition) for three independent experiments (*P<0.001 vs. solvent controls; (#P<0.001 vs. 6-OHDA).
Discussion
The study aimed to investigate the neuroprotective effect of Lovastatin and explain the mechanisms in experimental Parkinsonian cell models. The cell viability was obviously decreased in 6-OHDA-treated microglia cells (Figure 1). However, pre-incubation with lovastatin displayed a restoration of reduction of cell viability. In addition, our results showed that LDH and glutamate were significantly increased in 6-OHDA-induced microglia cells and the elevations were obviously prevented after lovastatin incubation, demonstrating that lovastatin displayed pronouncedly neuroprotective effects.
In order to explore if inflammatory mediators in microglia cells changed following 6-OHDA and lovastatin treatment, we measured the expression of TNF-α, IL-6 and IL-1β. Our study showed increased expression of TNF-α, IL-6 and IL-1β in 6-OHDA-induced microglia cells, implying these inflammatory mediators involved in our study. The elevations of TNF-α, IL-6 and IL-1β were significantly abolished following lovastatin treatment, strongly suggesting a direct anti-inflammatory property of lovastatin through by inhibiting inflammatory response. TNF-α, IL-6 and IL-1β in pathological brain processes including the mediation of neuronal death [25,26]. Simvastatin selectively reduces IL-1β expression and inhibits the activation of microglial cells and astrocytes after traumatic brain injury (TBI) in rat [27]. Atorvastatin can improve neurofunctional rehabilitation in rats through the suppression of cytokines-mediated inflammatory response [28].
In order to further verify that the neuroprotection is associated with inflammatory cytokine in this study, we focused specifically on 6-OHDA-treated microglia expressing TNF-α, IL-6 and IL-1β protein. We detected a significant increased TNF-α, IL-6 and IL-1β protein after 6-OHDA exposure and this increase was abolished following lovastatin treatment; while TNF-α, IL-6 and IL-1β proteins displayed a similar pattern after 6-OHDA neurotoxicity and lovastatin treatment (Figure 4), which demonstrated that TNF-α, IL-6 and IL-1β proteins was closely associated with inflammatory cytokine following 6-OHDA and lovastatin treatment. This result is consistent with other studies [29,30], which showing that pro-inflammatory mediator was involved lovastatin mediated neuroprotection. To our best of knowledge, this is the first time to describe the correlation of TNF-α, IL-6 and IL-1β and lovastatin in microglia.
In summary, our data reveal that lovastatin markedly attenuates inflammatory cytokines that were released by activated microglial. These results provide an insight into the mechanism of the beneficial effects of lovastatin in neurodegenerative disorders, particularly parkinson’s diseases, which presents the first evidence demonstrating the effects of lovastatin on pro-inflammatory mediator in 6-OHDA lesioned microglia cells and reveals lovastatin modulation effect, which provides an exciting and potential paradigm to ameliorate PD.
Conclusion
Lovastatin provided robust neuroprotection on dopaminergic neurodegeneration partially via lovastatin mediated anti-inflammatory mechanisms.
Acknowledgements
This work was supported by National Natural Science Foundations(U1304809) of China from Y J-Q.
Disclosure of conflict of interest
None.
References
- 1.Dolga AM, Nijholt IM, Ostroveanu A, Ten Bosch Q, Luiten PG, Eisel UL. Lovastatin induces neuroprotection through tumor necrosis factor receptor 2 signaling pathways. J Alzheimers Dis. 2008;13:111–122. doi: 10.3233/jad-2008-13201. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara T, Ayer R, Jadhav V, Chen W, Tsubokawa T, Zhang JH. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sureda FX, Junyent F, Verdaguer E, Auladell C, Pelegri C, Vilaplana J, Folch J, Canudas AM, Zarate CB, Palles M, Camins A. Antiapoptotic drugs: a therapautic strategy for the prevention of neurodegenerative diseases. Curr Pharm Des. 2011;17:230–245. doi: 10.2174/138161211795049732. [DOI] [PubMed] [Google Scholar]
- 4.Piau A, Nourhashemi F, Hein C, Caillaud C, Vellas B. Progress in the development of new drugs in Alzheimer’s disease. J Nutr Health Aging. 2011;15:45–57. doi: 10.1007/s12603-011-0012-x. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Yan J, Chen X, Li J, Yang Y, Weng J, Deng C, Yenari MA. Statins: multiple neuroprotective mechanisms in neurodegenerative diseases. Exp Neurol. 2011;230:27–34. doi: 10.1016/j.expneurol.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Undela K, Gudala K, Malla S, Bansal D. Statin use and risk of Parkinson’s disease: a meta-analysis of observational studies. J Neurol. 2013;260:158–165. doi: 10.1007/s00415-012-6606-3. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Lin CH, Wu RM, Lin MS, Lin JW, Chang CH, Lai MS. Discontinuation of statin therapy associates with Parkinson disease: A population-based study. Neurology. 2013;81:410–416. doi: 10.1212/WNL.0b013e31829d873c. [DOI] [PubMed] [Google Scholar]
- 8.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez H, Elgueta D, Montoya A, Pacheco R. Neuroimmune regulation of microglial activity involved in neuroinflammation and neurodegenerative diseases. J Neuroimmunol. 2014;274:1–13. doi: 10.1016/j.jneuroim.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Hilzendeger AM, Shenoy V, Raizada MK, Katovich MJ. Neuroinflammation in pulmonary hypertension: concept, facts, and relevance. Curr Hypertens Rep. 2014;16:469. doi: 10.1007/s11906-014-0469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicchetti F, Barker RA. The glial response to intracerebrally delivered therapies for neurodegenerative disorders: is this a critical issue? Front Pharmacol. 2014;5:139. doi: 10.3389/fphar.2014.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EA, Han AR, Choi J, Ahn JY, Choi SY, Cho SW. Anti-inflammatory mechanisms of N-adamantyl-4-methylthiazol-2-amine in lipopolysaccharide-stimulated BV-2 microglial cells. Int Immunopharmacol. 2014;22:73–83. doi: 10.1016/j.intimp.2014.06.022. [DOI] [PubMed] [Google Scholar]
- 13.Depboylu C, Stricker S, Ghobril JP, Oertel WH, Priller J, Hoglinger GU. Brain-resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T-lymphocytes in the MPTP mouse model of Parkinson disease. Exp Neurol. 2012;238:183–191. doi: 10.1016/j.expneurol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 14.Dutta G, Barber DS, Zhang P, Doperalski NJ, Liu B. Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J Neurochem. 2012;122:752–763. doi: 10.1111/j.1471-4159.2012.07826.x. [DOI] [PubMed] [Google Scholar]
- 15.Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol. 2007;150:963–976. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crotty S, Fitzgerald P, Tuohy E, Harris DM, Fisher A, Mandel A, Bolton AE, Sullivan AM, Nolan Y. Neuroprotective effects of novel phosphatidylglycerol- based phospholipids in the 6-hydroxydopamine model of Parkinson’s disease. Eur J Neurosci. 2008;27:294–300. doi: 10.1111/j.1460-9568.2007.06018.x. [DOI] [PubMed] [Google Scholar]
- 19.Schieven GL. The p38alpha kinase plays a central role in inflammation. Curr Top Med Chem. 2009;9:1038–1048. doi: 10.2174/156802609789630974. [DOI] [PubMed] [Google Scholar]
- 20.Van Eldik LJ, Thompson WL, Ralay Ranaivo H, Behanna HA, Martin Watterson D. Glia proinflammatory cytokine upregulation as a therapeutic target for neurodegenerative diseases: function-based and target-based discovery approaches. Int Rev Neurobiol. 2007;82:277–296. doi: 10.1016/S0074-7742(07)82015-0. [DOI] [PubMed] [Google Scholar]
- 21.Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. doi: 10.1111/j.1526-4610.2007.00854.x. discussion 1024-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schieven GL. The biology of p38 kinase: a central role in inflammation. Curr Top Med Chem. 2005;5:921–928. doi: 10.2174/1568026054985902. [DOI] [PubMed] [Google Scholar]
- 23.Akundi RS, Candelario-Jalil E, Hess S, Hull M, Lieb K, Gebicke-Haerter PJ, Fiebich BL. Signal transduction pathways regulating cyclooxygenase- 2 in lipopolysaccharide-activated primary rat microglia. Glia. 2005;51:199–208. doi: 10.1002/glia.20198. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Blanco J, Martín V, Herrera F, García-Santos G, Antolín I, Rodriguez C. Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem. 2008;107:127–140. doi: 10.1111/j.1471-4159.2008.05588.x. [DOI] [PubMed] [Google Scholar]
- 25.Michaluk P, Mikasova L, Groc L, Frischknecht R, Choquet D, Kaczmarek L. Matrix metalloproteinase-9 controls NMDA receptor surface diffusion through integrin beta1 signaling. J Neurosci. 2009;29:6007–6012. doi: 10.1523/JNEUROSCI.5346-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebrun-Julien F, Duplan L, Pernet V, Osswald I, Sapieha P, Bourgeois P, Dickson K, Bowie D, Barker PA, Di Polo A. Excitotoxic death of retinal neurons in vivo occurs via a non-cell-autonomous mechanism. J Neurosci. 2009;29:5536–5545. doi: 10.1523/JNEUROSCI.0831-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Mahmood A, Lu D, Wu H, Xiong Y, Qu C, Chopp M. Simvastatin attenuates microglial cells and astrocyte activation and decreases interleukin-1beta level after traumatic brain injury. Neurosurgery. 2009;65:179–185. doi: 10.1227/01.NEU.0000346272.76537.DC. discussion 185-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewen T, Qiuting L, Chaogang T, Tao T, Jun W, Liming T, Guanghong X. Neuroprotective effect of atorvastatin involves suppression of TNF-alpha and upregulation of IL-10 in a rat model of intracerebral hemorrhage. Cell Biochem Biophys. 2013;66:337–346. doi: 10.1007/s12013-012-9453-z. [DOI] [PubMed] [Google Scholar]
- 29.Townsend KP, Shytle DR, Bai Y, San N, Zeng J, Freeman M, Mori T, Fernandez F, Morgan D, Sanberg P, Tan J. Lovastatin modulation of microglial activation via suppression of functional CD40 expression. J Neurosci Res. 2004;78:167–176. doi: 10.1002/jnr.20234. [DOI] [PubMed] [Google Scholar]
- 30.Tringali G, Vairano M, Dello Russo C, Preziosi P, Navarra P. Lovastatin and mevastatin reduce basal and cytokine-stimulated production of prostaglandins from rat microglial cells in vitro: evidence for a mechanism unrelated to the inhibition of hydroxy-methyl-glutaryl CoA reductase. Neurosci Lett. 2004;354:107–110. doi: 10.1016/j.neulet.2003.09.066. [DOI] [PubMed] [Google Scholar]


