Abstract
Background: Previous study has detected the expression of miR-625 in esophageal squamous cell carcinoma (ESCC) and found that miR-625 was related to tumor depth, stage, and metastasis of ESCC. However, the prognostic value of miR-625 in ESCC has not yet been reported. Methods: Real-time quantitative PCR was employed to measure the expression level of miR-625 in clinical ESCC tissues. Survival curves were made using the Kaplan-Meier method, and the log rank test was used to analyze the differences between clinicopathological characteristics and survival in ESCC patients. Results: The expression level of miR-625 in ESCC tissues was significantly lower than that in adjacent non-tumor tissues (1.00 ± 0.38 vs. 3.25 ± 1.83, P < 0.0001). Low miR-625 expression was observed to be closely correlated with lymph node metastasis (P = 0.01), distant metastasis (P = 0.007), tumor differentiation (P = 0.04), and advanced TNM stage (P = 0.005). The 5-year overall survival rate in the low expression group was 38.1%, compared with 68.8% in the high expression group (log-rank test, P = 0.001). Multivariate Cox regression analysis showed that miR-625 expression level (HR = 3.72, 95% CI: 1.36-8.78, P = 0.005) was an independent factor in predicting the overall survival of ESCC patients. Conclusion: Our findings provide the convincing evidence for the first time that the down-regulation of miR-625 may serve as a novel molecular marker to predict the aggressive tumor progression and unfavorable prognosis of ESCC patients.
Keywords: MiRNA-625, esophageal cancer, ESCC, clinicopathological features, prognosis
Introduction
Esophageal cancer is the fourth leading cause of death from cancer in China, and esophageal squamous cell carcinoma (ESCC) is the dominant histological type [1-3]. The biology of ESCC was of aggressive local invasion, early metastasis, and resistance to chemotherapy. So far, the pathogenic mechanism was not fully elucidated resulting in esophageal carcinogenesis.
MicroRNAs (miRNAs) are an abundant class of small, endogenous, non-coding RNAs, and the miRNA products are small single-stranded RNAs of 19-22 nucleotides with a primary role of regulating the translation of many genes [4,5]. Many studies have shown that miRNAs can regulate a variety of cellular processes including cell proliferation, differentiation, and apoptosis [6]. Moreover, much evidence has indicated that miRNAs are thought to function as tumor suppressors or oncogenes in the pathogenesis of cancers [6-8].
The aberrant expression of miR-625 has been reported in several types of human cancers, including gastric cancer, breast cancer, acute lymphoblastic leukemia, and multiple myeloma [9-12]. Previously, Wang et al detected the expression of miR-625 in esophageal cancer and found that miR-625 was related to tumor depth, stage, and metastasis of esophageal cancer. Furthermore, they identified that miR-625 could regulate the proliferation and invasion of esophageal cancer cells by targeting Sox 2 [13]. However, the prognostic value of miR-625 in ESCC has not yet been reported.
Material and methods
Patients and tissue samples
This study was approved by the Research Ethics Committee of the Affiliated Hospital of Qingdao University. Written informed consent was obtained from all of the patients. All specimens were handled and made anonymous according to the ethical and legal standards. 169 pairs of ESCC tissues and their corresponding non-tumor adjacent tissues were obtained from patients who underwent surgery at the Thoracic Surgery Department of Affiliated Hospital of Qingdao University (Qingdao, Shandong, China) between 2008 and 2013 and were subsequently diagnosed with ESCC based on histopathological evaluation. Fresh samples were snap-frozen, put in liquid nitrogen immediately after surgery, and were stored at -80°C until used. Corresponding non-tumor adjacent tissues were obtained from a part of the resected specimen that was the farthest distance from the tumor. No previous local or systemic treatment had been conducted on these patients before the operation. All patients were staged according to the criteria of the International Union Against Cancer. The clinicopathologic information was shown in Table 1.
Table 1.
Association of miR-625 expression with different clinicopathological features of ESCC patients
| Variables | Cases (n) | miR-625 expression | P value | |
|---|---|---|---|---|
|
|
||||
| Low (n = 86) | High (n = 83) | |||
| Gender | ||||
| Male | 97 | 45 | 52 | 0.63 |
| Female | 72 | 41 | 31 | |
| Age (years) | ||||
| < 55 | 68 | 31 | 37 | 0.34 |
| ≥ 55 | 101 | 55 | 46 | |
| Tumor location | ||||
| Middle | 87 | 48 | 39 | 0.54 |
| Lower | 82 | 38 | 44 | |
| Differentiation | ||||
| Well | 58 | 20 | 38 | 0.04 |
| Moderate | 62 | 37 | 25 | |
| Poor | 49 | 29 | 20 | |
| Depth | ||||
| T1, 2 | 80 | 31 | 49 | 0.27 |
| T3, 4 | 89 | 55 | 34 | |
| Lymph node metastasis | ||||
| Yes | 57 | 39 | 18 | 0.01 |
| No | 112 | 47 | 65 | |
| Distant metastasis | ||||
| Yes | 34 | 21 | 13 | 0.007 |
| No | 135 | 65 | 70 | |
| TNM stage | ||||
| I + II | 98 | 34 | 64 | 0.005 |
| III + IV | 71 | 52 | 19 | |
Evaluation of miR-625 expression by quantitative RT-PCR
Total RNA was extracted from ESCC tissues and matched normal adjacent tissues by homogenizing tissue in Trizol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. Primers for miR-625 and endogenous control U6 snRNA were obtained from Applied Biosystems (Foster City, California, USA). The concentration and purity of RNA were determined spectrophotometrically using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, Delaware, USA). cDNA was generated using the PrimeScript RT reagent kit (Takara Co. Ltd, Dalian, China) in a 20 μl final reaction volume containing 0.5 μg of RNA, 0.5 μl Prime-Script RT enzyme mix, and 4 μl 5 × PrimeScript buffer, and 1 ul RT primer, and incubated at 42°C for 60 min and at 85°C for 5 min. Quantitative real-time PCR assay was performed to evaluate miR-625 expression using SYBR Premix Ex Taq (Takara Co. Ltd) and measured in a LightCycler 480 System (Roche, Basel, Switzerland). The amplification profile was denatured at 95°C for 10 min, followed by 45 cycles of denaturation at 95°C for 15 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min. Relative quantification of miRNA expression was performed using the 2-ΔΔCT. The raw data were presented as the relative quantity of target miRNA, normalized with respect to U6 snRNA and relative to a calibrator sample.
Statistical analysis
All computations were carried out using the software of SPSS version 18.0 for Windows (SPSS, Inc, Chicago, IL). Data were expressed as mean ± SD. Fisher’s exact test, chi-square, and two-sample t-tests were used to evaluate the statistical differences among the groups with different clinicopathological data. Survival curves were made using the Kaplan-Meier method, and the log rank test was used to analyze the differences between clinicopathological characteristics and survival in ESCC patients. A Cox proportional hazards model was used for multivariate analysis. Differences were considered statistically significant when P was less than 0.05.
Results
The expression of miR-625 in ESCC tissues
miR-625 expression level was detected in 169 paired ESCC and adjacent non-tumor tissues by qRT-PCR. As shown in Figure 1, after normalization to U6 expression levels, the expression level of miR-625 in ESCC tissues was significantly lower than that in adjacent non-tumor tissues (1.00 ± 0.38 vs. 3.25 ± 1.83, P < 0.0001). Accordingly, the median fold change of miR-625 was used as a cutoff value todivide all 169 patients into two groups: the low expression group (n = 86) and the high expression group (n = 83).
Figure 1.
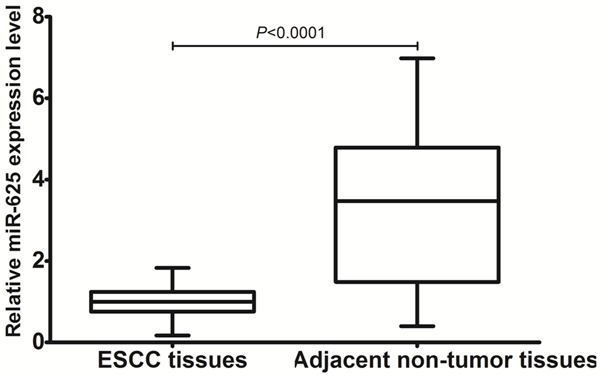
Comparison of miR-625 expression levels between ESCC tissues and adjacent non-tumor tissues.
Association of miR-625 expression with clinicopathological characteristics of ESCC patients
Table 1 summarized the association between miR-625 expression and clinicopathological characteristics of ESCC patients. Low miR-625 expression was observed to be closely correlated with lymph node metastasis (P = 0.01), distant metastasis (P = 0.007), tumor differentiation (P = 0.04), and advanced TNM stage (P = 0.005). In contrast, there was no association between miR-625 expression and other clinical factors, such as gender, age, tumor depth, and tumor location (all P > 0.05).
miR-625 down-regulation associates with poor prognosis of ESCC patients
To evaluate the prognostic value of miR-625 expression in ESCC, survival curves were constructed by Kaplan-Meier method and compared by the log-rank test. As shown in Figure 2, ESCC patients with low miR-625 expression level had shorter overall survival than those with high miR-625 expression level. The 5-year overall survival rate in the low expression group was 38.1%, compared with 68.8% in the high expression group (log-rank test, P = 0.001).
Figure 2.
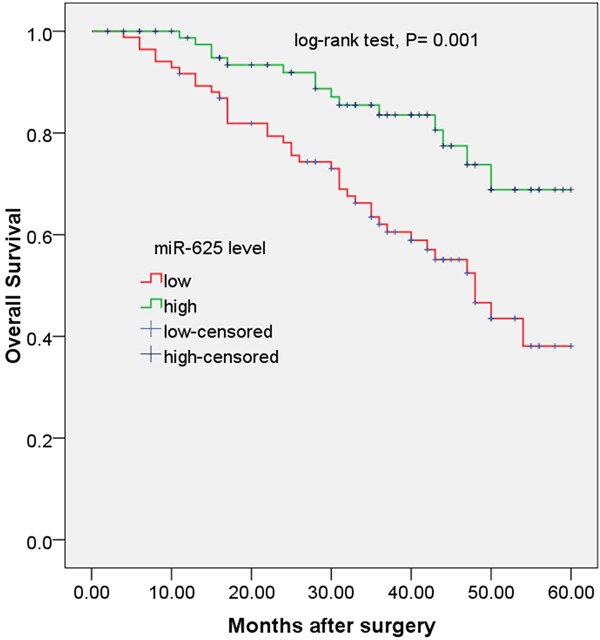
Kaplan-Meier curves for overall survival in patients with ESCC divided according to miR-625 expression.
To determine the possibility of miR-625 as an independent risk factor for poor prognosis, both clinicopathological factors and the level of miR-625 expression were evaluated by multivariate Cox regression analysis. Results showed that distant metastasis (HR = 4.65, 95% CI: 2.56-9.78: P = 0.01), clinical stage (HR = 2.91, 95% CI: 1.24-10.88, P = 0.03), and miR-625 expression level (HR = 3.72, 95% CI: 1.36-8.78, P = 0.005) were independent factors in predicting the overall survival of ESCC patients (shown in Table 2).
Table 2.
Multivariate Cox regression analysis of overall survival in 169 patients with ESCC
| Variable | Hazard ratio | 95% CI | P-value |
|---|---|---|---|
| Gender | 1.25 | 0.35-4.82 | 0.52 |
| Age | 2.54 | 0.86-4.56 | 0.41 |
| Tumor location | 0.69 | 0.46-3.71 | 0.49 |
| Differentiation | 3.16 | 0.78-6.93 | 0.07 |
| Depth | 2.45 | 0.81-9.12 | 0.11 |
| Lymph node metastasis | 2.97 | 0.85-10.02 | 0.06 |
| Distant metastasis | 4.65 | 2.56-9.78 | 0.01 |
| TNM stage | 2.91 | 1.24-10.88 | 0.03 |
| miR-625 expression | 3.72 | 1.36-8.78 | 0.005 |
Discussion
Progress in perioperative management and definitive or adjuvant therapy has led to improved survival of ESCC patients. However, for patients with advanced disease, prognosis remains poor [14]. Local ESCCs directly invade other organs, presenting serious obstacles to radical resection, a characteristic which enhances local recurrence. Moreover, ESCCs cause early lymphatic and hematogenous disseminations more frequently compared to other solid gastrointestinal cancers [15]. Therefore, clinical indicators that accurately predict ESCC progression and prognosis are essential for improving patient survival.
The function of miRNAs in carcinogenesis has been extensively studied and well established [7,16]. Aberrant expression of miRNAs has been demonstrated to be involved in the initiation, progression, and metastasis of human cancers [17]. Some miRNAs are over-expressed in tumors and act as oncogenes, promoting carcinogenesis by targeting tumor suppressors. The other miRNAs are down-regulated in tumors and generally par-ticipate in oncogene overexpression. Furthermore, aberrant miRNA expression might be of potential use as a diagnostic and prognostic biomarker for cancer [18-20]. The aberrant expression of miR-625 has been reported in several types of human cancers, including gastric cancer, breast cancer, acute lymphoblastic leukemia, and multiple myeloma [9-12]. Previously, Wang et al detected the expression of miR-625 in esophageal cancer and found that miR-625 was related to tumor depth, stage, and metastasis of esophageal cancer. Furthermore, they identified that miR-625 could regulate the proliferation and invasion of esophageal cancer cells by targeting Sox2 [13]. However, the prognostic value of miR-625 in ESCC has not yet been reported. In the present study, miR-625 expression level was detected in 169 paired ESCC and adjacent non-tumor tissues by qRT-PCR. We found that the expression level of miR-625 in ESCC tissues was significantly lower than that in adjacent non-tumor tissues. Low miR-625 expression was observed to be closely correlated with lymph node metastasis (P = 0.01), distant metastasis (P = 0.007), tumour differentiation (P = 0.04), and advanced TNM stage (P = 0.005). To evaluate the prognostic value of miR-625 expression in ESCC, survival curves were constructed by Kaplan-Meier method and compared by the log-rank test. We found that ESCC patients with low miR-625 expression level had shorter overall survival than those with high miR-625 expression level. The 5-year overall survival rate in the low expression group was 38.1%, compared with 68.8% in the high expression group (log-rank test, P = 0.001). To determine the possibility of miR-625 as an independent risk factor for poor prognosis, both clinicopathological factors and the level of miR-625 expression were evaluated by multivariate Cox regression analysis. Results showed that distant metastasis, clinical stage, and miR-625 expression level were independent factors in predicting the overall survival of ESCC patients, suggesting that the down-regulation of miR-625 may serve as a novel molecular marker to predict the aggressive tumor progression and unfavorable prognosis of ESCC patients.
Previously, in the study by Lou et al, they measured the differential expression of miR-625 in colorectal tumor tissues, adjacent non-tumor tissues, and five colorectal cancer cell lines and investigated the clinicopathological and prognostic values of miR-625 in patients with colorectal cancer. The results showed that miR-625 was associated with advanced lymphatic invasion, liver metastasis, and reduced overall survival of colorectal cancer patients. Furthermore, they designed and transfected miR-625 mimics into the colorectal cancer cell line HCT116 and found that miR-625 can regulate the invasion and metastasis of colorectal cancer cells [18]. Wang et al. found that miR-625 was significantly downregulated and negatively correlated with lymph node metastasis in gastric cancer [10]. Our findings were in line with theirs.
In conclusion, our findings provide the convincing evidence for the first time that the down-regulation of miR-625 may serve as a novel molecular marker to predict the aggressive tumor progression and unfavorable prognosis of ESCC patients. Further studies and more samples will be required to confirm the prognostic value of miR-625 in ESCC.
Disclosure of conflict of interest
None.
References
- 1.Zhao P, Dai M, Chen W, Li N. Cancer trends in China. Japanese Journal of Clinical Oncology. 2010;40:281–285. doi: 10.1093/jjco/hyp187. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30:1415–1425. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 6.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–1771. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Ho-rvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 8.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 9.Si H, Sun X, Chen Y, Cao Y, Chen S, Wang H, Hu C. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139:223–229. doi: 10.1007/s00432-012-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang M, Li C, Nie H, Lv X, Qu Y, Yu B, Su L, Li J, Chen X, Ju J, Yu Y, Yan M, Gu Q, Zhu Z, Liu B. Down-regulated miR-625 suppresses invasion and metastasis of gastric cancer by targeting ILK. FEBS Letters. 2012;586:2382–2388. doi: 10.1016/j.febslet.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 11.Huang JJ, Yu J, Li JY, Liu YT, Zhong RQ. Circulating microRNA expression is associated with genetic subtype and survival of multiple myeloma. Med Oncol. 2012;29:2402–2408. doi: 10.1007/s12032-012-0210-3. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Liang YN, Luo XQ, Liu XD, Guo HX. [Association of miRNAs expression profiles with prognosis and relapse in childhood acute lymphoblastic leukemia] . Zhonghua Xue Ye Xue Za Zhi. 2011;32:178–181. [PubMed] [Google Scholar]
- 13.Wang Z, Qiao Q, Chen M, Li X, Wang Z, Liu C, Xie Z. miR-625 down-regulation promotes proliferation and invasion in esophageal cancer by targeting Sox2. FEBS Letters. 2014;588:915–921. doi: 10.1016/j.febslet.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 14.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Australasian Gastro-Intestinal Trials G: Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. The Lancet Oncology. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 15.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 16.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 18.Lou X, Qi X, Zhang Y, Long H, Yang J. Decreased expression of microRNA-625 is associated with tumor metastasis and poor prognosis in patients with colorectal cancer. J Surg Oncol. 2013;108:230–235. doi: 10.1002/jso.23380. [DOI] [PubMed] [Google Scholar]
- 19.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng DX, Luo M, Qiu LW, He YL, Wang XF. Prognostic implications of microRNA-100 and its functional roles in human epithelial ovarian cancer. Oncol Rep. 2012;27:1238–1244. doi: 10.3892/or.2012.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]


