Abstract
Vitamin D receptor (VDR) is a receptor of vitamin D3, which plays a pivotal role in regulating cell proliferation and differentiation, lymphocyte activation and cytokine production, and is associated with TB susceptibility. Growing studies explored the association of TaqI polymorphism of VDR with tuberculosis (TB) susceptibility. However, the results were inconsistent and conflicting. To assess the relationship between the VDR TaqI gene polymorphism and the risk of TB, a meta-analysis was performed. Databases including PubMed and EMbase were systematically searched for genetic association studies of TaqI polymorphism of VDR and tuberculosis until February 15, 2015. Data were extracted by two independent authors and pooled odds ratio (OR) with 95% confidence interval (CI) was calculated to assess the strength of the association between VDR TaqI gene polymorphism and TB risk, meta-regression and subgroup analyses were performed to identify the source of heterogeneity. Thirty-eight studies with a total of 6881 cases and 7511 controls were reviewed in the present meta-analysis. A statistically significant correlations were observed between VDR TaqI gene polymorphism and TB risk in South and West Asians (t vs. T: OR=1.27, 95% CI=1.07-1.51, P=0.007; tt vs. TT: OR=1.59, 95% CI=1.11-2.26, P=0.011; tt vs. Tt + TT: OR=1.43, 95% CI=1.17-1.73, P=0.000; tt + Tt vs. TT: OR=1.32, 95% CI=1.05-1.67, P=0.019). Heterogeneity between studies was not pronounced, and meta-regression found no source contributed to heterogeneity. However, after stratified analysis with respect to genotyping methods and sample size, significant association was found in “small” studies (<500 participants) and studies with “PCR-RFLP” methods. Synthesis of the available studies suggests that t allele of the VDR TaqI polymorphism is significantly associated with an increased TB risk in South and West Asians.
Keywords: Tuberculosis, Vitamin D receptor, TaqI polymorphisms
Introduction
Tuberculosis (TB) is one of the most common infectious diseases and the leading cause of mortality worldwide, with an estimated 9 million new cases and 1.5 million deaths occurred in 2013. According to World Health Organization (WHO) report, approximately 56% of new cases occurred in the South-East Asia and Western Pacific Regions [1]. It is well known that tuberculosis susceptibility may be influenced by multiple genetic, socio-economic and environmental factors [2,3], which contains single nucleotide polymorphisms (SNP) as a major factor.
Vitamin D (VitD) is well known to play a critical role in modulating monocyte and macrophage activity and influencing human innate immunity to certain infectious agents including M. tuberculosis [4]. Vitamin D receptor (VDR) is a nuclear hormone receptor, which upon binding to vitamin D3, interacts with Vitamin D response elements and signals other target genes. It is highly expressed on dendritic cells, activated T lymphocytes and macrophages. After binding with Vitamin D, VDR could modulate cytokine responses by T cells [5,6], and thus represents antibacterial responses in innate immunity [7]. Many recent studies have demonstrated the critical role of VDR in the inflammatory-related immune response to active TB disease [8-10]. Human VDR gene is located on chromosome 12q13.11 and contains 14 exons [11]. The VDR polymorphisms located in coding region and 3’ untranslated region are FokI, TaqI, ApaI and BsmI. Accumulated evidence has suggested that polymorphisms in the VDR gene may influence the expression and function of VDR and subsequent downstream vitamin D-mediated effect [12]. To date, several meta-analyses focused on the association of VDR TaqI polymorphisms with tuberculosis risk across different ethnicities [13-15], however, due to the limitations of sample size and broadly statistical analysis, the results were varied and inconsistent among different employed genetic models. In addition, the previous studies did not cover all eligible publications related to tuberculosis and thus resulted in biased effect sizes. To ascertain the authentic effect of VDR TaqI polymorphisms on susceptibility to tuberculosis, we conducted a meta-analysis including all eligible case-control studies focused on the relationship between the VDR TaqI polymorphism and tuberculosis risk.
Materials and methods
Literature Search
A systematic search was conducted using the databases of the US National Institutes of Health (PubMed), Web of Science and Embase databases (last search was updated on February 15 2015), with the combination of terms like: ‘VDR’OR ‘Vitamin D receptor’ OR ‘FokI’ OR ‘rs10735810’ AND ‘polymorphism’ OR ‘mutation’ OR ‘SNP’ OR ‘Single Nucleotide Polymorphism’ AND ‘tuberculosis’. To identify the extra eligible studies, the relevant published studies and review articles were manually examined. The search in these databases was limited to articles relating to humans. No language restrictions were applied.
The identified studies in our meta-analysis met all of the following criteria: (1) studies had to assess the association between Vitamin D receptor TaqI polymorphisms and tuberculosis risk; (2) case-control studies or cohort design, and studies included available genotype frequencies to calculate odds ratio (OR) and 95% confidence interval (CI); (3) independent studies using original data. Studies were excluded for the following criteria: (1) the studies not providing genotype distribution or allele frequency data; (2) reviews or case reports, case studies without control subjects; (3) duplicated previous publications. At last, 38 case-control published studies from PubMed, Web of Science and Embase were available. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist was available as supplementary material, as displayed in Checklist S1.
Data extraction
Two investigators (CY and WXJ) independently performed the required data extraction, and then conducted group discussion to resolve the disagreements. The following data were extracted from each study: publication year, the name of first author, country, ethnicity, study design, genotyping method, diagnosis method of cases, the tuberculosis type, number of cases and controls, genotype and allele frequencies for cases and controls, HIV status of cases and controls, and source of genotyping. According to the source of controls, all eligible studies were defined as hospital-based (HB) and population-based (PB). If data concerning the genotype distributions were not displayed regarding the included studies, the primary author was contacted via electronic mail to obtain the missing data.
Statistical analysis
Minor allele frequency was calculated manually based on genotypic distribution among cases and controls. We assessed Hardy-Weinberg Equilibrium (HWE) among control population for each study using Hardy-Weinberg Equilibrium Online Calculator (http://www.changbioscience.com/genetics/hardy.html). And a P value of >0.05 was considered to meet HWE.
All statistical analyses in this meta-analysis were carried out using the software Stata 12.0 (Stata Corporation, College Station, TX, USA), with two-sided p values. The extracted data from all publications were tested using five genetic models i.e. allele model (t vs. T), homozygote model (tt vs. TT), heterozygote model (Tt vs. TT), dominant model (tt + Tt vs. TT) and recessive model (tt vs. Tt + TT). Odds ratios (ORs) with a corresponding 95% confidence interval (CI) were calculated (for all five genetic models) to assess the strength of association between VDR TaqI polymorphism and the TB risk. The significance of pooled OR was measured by the Z-test (P<0.05 was considered statistically significant). Heterogeneity assumption was assessed by the x2-based Q-statistics and Higgins I2 test. Those resulting with I2>50% were identified as a heterogeneous group, then ORs were pooled according to random effect model (Mantel-Haenszel method) [16]. Otherwise the fixed effect model was adopted (DerSimonian-Laird method) [17].
Subgroup analyses were conducted based on these genetic models to define the sources of heterogeneity, according to ethnicities, sample size, tuberculosis type, HWE, the source of controls as well as for the genotyping methods. A meta-regression was used to illustrate the potential reasons for heterogeneity between the studies. We classified the studies that were conducted in Asia into two groups: East and Southeast Asia (China, South Korean, South Sumatera, Indonesia and Cambodia) and South and West Asia (India and Iran). As a result, the enrolled studies were classified into five subgroups based on ethnicity: East and Southeast Asians, South and West Asians, Africans, Europeans and Americans. Studies with more than 500 participants were defined as “large”, and studies with less 500 participants were defined as “small”.
Sensitivity analysis was conducted to evaluate stability of the results by deleting of a single study at a time, the pooled ORs were recalculated to determine whether individual study could influence the overall results. Furthermore, publication bias was identified by examining the Begg’s funnel plots [18] and Egger’s regression test [19].
Results
Characteristics of eligible studies
A total of 413 potential studies were identified by preliminary searching PubMed, Embase and Web of Science, among which 38 case-control studies were selected according to the inclusion and exclusion criteria, involving a total of 6881 tuberculosis patients and 7511 control subjects in this meta-analysis [20-57]. The detailed literature search strategy and included or excluded studies were explained in Figure 1. The baseline characteristics, such as author name, publication year, region, ethnicity, design, genotyping method, numbers about cases and controls were depicted in Table 1. The publication year of eligible studies ranged from 1999 to 2014. The study participants were broadly classified according to the predominant ancestry, including East and Southeast Asians, South and West Asians, Africans, Europeans and Americans. Three studies adopted hospital-based control [37,45,46], while the other thirty-five studies adopted population-based control. Thirty were pulmonary TB studies, two were extra-pulmonary TB studies [48,53] and the other six studies were pulmonary and extra-pulmonary merged studies [23,30,35,36,45,56]. HIV status of the studied population was considered in twenty-six studies. All of them adopted blood samples for genotyping. Genotyping for Vitamin D receptor TaqI polymorphism across all studies, twenty-nine were conducted using PCR-RFLP assay [20,21,23,25-38,40,42,44,46-53,56], and the other nine studies were merged into the “other methods” group. The results of HWE test in the control population and genotype frequencies of TaqI polymorphisms were recalculated and extracted from all eligible publications, and were shown in Table 2. Twenty-nine of the eligible studies met the HWE (P>0.05), except for nine study [21,23,25,26,28,29,31,34,40].
Figure 1.
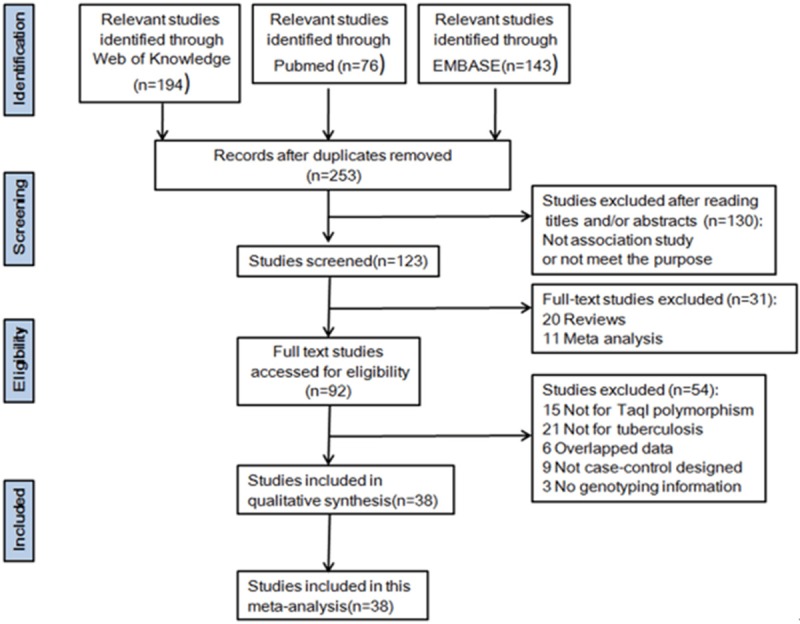
Flow diagram of search strategy and study selection process (TIF).
Table 1.
Main characteristics of included studies summarized for the meta-analysis
| Year | First Author | Country | Ethnicity | Study design | Tuberculosis | controls | HIV status | Source of genotyping | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Part of the body | Sample size | Diagnosis method | Genotyping method | Sample size | ||||||||
| 2014 | Arji N | Morocco | African | PB | Pulmonary tuberculosis | 274 | AFB smear and culture | PCR-RFLP | Healthy persons | 203 | Negative | blood |
| 2013 | Wu | China | ES Asian | PB | Pulmonary tuberculosis | 213 | Clinical symptoms bacteriology X-ray | PCR-RFLP | Healthy persons | 211 | Negative | blood |
| 2013 | Alexandra | Romania | European | PB | Pulmonary tuberculosis | 68 | Not available | ARMS-PCR | Healthy persons | 110 | Negative | blood |
| 2012 | Rathored | India | SW Asian | PB | MDR tuberculosis and drug sensitive pulmonary tuberculosis | 692 | AFB smear and culture | PCR-RFLP | Healthy persons | 205 | Negative | blood |
| 2011 | J Kim | South Korean | ES Asian | PB | Pulmonary (98) and extra pulmonary tuberculosis (62) | 160 | AFB smear and culture | Pyro sequencing | Healthy persons | 156 | Not available | blood |
| 2011 | T Kang | South Korean | ES Asian | PB | Pulmonary tuberculosis | 103 | AFB smear and culture | PCR-RFLP | Healthy persons | 105 | Not available | blood |
| 2011 | Sudarto | South Sumatera, Indonesia | ES Asian | PB | Pulmonary tuberculosis | 40 | positive acid-fast bacilli sputum examination | PCR-RFLP | Healthy persons | 40 | Not available | blood |
| 2011 | A Singh | India | SW Asian | PB | Pulmonary tuberculosis | 101 | AFB smear or culture | PCR-RFLP | Healthy persons | 225 | Negative | blood |
| 2011 | Sharma | India | SW Asian | PB | Pulmonary tuberculosis | 474 | AFB smear or culture | PCR-RFLP | Healthy persons | 607 | Not available | blood |
| 2011 | Wang X | China | ES Asian | PB | Pulmonary tuberculosis | 213 | AFB smear or culture | PCR-RFLP | Healthy persons | 211 | Not available | blood |
| 2011 | Ates | Turkey | European | PB | Pulmonary (98) and extra pulmonary tuberculosis (30) | 128 | AFB smear or culture | PCR-RFLP | Healthy persons | 80 | Not available | blood |
| 2010 | Marashian | Iran | SW Asian | PB | Pulmonary tuberculosis | 164 | AFB smear and X-ray | PCR-RFLP | contacts | 50 | Not available | blood |
| 2009 | Banoei | Iran | SW Asian | PB | Pulmonary tuberculosis | 60 | Confirmed in Massih Danes hvari | PCR-RFLP | Healthy subjects | 62 | Negative | blood |
| 2009 | Vidyarani | India | SW Asian | PB | Pulmonary tuberculosis | 40 | AFB smear and culture | PCR-RFLP | Healthy subject | 49 | Not available | blood |
| 2009 | Selvaraj | India | SW Asian | PB | Pulmonary tuberculosis | 65 | Clinical symptom, AFB smear and culture | PCR-RFLP | Healthy subjects | 60 | Negative | blood |
| 2009 | Alagarasu | India | SW Asian | PB | Pulmonary (187) and extra pulmonary tuberculosis (30) | 217 | AFB smear, clinical criteria and X-ray | PCR-RFLP | Healthy controls | 144 | Cases (51%), Controls (0) | blood |
| 2009 | Meng XJ | China | ES Asian | PB | Pulmonary (185 and extra pulmonary tuberculosis (39) | 224 | AFB smear or culture | PCR-RFLP | Healthy controls | 225 | Negative | blood |
| 2009 | Jiao WW | China | ES Asian | HB | Pulmonary tuberculosis | 125 | Clinical symptom, AFB smear and X-ray | PCR-RFLP | Healthy controls | 446 | Negative | blood |
| 2008 | Selvaraj | India | SW Asian | PB | Pulmonary tuberculosis | 51 | AFB smear and culture | PCR-RFLP | Normal health subjects | 60 | Negative | blood |
| 2008 | Liu Y.-D | China | ES Asian | PB | Pulmonary tuberculosis | 60 | AFB smear and culture | SNaPshot | Normal health subjects | 30 | Negative | blood |
| 2007 | Wilbur | Paraguay | American | PB | Pulmonary tuberculosis | 54 | Clinical symptoms, PPD test | PCR-RFLP | No symptoms | 124 | Not available | blood |
| 2007 | Olesen | Guinea-Bissau | African | PB | Pulmonary tuberculosis | 320 | AFB smear and clinical criteria | TaqMan | Healthy controls | 344 | HIV positive in 33% case sand negative in controls | blood |
| 2007 | Babb | South Africa | African | PB | Pulmonary tuberculosis | 249 | AFB smear and XRay | PCR-RFLP | No clinical history or symptoms of TB | 352 | Negative | blood |
| 2007 | Soborg | Tanzanian | African | PB | Pulmonary tuberculosis | 435 | Culture | PCR-SSP | Culture negative | 416 | HIV positive in 44% cases and 18% controls | blood |
| 2006 | Chen XR | China | ES Asian | PB | Pulmonary tuberculosis | 140 | Clinical symptoms, AFB smear and XRay | PCR-RFLP | household contacts | 139 | Negative | blood |
| 2006 | Lombard | Venda | African | HB | Pulmonary and meningeal tuberculosis | 66 | AFB smear | ARMS-PCR | Healthy controls with no history of TB | 86 | Negative | blood |
| 2004 | Bornman | Gambia, Guinea- Bissau, Guinea | African | HB | Pulmonary tuberculosis | 416 | AFB or culture | PCR-RFLP | Healthy community control subjects | 718 | Cases (12.5%), controls (6.8%) | blood |
| 2004 | Fitness | Malawi | African | PB | Pulmonary tuberculosis | 386 | AFB smear, culture and histology | PCR-RFLP | Healthy controls | 624 | Cases (67.6%), Controls (13.1%) | blood |
| 2004 | Selvaraja | India | SW Asian | PB | Spinal tuberculosis patients | 64 | X-ray and Clinical criteria | PCR-RFLP | 77 were contacts and 26 were normal healthy subjects. | 103 | Not available | blood |
| 2004 | Selvarajb | India | SW Asian | PB | Pulmonary tuberculosis | 46 | AFB smear, culture and and radiographic abnormalities | PCR-RFLP | clinically normal | 64 | Negative | blood |
| 2004 | Roth | Peru | American | PB | Pulmonary tuberculosis | 100 | AFB smear | PCR-RFLP | Two healthy controls, 1PPD+ and 1PPD- | 201 | Negative | blood |
| 2004 | Liu | China | ES Asian | PB | Pulmonary tuberculosis | 120 | AFB smear, culture and X-ray | PCR-RFLP | normal controls | 240 | Negative | blood |
| 2002 | Delgado | Cambodia | ES Asian | PB | Pulmonary tuberculosis | 358 | AFB smear | PCR-RFLP | contacts with no TB | 106 | Negative | Blood |
| 2000 | Selvaraja | India | SW Asian | PB | Spinal tuberculosis | 66 | Culture and X-ray | PCR-RFLP | contacts with no TB | 80 | Not available | Blood |
| 2000 | Selvarajb | India | SW Asian | PB | Pulmonary tuberculosis | 44 | AFB smear and culture | PCR-SSOP | contacts with no TB | 66 | Not available | Blood |
| 2000 | Selvarajc | India | SW Asian | PB | Pulmonary tuberculosis | 200 | X-ray and Clinical criteria | PCR-SSOP | patient contacts | 108 | Not available | Blood |
| 2000 | Wilkinson | India | SW Asian | PB | Pulmonary tuberculosis (27) and military tuberculosis (64) | 91 | Biopsy or culture Tuberculosis | PCR-RFLP | contacts with no TB | 116 | Negative | Blood |
| 1999 | Bellamy | Gambia | African | PB | Pulmonary tuberculosis | 408 | AFB smear | PCR-SSCP | Male donors | 414 | Negative | Blood |
a,b,c: To differentiate the different articles by the same author (Selvaraj) in the same year (2004, 2000). PB: population-based; HB: hospital-based; AFB, Acid-fast bacilli; HIV, human immunodeficiency virus; MDR, multi-drug resistance for isoniazide and rifampicin; PPD, purified protein derivative; SNPs, single nucleotide polymorphism; TB, tuberculosis; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Table 2.
Distribution of gene polymorphism of studies included in the meta-analysis
| Year | First Author | Case | Control | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| genotype | Minor allele | genotype | Minor allele | HWE | ||||||
|
|
|
|
|
|
||||||
| TT | Tt | tt | MAF | TT | Tt | tt | MAF | p-value | ||
| 2014 | Arji N | 137 | 79 | 58 | 0.36 | 109 | 48 | 46 | 0.34 | 0.9185 |
| 2013 | Fang Wu | 191 | 19 | 3 | 0.06 | 183 | 23 | 5 | 0.08 | 0.0004 |
| 2013 | Alexandra | 16 | 52 | 0 | 0.38 | 43 | 48 | 19 | 0.39 | 0.3802 |
| 2012 | J. Rathored | 290 | 285 | 117 | 0.38 | 97 | 79 | 29 | 0.33 | 0.0002 |
| 2011 | J Kim | 143 | 16 | 1 | 0.06 | 137 | 18 | 1 | 0.06 | 0.6319 |
| 2011 | T Kang | 134 | 14 | 1 | 0.05 | 148 | 85 | 8 | 0.05 | 0.0022 |
| 2011 | Sudarto | 14 | 12 | 14 | 0.50 | 17 | 9 | 14 | 0.46 | 0.0120 |
| 2011 | A. Singh | 61 | 30 | 10 | 0.25 | 132 | 60 | 33 | 0.28 | 0.0563 |
| 2011 | Sharma | 138 | 95 | 42 | 0.33 | 258 | 275 | 47 | 0.32 | 0.0002 |
| 2011 | Wang X | 191 | 19 | 3 | 0.06 | 183 | 23 | 5 | 0.08 | 0.0004 |
| 2011 | Ates | 49 | 65 | 14 | 0.36 | 30 | 39 | 11 | 0.38 | 0.7659 |
| 2010 | Marashian | 63 | 93 | 8 | 0.33 | 26 | 24 | 0 | 0.24 | 0.0256 |
| 2009 | Banoei | 8 | 33 | 19 | 0.59 | 33 | 24 | 5 | 0.27 | 0.8288 |
| 2009 | Vidyarani | 15 | 18 | 7 | 0.40 | 27 | 18 | 4 | 0.27 | 0.6863 |
| 2009 | Selvaraj | 24 | 33 | 8 | 0.38 | 27 | 21 | 12 | 0.38 | 0.0497 |
| 2009 | Alagarasu | 82 | 95 | 38 | 0.40 | 70 | 62 | 14 | 0.31 | 0.9597 |
| 2009 | Meng XJ | 154 | 66 | 4 | 0.17 | 170 | 50 | 5 | 0.13 | 0.5640 |
| 2009 | Jiao WW | 113 | 12 | 0 | 0.05 | 387 | 58 | 1 | 0.07 | 0.4423 |
| 2008 | Selvaraj | 18 | 23 | 10 | 0.42 | 34 | 22 | 4 | 0.25 | 0.8633 |
| 2008 | Liu Y.-D | 54 | 5 | 1 | 0.06 | 24 | 6 | 0 | 0.10 | 0.5428 |
| 2007 | Wilbur | 22 | 28 | 4 | 0.33 | 59 | 58 | 5 | 0.28 | 0.0438 |
| 2007 | Olesen | 150 | 145 | 25 | 0.30 | 161 | 150 | 34 | 0.32 | 0.9132 |
| 2007 | Babb | 136 | 94 | 19 | 0.27 | 190 | 140 | 22 | 0.26 | 0.5723 |
| 2007 | Soborg | 247 | 172 | 19 | 0.24 | 233 | 162 | 30 | 0.26 | 0.7997 |
| 2006 | Chen XR | 137 | 3 | 0 | 0.01 | 134 | 5 | 0 | 0.02 | 0.8290 |
| 2006 | Lombard | 51 | 30 | 5 | 0.23 | 47 | 34 | 1 | 0.22 | 0.0571 |
| 2004 | Bornman | 174 | 132 | 37 | 0.30 | 331 | 253 | 50 | 0.28 | 0.8644 |
| 2004 | Fitness | 261 | 154 | 22 | 0.23 | 384 | 241 | 47 | 0.25 | 0.2791 |
| 2004 | Selvaraja | 27 | 28 | 9 | 0.36 | 40 | 48 | 14 | 0.37 | 0.9470 |
| 2004 | Selvarajb | 13 | 23 | 10 | 0.47 | 27 | 27 | 10 | 0.37 | 0.8388 |
| 2004 | Roth | 90 | 10 | 0 | 0.05 | 169 | 31 | 1 | 0.08 | 0.9928 |
| 2004 | Liu | 105 | 12 | 3 | 222/18 | 203 | 32 | 5 | 438/42 | 0.4821 |
| 2002 | Delgado | 325 | 30 | 3 | 680/36 | 96 | 10 | 0 | 202/10 | 0.6103 |
| 2000 | Selvaraja | 27 | 30 | 9 | 84/48 | 32 | 38 | 10 | 102/58 | 0.8042 |
| 2000 | Selvarajb | 15 | 21 | 8 | 51/37 | 22 | 37 | 7 | 81/51 | 0.1386 |
| 2000 | Selvarajc | 79 | 90 | 31 | 248/152 | 51 | 42 | 15 | 144/72 | 0.1939 |
| 2000 | Wilkinson | 39 | 46 | 6 | 124/58 | 45 | 58 | 13 | 148/84 | 0.3750 |
| 1999 | Bellamy | 204 | 177 | 27 | 585/231 | 188 | 177 | 49 | 553/275 | 0.4603 |
a,b,c: To differentiate the different articles by the same author (Selvaraj) in the same year (2004, 2000). HWE, Hardy-Weinberg equilibrium; MAF, minor allele frequency.
Quantitative data synthesis
Pooled analysis. Table 2 shows the genotype distribution and allele frequencies in the original 38 studies. The overall frequency of t allele in TaqI polymorphism was 25.3% in cases and 23.9% in controls. This analysis, using five different genetic models showed low heterogeneity (I2 range =37-47.5% for all comparisons). The significant association has not been detected in all the five genetic models between TaqI polymorphism and risk of tuberculosis (Table 3).
Table 3.
Meta-analysis results
| t vs. T | tt vs. TT | tt vs. Tt + TT | Tt vs. TT | tt + Tt vs. TT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| N | OR (95% CI) | Heterogeneity (I2, PQ) | OR (95% CI) | Heterogeneity (I2, PQ) | OR (95% CI) | Heterogeneity (I2, PQ) | OR (95% CI) | Heterogeneity (I2, PQ) | OR (95% CI) | Heterogeneity (I2,PQ) | |
| Total | 38 | 1.03 (0.97, 1.09)F | 47.5%; 0.001 | 1.09 (0.96, 1.25)F | 46.9%, 0.001 | 1.05 (0.92, 1.19)F | 42.2%, 0.004 | 1.03 (0.96, 1.12)F | 37%, 0.013 | 1.04 (0.96, 1.12)F | 40.3%, 0.006 |
| Ethnicities | |||||||||||
| ES Asians | 11 | 0.93 (0.77, 1.11)F | 0%; 0.707 | 0.93 (0.55, 1.57)F | 0%, 0.996 | 0.89 (0.53, 1.48)F | 0%, 0.998 | 0.94 (0.76, 1.16)F | 0%, 0.494 | 0.93 (0.76, 1.14)F | 0%, 0.587 |
| SW Asians | 15 | 1.27 (1.07, 1.51)* | 63.0%; 0.001 | 1.59 (1.11, 2.26)* | 56.5%, 0.004 | 1.43 (1.17, 1.73)*,F | 46%, 0.026 | 1.25 (0.99, 1.59) | 57.8%, 0.003 | 1.32 (1.05, 1.67)* | 61.6%, 0.001 |
| Africans | 8 | 0.95 (0.87, 1.03)F | 0%; 0.432 | 0.86 (0.64, 1.16) | 51.2%, 0.045 | 0.86 (0.64, 1.15) | 51.9%, 0.042 | 0.98 (0.88, 1.10)F | 0%, 0.927 | 0.96 (0.86, 1.07)F | 0%, 0.884 |
| Americans | 2 | 0.92 (0.43, 1.97) | 67.9%; 0.078 | 1.69 (0.48, 5.96)F | 0%, 0.488 | 1.56 (0.46, 5.33)F | 0%, 0.559 | 0.91 (0.43, 1.91) | 54.2%, 0.14 | 0.92 (0.40, 2.09) | 63.7%, 0.097 |
| Europeans | 2 | 0.94 (0.70, 1.27)F | 0%; 0.894 | 0.32( 0.03,3.85) | 66.2%, 0.086 | 0.20 (0.01, 6.66) | 82.5%, 0.017 | 1.70 (0.61, 4.74) | 79.9%, 0.026 | 1.39 (0.66, 2.95) | 65.1%, 0.091 |
| Sample size | |||||||||||
| Largea | 9 | 0.97 (0.89, 1.04)F | 29.0%; 0.187 | 0.96 (0.70, 1.31) | 61.7%, 0.008 | 0.98 (0.70, 1.37) | 68.7%, 0.001 | 0.94 (0.85, 1.04)F | 12.1%, 0.334 | 0.94 (0.85, 1.04)F | 0%, 0.530 |
| Smallb | 29 | 1.12 (1.03, 1.23)*,F | 47.2%; 0.003 | 1.24 (1.02, 1.52)*,F | 39.1%, 0.019 | 1.09 (0.91, 1.31)F | 25.6%, 0.109 | 1.18 (1.05, 1.33)*,F | 33.4%, 0.043 | 1.17 (1.05, 1.31)*,F | 41%, 0.012 |
| Genotyping method | |||||||||||
| PCR-RFLP | 29 | 1.10 (0.98, 1.23) | 51.8%; 0.001 | 1.26 (1.08, 1.46)*,F | 40.6%, 0.015 | 1.20 (1.04, 1.39)*,F | 29.5%, 0.073 | 1.04 0.94, 1.14)F | 37.3%, 0.024 | 1.06 (0.97, 1.16)F | 45.1%, 0.005 |
| Other methods | 9 | 0.92 (0.83, 1.03)F | 0%; 0.594 | 0.73 (0.56, 0.95)*,F | 19.4%, 0.27 | 0.69 (0.54, 0.90)*,F | 45.6%, 0.065 | 1.03 (0.89, 1.19)F | 42.8%, 0.082 | 0.97 (0.85, 1.12)F | 19.4%, 0.270 |
| Source of controls | |||||||||||
| Contactsc | 11 | 1.06 (0.93, 1.21)F | 0.0%; 0.526 | 1.15 (0.83, 1.60)F | 0%, 0.857 | 1.13 (0.82, 1.55)F | 0%, 0.875 | 1.05 (0.88, 1.25)F | 0%, 0.510 | 1.06 (0.90, 1.25)F | 0%, 0.478 |
| Healthyd | 27 | 1.06 (0.95, 1.18) | 57.5%; 0.000 | 1.16 (0.89, 1.53) | 58.8%, 0.000 | 1.07 (0.84, 1.38) | 55%, 0.000 | 1.03 (0.94, 1.12)F | 47.4%, 0.004 | 1.08 (0.94, 1.23) | 50.2%, 0.002 |
| HWE | |||||||||||
| PHWE>0.05 | 29 | 1.05 (0.94, 1.17) | 54.9%; 0.000 | 1.14 (0.87, 1.48) | 53.9%, 0.000 | 0.97 (0.84, 1.12)F | 43.3%, 0.008 | 1.05 (0.96, 1.15)F | 34.9%, 0.034 | 1.04 (0.95, 1.13)F | 45.1%, 0.005 |
| PHWE<0.05 | 9 | 1.09 (0.96, 1.23)F | 0%; 0.476 | 1.35 (1.02, 1.78)*,F | 0%, 0.569 | 1.33 (1.02, 1.73)*,F | 27.3%, 0.201 | 0.97 (0.82, 1.16)F | 47.2%, 0.056 | 1.03 (0.87, 1.21)F | 27.5%, 0.200 |
| Tuberculosis type | |||||||||||
| pulmonary | 29 | 1.05 (0.95, 1.17) | 53.2%; 0.000 | 1.15 (0.90, 1.48) | 52.6%, 0.001 | 1.03 (0.90, 1.18)F | 48.5%, 0.002 | 1.03 (0.94, 1.12)F | 46%, 0.003 | 1.02 (0.94, 1.11)F | 47.9%, 0.002 |
| Extra and pulmonary | 7 | 1.13 (0.95, 1.33)F | 30%; 0.21 | 1.29 (0.84, 1.96)F | 39.6%, 0.141 | 1.22 (0.81, 1.82)F | 35.7%, 0.169 | 1.12 (0.90, 1.39)F | 0%, 0.545 | 1.14 (0.93, 1.41)F | 0.9%, 0.410 |
| extra | 2 | 0.97 (0.70, 1.36)F | 0%; 0.855 | 1.00 (0.49, 2.04)F | 0%, 0.876 | 1.06 (0.55, 2.06)F | 0%, 0.915 | 0.90 (0.55, 1.46)F | 0%, 0.873 | 0.92 (0.58, 1.46)F | 0%, 0.855 |
Abbreviations: N: number of studies included; OR: odds ratio; Ph: p value for heterogeneity; PQ: Cochran’s Q statistics; I2: Higgin’s I2 statistics.
Results derived using Fixed effects for analysis. Random effects were used for all other calculations.
OR with statistical significance;
studies with more than 500 participants;
studies with less than 5000 participants;
studies with controls from patient contacts;
studies with controls from healthy person.
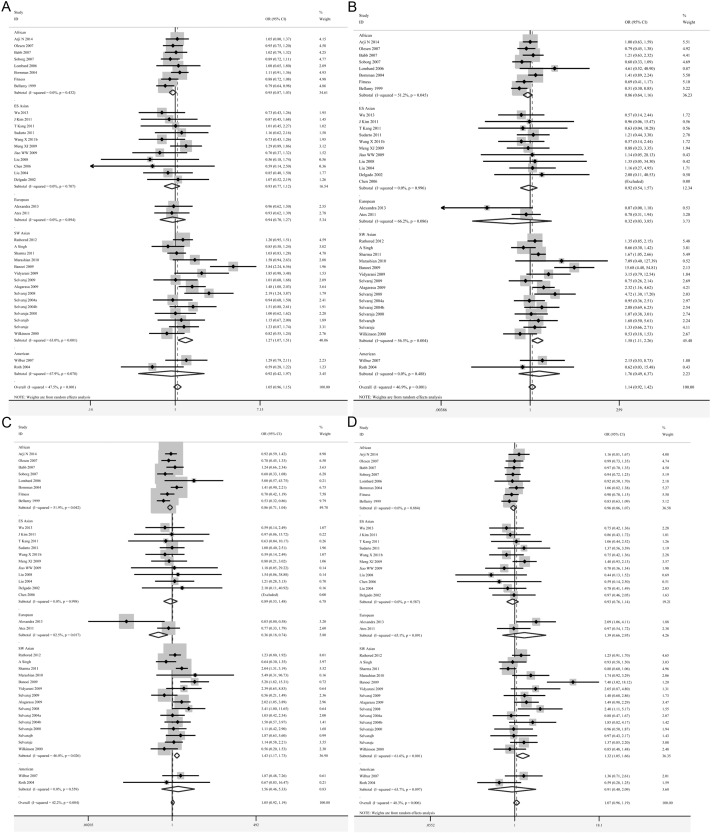
Subgroup analysis. Subgroup meta-analyses according to different races (Africans, East and Southeast Asians, South and West Asians, Americans and Europeans) have been conducted based on the five genetic models. 15 studies belonged to the South and West Asians [23,27,28,31-35,38,48,49,53-56], 11 to the East and Southeast Asians [21,24-26,36,37,39,44,51,52], 8 to the Africans [20,41-43,45-47,57], 2 to the Americans [40,50] and 2 to the Europeans [22,30] group. Appropriate effects were used for further analysis according to the heterogeneity. We found significant positive correlations between the t allele polymorphisms and increased risks of tuberculosis in South and West Asians (t vs. T: OR=1.27, 95% CI=1.07-1.51, P=0.007; tt vs. TT: OR=1.59, 95% CI=1.11-2.26, P=0.011; tt vs. Tt + TT: OR=1.43, 95% CI=1.17-1.73, P=0.000; tt + Tt vs. TT: OR=1.32, 95% CI=1.05-1.67, P=0.019) (Table 3; Figure 2A-D). However, the Africans, East and Southeast Asians, Americans and Europeans groups showed no significant difference in all five genetic models (Table 3).
Figure 2.
Forest plots showing the association of the TaqI polymorphisms with risk of tuberculosis for five ancestral subgroups. A. t vs. T; B. tt vs. TT; C. tt vs. Tt + TT; D. tt + Tt vs. TT (TIF).
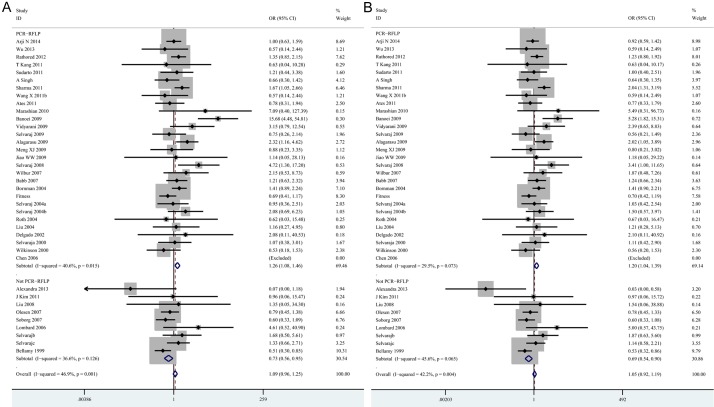
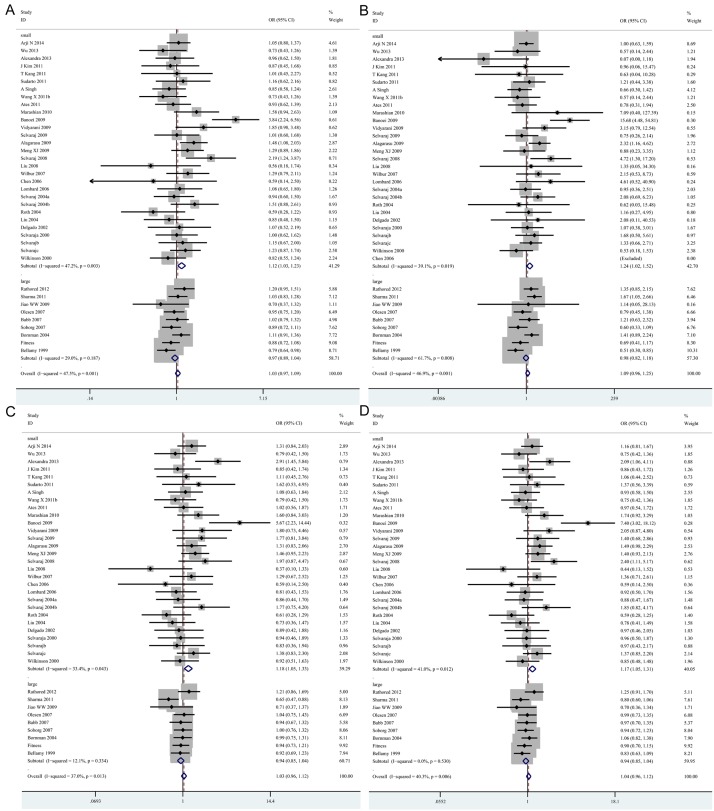
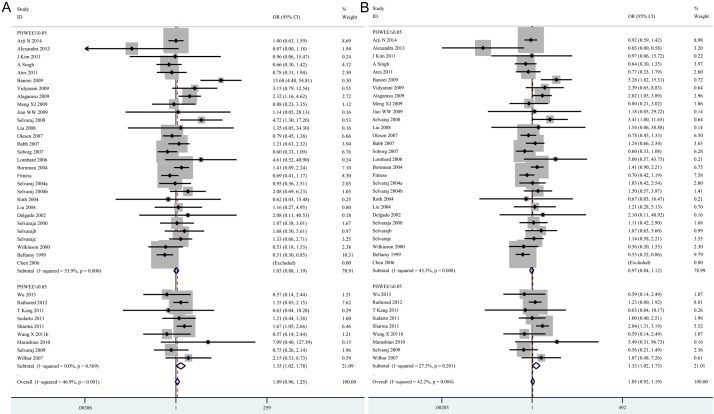
In a further stratified analysis by genotyping methods, significant associations were observed in the studies using PCR-RLFP for homozygote model (tt vs. TT: OR=1.26, 95% CI=1.08-1.46, P=0.004) and recessive model (tt vs. Tt + TT: OR=1.20, 95% CI=1.04-1.39, P=0.014). In contrast, decreased risks of tuberculosis was found in studies using other methods for homozygote model (tt vs. TT: OR=0.73, 95% CI=0.56-0.95, P=0.019) and recessive model (tt vs. Tt + TT: OR=0.69, 95% CI=0.54-0.90, P=0.005) (Table 3; Figure 3A, 3B). In the stratified analysis by sample size, statistically significant associations were found in the “small” studies for allele model (t vs. T: OR=1.12, 95% CI=1.03-1.23, P= 0.010), homozygote model (tt vs. TT: OR=1.24, 95% CI=1.02-1.52, P=0.030), heterozygote model (Tt vs. TT: OR=1.18, 95% CI=1.05-1.33, P=0.007) and dominant model (tt + Tt vs. TT: OR=1.17, 95% CI=1.05-1.31, P=0.006), respectively (Table 3; Figure 4A-D). However, there was no significant difference in “big” studies [23,28,37,41-43,46,47,57] for all five models. In subgroup analyses according to HWE in controls, significant associations were observed in the studies not in HWE for homozygote model (tt vs. TT: OR=1.35, 95% CI=1.02-1.78, P=0.034) and recessive model (tt vs. Tt + TT: OR=1.33, 95% CI=1.02-1.73, P=0.035) (Table 3; Figure 5A, 5B). However, when stratified by source of control and tuberculosis type, statistical significant association was not detected in all subgroups.
Figure 3.
Forest plots showing the association of the TaqI polymorphisms with risk of tuberculosis for genotyping methods. A. tt vs. TT; B. tt vs. Tt + TT (TIF).
Figure 4.
Forest plots showing the association of the TaqI polymorphisms with risk of tuberculosis for sample sizes. A. t vs. T; B. tt vs. TT; C. Tt vs. TT; D. tt + Tt vs. TT (TIF).
Figure 5.
Forest plots showing the association of the TaqI polymorphisms with risk of tuberculosis for HWE. A. tt vs. TT; B. tt vs. Tt + TT (TIF).
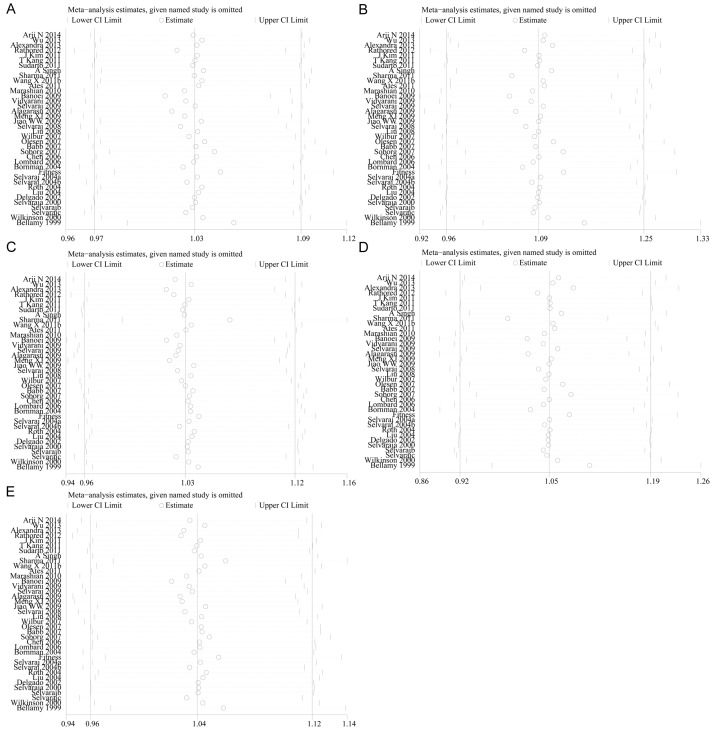
Sensitivity analysis
Sensitivity analysis was conducted to evaluate the root of heterogeneity in every genetic model. The pooled OR in none of the studied genetic models affected by excluding studies one after another (Figure 6). This indicates that this meta-analysis is reliable in nature.
Figure 6.
Sensitivity analysis for heterogeneity. A. t vs. T; B. tt vs. TT; C. Tt vs. TT; D. tt vs. Tt + TT; E. tt + Tt vs. TT (TIF).
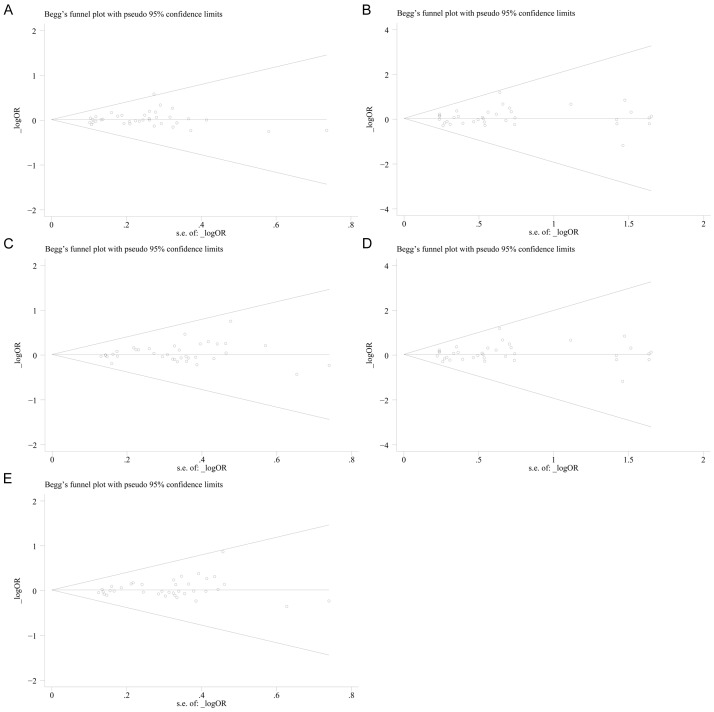
Publication bias
The Begg’s funnel plot and the Egger’s linear regression test were performed to evaluate the publication bias of all included studies. The funnel plots seemed symmetrical under all the five genetic models (Figure 7A. t vs. T: z=0.16, P=0.870; Figure 7B. tt vs. TT: z=0.55, P=0.583; Figure 7C. Tt vs. TT: z=0.49, P=0.624; Figure 7D. tt + Tt vs. TT: z=1.29, P=0.195; Figure 7E. tt vs. Tt + TT: z=0.58, P=0.565). Egger’s test also suggested that there was no significant publication bias under all the genetic models (Figure 8A. t vs. T: t=0.85, P=0.402; Figure 8B. tt vs. TT: t=0.83, P=0.413; Figure 8C. Tt vs. TT: t=1.41, P=0.168; Figure 8D. tt + Tt vs. TT: t=1.58, P=0.124; Figure 8E. tt vs. Tt + TT: t=0.89, P=0.377)
Figure 7.
Begg’s funnel plots for publication bias test. A. t vs. T; B. tt vs. TT; C. Tt vs. TT; D. tt vs. Tt + TT; E. tt + Tt vs. TT (TIF).
Figure 8.
Egger’s linear regression plots for publication bias test. A. t vs. T; B. tt vs. TT; C. Tt vs. TT; D. tt vs. Tt + TT; E. tt + Tt vs. TT (TIF).
Discussion
Extensive evidence regarding the potential association between VDR TaqI polymorphism and TB risk, however, the results from published studies remain controversial. The discrepancies may be partly attributed to differing genetic backgrounds and environment among various populations. Up till now, there were three meta-analyses investigating the correlation between VDR TaqI polymorphism and TB risk. Nevertheless, due to the limitations of relatively smaller sample size, it failed detect any genetic associations either in the overall analysis or in subgroup analyses stratified by ethnicity [13-15]. To adjust for potential confounding factors, we examined the data in this meta-analysis, adding more recently published studies, by regional stratification, sample size and genotyping methods, to evaluate the association between VDR TaqI polymorphisms and TB risk more comprehensively and rigorously. Our pooled meta-analysis found there to be no significant evidence on the association between the VDR TaqI polymorphism and human tuberculosis risk, but subgroup results suggested an ethnic-specific increased risk in genotypes carrying the minor (t) allele in the SW Asian population, whereas the tuberculosis risk was positively related to the “small” sample size and negatively related to the “others” genotyping methods.
TB is a serious public health problem in worldwide which prevention and control dependent on many factors such as early diagnosis, drug resistance, vaccine and HIV co-infection. In the past decades, the association between genetic factors and host susceptibility to TB has been widely studied. VDR is known as an intracellular hormone receptor. This receptor exerts immune modulatory effects in regulating cell proliferation and differentiation, lymphocyte activation and cytokine production, and is associated with TB susceptibility [4]. Underlying mechanisms have been proposed based on its function as vitamin D receptor. Vitamin D is an immunomodulator hormone that enhances macrophage phagocytosis of live M. tuberculosis and induces the expression of antimicrobial peptide cathelicidin which restricts the growth of M. tuberculosis in monocytes [7]. Vitamin D exerts its actions through vitamin D receptor (VDR), which variants may influence VDR activity and subsequent downstream vitamin D-mediated effect [12]. One of the most widely studied polymorphism in human VDR gene is TaqI, which is located in exon 9. This gene polymorphism is located within the 3’ untranslated region which is known to be involved in regulation of gene expression, especially through regulation of VDR mRNA stability, thus affects the circulating 25-hydroxyvitamin D levels. Positive association between VDR TaqI polymorphisms and the tuberculosis infections has provided strong evidence for this hypothesis. However, this polymorphism might have diverse roles in different ethnic populations: significantly associations with TB were observed in SW Asian population, but not among ES Asians, Africans, Europeans and Americans. It might be owing to pertinent environmental factors which were able to influence serum vitamin D concentrations on different populations, including dietary factors, intensity and hours of sunlight. Additionally, the different genotype frequencies of VDR TaqI polymorphisms between populations may contribute to inconsistent associations with tuberculosis risk. The other stratified analyses suggested sample size might partly affect the association between VDR TaqI gene polymorphism and TB risk. There was a significantly increased TB risk of four genetic models of VDR TaqI gene polymorphism in “small” studies, but insignificant association was found in “large” studies. It was worth noting that seven out of nine of the “large” studies were based on Africans in which a trend of reduced TB risk was observed in t allele genotype. Additionally, small sample size studies tend to overestimate the influence of genetic factors [58]. In this meta-analysis, we also found an increased TB risk of two genetic models in studies with PHWE<0.05. It is probable that studies without HWE in controls hint a non-random inclusion or genotyping error, which may led to misleading results. Interestingly, we observed that significant association was reversed in “other methods” studies relative to in “PCR-RFLP” studies. It may be due to high detection rate of PCR-RFLP methods. Thus, more “large” studies in agreement with HWE based on SW Asians which used PCR-RFLP methods are required to quantify this effect size reliably.
In the present study, the subgroup analyses suggested ethnicities, source of controls and tuberculosis type might partly explain the moderate heterogeneity between studies observed under some genetic models. Moreover, the heterogeneity was not remarkably decreased upon exclusion of the studies that deviated from the HWE (Table S2). Further meta-regression analyses were performed as well to identify potential sources of the heterogeneity (Table S1). However, we could not find the source among publication years, ethnicity, sample sizes, HWE, genotype methods and source of controls. We could not further explore the source of heterogeneity because not all necessary information could be obtained from all the studies included. However, eligible studies were conducted in 19 countries in this meta-analysis, thus the cause of heterogeneity may partly due to the environmental factors quite different in these countries which may influence VDR gene expression and modulate genotype-related risk, gene-environment interaction. Additionally, the different experimental designs, diagnosis standards, ages of participants and HIV status also may contribute to the heterogeneity.
Current systematic review has several limitations that require careful consideration. First, the interactions of environmental risk factors, other co-variables and host cells might elucidate the mechanism by which TaqI polymorphism increase TB risk. More original data need to be obtained to interpret the gene environment interactions. Second, only articles in English or Chinese were included, which may impeded the completeness of evidence and deviate the results. Third, relevant stratifications could not be made for many studies due to incomplete information (e.g., by diagnosis standards, ages of participants or HIV status). In addition, in the subgroup analysis according to regional geography, only 2 studies concerning the relationship between the TaqI polymorphism and the Americans and Europeans were included; such a small sample size makes the analyses be prone to bias. Thus, further studies on the association of the TaqI polymorphism with TB risk are warranted to verify current findings. Fourth, some of the included studies did not mention whether their study populations were in HWE. Based on the data supplied by the articles and own calculations, significant deviations from HWE (P<0.05) in controls were observed for nine studies on TaqI polymorphisms. Their results should be interpreted with greater caution. We therefore repeated the meta-analyses after exclusion of these studies. However, this exclusion did not materially affect the results (Table S2).
In conclusion, results from this meta-analysis demonstrate that VDR TaqI polymorphism is associated with increased TB risk in SW Asians, while the relationship between tuberculosis risk and Americans and Europeans need to be proved in future large scale studies. However, due to the moderate strength of the associations, their values to be used for risk prediction should be considered cautiously and future large scale case-control studies are required to validate these findings.
Acknowledgements
The study was supported by key project of 309th Hospital to Y.C. (2014ZD-004). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.World Health Organization. Global tuberculosis Report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.Hill AV. Aspects of genetic susceptibility to human infectious diseases. Annu Rev Genet. 2006;40:469–486. doi: 10.1146/annurev.genet.40.110405.090546. [DOI] [PubMed] [Google Scholar]
- 3.Pacheco AG, Moraes MO. Genetic polymorphisms of infectious diseases in case-control studies. Dis Markers. 2009;27:173–186. doi: 10.3233/DMA-2009-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haussler MR, Whitfield GK, Hausler CA. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:324–49. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 5.Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371:1–12. doi: 10.1016/j.cca.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Grange JM, Davies PD, Brown RC, Woodhead JS, Kardjito T. A study of vitamin D levels in Indonesian patients with untreated pulmonary tuberculosis. Tubercle. 1985;66:187–91. doi: 10.1016/0041-3879(85)90035-2. [DOI] [PubMed] [Google Scholar]
- 7.Sasidharan PK, Rajeev E, Vijayakumari V. Tuberculosis and vitamin D deficiency. J Assoc Physicians India. 2002;50:554–8. [PubMed] [Google Scholar]
- 8.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K. IFN-gamma- and TNF-independen t vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 9.Chandra G, Selvaraj P, Jawahar MS, Banurekha VV, Narayanan PR. Effect of vitamin D3 on phagocytic potential of macrophages with live Mycobacterium tuberculosis and lymphoproliferative response in pulmonary tuberculosis. J Clin Immunol. 2004;24:249–57. doi: 10.1023/B:JOCI.0000025446.44146.52. [DOI] [PubMed] [Google Scholar]
- 10.Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1, 25 Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40:128–34. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Kang TJ, Jin SH, Yeum CE, Lee SB, Kim CH, Lee SH, Kim KH, Shin ES, Chae GT. Vitamin D receptor gene Taq I, Bsm I and Fok I polymorphisms in Korean patients with tuberculosis. Immune Netw. 2011;11:253–257. doi: 10.4110/in.2011.11.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellamy R, Ruwende C, Corrah T. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 13.Lewis SJ, Baker I, Davey Smith G. Meta-analysis of vitamin D receptor polymorphisms and pulmonary tuberculosis risk. Int J Tuberc Lung Dis. 2005;9:1174–1177. [PubMed] [Google Scholar]
- 14.Gao L, Tao Y, Zhang L. Vitamin D receptor genetic polymorphisms and tuberculosis: updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2010;14:15–23. [PubMed] [Google Scholar]
- 15.Areeshi MY, Mandal RK, Akhter N, Panda AK, Haque S. Evaluating the Association between TaqI Variant of Vitamin D Receptor Gene and Susceptibility to Tuberculosis: A Meta-analysis. Toxicol Int. 2014;21:140–7. doi: 10.4103/0971-6580.139791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;3:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arji N, Busson M, Iraqi G, Bourkadi JE, Benjouad A. Genetic diversity of TLR2, TLR4, and VDR loci and pulmonary tuberculosis in Moroccan patients. J Infect Dev Ctries. 2014;15:430–40. doi: 10.3855/jidc.3820. [DOI] [PubMed] [Google Scholar]
- 21.Wu F, Zhang W, Zhang L, Wu J, Li C, Meng X, Wang X, He P, Zhang J. NRAMP1, VDR, HLA-DRB1, and HLA-DQB1 gene polymorphisms in susceptibility to tuberculosis among the Chinese Kazakh population: a case-control study. Biomed Res Int. 2013;2013:484535. doi: 10.1155/2013/484535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexandra SG, Georgiana DC, Nicoleta C, Daniela PM, Traian S, Veronica S. Apa I and Taq I polymorphisms of VDR (vitamin D receptor) gene in association with susceptibility to tuberculosis in the Romanian population. Romanian Biotechnological Letters. 2013;18:7956–7962. [Google Scholar]
- 23.Rathored J, Sharma SK, Singh B, Banavaliker JN, Sreenivas V. Risk and outcome of multidrug-resistant tuberculosis: vitamin D receptor polymorphisms and serum 25(OH)D. Int J Tuberc Lung Dis. 2012;16:1522–1528. doi: 10.5588/ijtld.12.0122. [DOI] [PubMed] [Google Scholar]
- 24.Kim JS, Ahn JH, Park CK, Yoon HK, Kang JY. Influence of Vitamin D Receptor Polymorphism on Tuberculosis Among South Korean. Chest. 2011;140:780A. [Google Scholar]
- 25.Kang TJ, Jin SH, Yeum CE, Lee SB, Kim CH. Vitamin D Receptor Gene Taq I, Bsm I and Fok I Polymorphisms in Korean Patients with Tuberculosis. Immune Network. 2011;11:253–257. doi: 10.4110/in.2011.11.5.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sudarto , Ahmad Z, Arrahmanda K, Ergan D, Yuwono Effect of TaqI vitamin D receptor gene polymorphism on the incidence of pulmonary tuberculosis. Respirology. 2011;16S:78. [Google Scholar]
- 27.Singh A, Gaughan JP, Kashyap VK. SLC11A1 and VDR gene variants and susceptibility to tuberculosis and disease progression in East India. Int J Tuberc Lung Dis. 2011;15:1468–1474. i. doi: 10.5588/ijtld.11.0089. [DOI] [PubMed] [Google Scholar]
- 28.Sharma PR, Singh S, Jena M, Mishra G, Prakash R. Coding and non-coding polymorphisms in VDR gene and susceptibility to pulmonary tuberculosis in tribes, castes and Muslims of Central India. Infect Genet Evol. 2011;11:1456–1461. doi: 10.1016/j.meegid.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Yang YJ, WF , ZL , Zhang WJ. Case-control study of gene polymorphisims with susceptibility to tuberculosis in Hazakhs of Xinjiang Chinese. Journal of Zoonoses. 2011;27:206–211. [Google Scholar]
- 30.Ates O, Dolek B, Dalyan L, Musellim B, Ongen G, Topal-Sarikaya A. The association between BsmI variant of vitamin D receptor gene and susceptibility to tuberculosis. Mol Biol Rep. 2011;38:2633–2636. doi: 10.1007/s11033-010-0404-8. [DOI] [PubMed] [Google Scholar]
- 31.Marashian SM, Farnia P, Setf S, Anooshen S, Velayati AA. Evaluating the role of vitamin D receptor polymorphisms on susceptibility to tuberculosis among Iranian patients: a case-control study. Tuberk Toraks. 2010;58:147–153. [PubMed] [Google Scholar]
- 32.Banoei MM, Mirsaeidi MS, Houshmand M, Tabarsi P, Ebrahimi G. Vitamin D receptor homozygote mutant tt and bb are associated with susceptibility to pulmonary tuberculosis in the Iranian population. Int J Infect Dis. 2010;14:E84–E85. doi: 10.1016/j.ijid.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Vidyarani M, Selvaraj P, Raghavan S, Narayanan PR. Regulatory role of 1, 25-dihydroxyvitamin D3 and vitamin D receptor gene variants on intracellular granzyme A expression in pulmonary tuberculosis. Exp Mol Pathol. 2009;86:69–73. doi: 10.1016/j.yexmp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Selvaraj P, Prabhu Anand S, Harishankar M, Alagarasu K. Plasma 1,25 dihydroxy vitamin D3 level and expression of vitamin d receptor and cathelicidin in pulmonary tuberculosis. J Clin Immunol. 2009;29:470–478. doi: 10.1007/s10875-009-9277-9. [DOI] [PubMed] [Google Scholar]
- 35.Alagarasu K, Selvaraj P, Swaminathan S, Narendran G, Narayanan PR. 5’ Regulatory and 3’ Untranslated Region Polymorphisms of Vitamin D Receptor Gene in South Indian HIV and HIV-TB Patients. J Clin Immunol. 2009;29:196–204. doi: 10.1007/s10875-008-9234-z. [DOI] [PubMed] [Google Scholar]
- 36.Meng XJ, Liu S, Zhang XQ, Fan YJ, Li CZ. Association of the polymorphism for the vitamin D receptor gene and the susceptibility to pulmonary tuberculosis in population of Chinese Uighurs. Chinese Journal of Zoonoses. 2009;25:507–510. [Google Scholar]
- 37.Jiao WW, Li ZN, Sun L, Zhao SY, Li HM, Jiao AX, Guo YJ. Vitamin D receptor gene polymorphisms and susceptibility to pediatric tuberculosis among the Chinese Han population. Chinese Journal of Practical Pediatrics Apr. 2009;24:264–266. [Google Scholar]
- 38.Selvaraj P, Vidyarani M, Alagarasu K, Prabhu Anand S, Narayanan PR. Regulatory role of promoter and 3’ UTR variants of vitamin D receptor gene on cytokine response in pulmonary tuberculosis. J Clin Immunol. 2008;28:306–313. doi: 10.1007/s10875-007-9152-5. [DOI] [PubMed] [Google Scholar]
- 39.Liu YD, Xiao HP, Sha W, Zheng RJ, Liu ZB, et al. Distribution of vitamin D receptor gene polymorphisms among new and recurrent pulmonary tuberculosis. Chin J Infect Chemot her. 2008;8:289–292. [Google Scholar]
- 40.Wilbur AK, Kubatko LS, Hurtado AM, Hill KR, Stone AC. Vitamin D receptor gene polymorphisms and susceptibility M. tuberculosis in native Paraguayans. Tuberculosis (Edinb) 2007;87:329–337. doi: 10.1016/j.tube.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Olesen R, Wejse C, Velez DR, Bisseye C, Sodemann M. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 2007;8:456–467. doi: 10.1038/sj.gene.6364410. [DOI] [PubMed] [Google Scholar]
- 42.Babb C, van der Merwe L, Beyers N, Pheiffer C, Walzl G. Vitamin D receptor gene polymorphisms and sputum conversion time in pulmonary tuberculosis patients. Tuberculosis (Edinb) 2007;87:295–302. doi: 10.1016/j.tube.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Søborg C, Andersen AB, Range N, Malenganisho W, Friis H. Influence of candidate susceptibility genes on tuberculosis in a high endemic region. Mol Immunol. 2007;44:2213–2220. doi: 10.1016/j.molimm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Chen XR, Feng YL, Ma Y, Zhang ZD, Li CY. Study on the Association of Two Polymorphisms of the Vitamin D Receptor (VDR) Gene with the Susceptibility to Pulmonary Tuberculosis (PTB) in Chinese Tibetans. J Si chuan Univ (Med Sci Edi) 2006;37:847–851. [PubMed] [Google Scholar]
- 45.Lombard Z, Dalton DL, Venter PA, Williams RC, Bornman L. Association of HLA-DR, -DQ, and vitamin D receptor alleles and haplotypes with tuberculosis in the Venda of South Africa. Hum Immunol. 2006;67:643–654. doi: 10.1016/j.humimm.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Bornman L, Campbell SJ, Fielding K, Bah B, Sillah J. Vitamin D receptor polymorphisms and susceptibility to tuberculosis in West Africa: A case-control and family study. J Infect Dis. 2004;190:1631–1641. doi: 10.1086/424462. [DOI] [PubMed] [Google Scholar]
- 47.Fitness J, Floyd S, Warndorff DK, Sichali L, Malema S, Crampin AC, Fine PE, Hill AV. Large-scale candidate gene study of tuberculosis susceptibility in the karonga district of northern Malawi. Am J Trop Med Hyg. 2004;71:341–349. [PubMed] [Google Scholar]
- 48.Selvaraj P, Kurian SM, Chandra G, Reetha AM, Charles N. Vitamin D receptor gene variants of BsmI, ApaI, TaqI, and FokI polymorphisms in spinal tuberculosis. Clin Genet. 2004;65:73–76. doi: 10.1111/j..2004.00183.x. [DOI] [PubMed] [Google Scholar]
- 49.Selvaraj P, Chandra G, Jawahar MS, Rani MV, Rajeshwari DN, Narayanan PR. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and FokI polymorphisms on macrophage phagocytosis and lymphoproliferative response to mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol. 2004;24:523–532. doi: 10.1023/B:JOCI.0000040923.07879.31. [DOI] [PubMed] [Google Scholar]
- 50.Roth DE, Soto G, Arenas F, Bautista CT, Ortiz J. Association between vitamin D receptor gene polymorphisms and response to treatment of pulmonary tuberculosis. J Infect Dis. 2004;190:920–927. doi: 10.1086/423212. [DOI] [PubMed] [Google Scholar]
- 51.Liu W, Cao WC, Zhang CY, Tian L, Wu XM. VDR and NRAMP1 gene polymorphisms in susceptibility to pulmonary tuberculosis among the Chinese Han population: a case-control study. Int J Tuberc Lung Dis. 2004;8:428–434. [PubMed] [Google Scholar]
- 52.Delgado JC, Baena A, Thim S, Goldfeld AE. Ethnic-Specific Genetic Associations with Pulmonary Tuberculosis. J Infect Dis. 2002;186:1463–8. doi: 10.1086/344891. [DOI] [PubMed] [Google Scholar]
- 53.Selvaraj P, Kurian SM, Reetha AM, Charles N, Narayanan PR. Vitamin D receptor and interleukin-1 receptor antagonist gene polymorphism in spinal tuberculosis. Current Science. 2000;79:986–989. [Google Scholar]
- 54.Selvaraj P, Kurian SM, Uma H, Reetha AM, Narayanan PR. Influence of non-MHC gene on lymphocyte response to Mycobacterium tuberculosis antigens and tuberculin reactive status in pulmonary tuberculosis. Indian J Med Res. 2000;112:86–92. [PubMed] [Google Scholar]
- 55.Selvaraj P, Narayanan PR, Reetha AM. Association of vitamin D receptor genotypes with the susceptibility to pulmonary tuberculosis in female patients and resistance in female contacts. Indian J Med Res. 2000;111:172–179. [PubMed] [Google Scholar]
- 56.Wilkinson RJ, Llewelyn M, Toossi Z, Patel P, Lalvani A. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. Lancet. 2000;355:618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 57.Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, Hil AV. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the Vitamin D receptor gene. J Infect Dis. 1999;179:721–4. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 58.Luo C, Zou P, Ji G, Gu A, Zhao P, Zhao C. The aryl hydrocarbon receptor (AhR) 1661G > A polymorphism in human cancer: A meta-analysis. Gene. 2013;513:225–230. doi: 10.1016/j.gene.2012.09.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.