Abstract
Background: Acid-suppressive medications are widely used for the management of acid-related disorders. It has been reported that acid-suppressive medication users were at increased risk of fracture, but such an association was inconsistent among observational studies. The purpose of our analysis was to assess the relationship between use of antacid drugs and fracture risk. Methods: We systematically searched electronic database and manually examined the reference lists of previous reviews for potentially eligible studies. Given the heterogeneity across studies, random effects models were used to calculate summary estimates. Subgroup analysis and sensitivity analysis were conducted to explore the potential heterogeneity. Results: 18 studies met our inclusion criteria. PPI and H2RA were associated with increased risk of hip fracture, with substantial heterogeneity (PPI: 1.216, 1.134-1.304, I2=71.3%; H2RA: 1.128, 1.022-1.245, I2=72.1%). High risk of spine fracture was observed in PPI users (1.216, 95% CI: 1.134-1.304) but not H2RA users. When considering 5 studies conducted among postmenopausal women, the RR was 1.376, (95% CI: 1.043-1.816) with modest heterogeneity (I2=57.7%). Subgroup analysis and sensitivity analysis found consistent association between hip fracture risk and PPI use but not H2RA use. Positive association for H2RA use lost its significance when considering case-control studies and European studies. Conclusion: Results of this updated meta-analysis provided evidence to support that acid-suppressive medications were associated with increased risk of fracture, especially hip fracture.
Keywords: Fracture risk, acid-suppressive medication, PPI, H2RA
Introduction
Acid-suppressive medications are widely used for the management of acid-related disorders [1]. Despite outstanding efficacy and negligible short-term adverse-effects, increased concerns have been raised regarding the side effects of chronic use of PPI and H2RA [2,3,40,41]. Given the wide-spread prescription of acid-suppressive drugs, its safety profile is of great significance. Mounting epidemiological evidences have associated PPI or H2RA with possible risk of fracture. Fracture is considered to be one of the major public health burdens, especially among the elders. Considerable mobility and mortality was reported to correlate with fracture [4-6]. Observational studies evaluating the association between antacid drugs use and fracture risk reached conflicting conclusions. Meta-analysis on this issue have been conducted and found that acid-inhibiting drugs were associated with increased risk of fracture. However, previous reviews either focused on the effect of PPI, but not H2RA or reported on the risk of single fracture [7,8]. Although some meta-analyses published in 2011 have evaluated the effects of both PPI and H2RA on fracture risk [7-11]. Since the publication of previous meta-analysis, several studies with long duration of follow-up and well controlled confounders have been carried out, of which 3 published in 2014, again with inconsistent findings and they were not synthesized in the prior reviews [12-17].
To obtain a better understanding of associations between acid-suppressive medications and fracture risk, we updated previous meta-analysis with data from new observational studies published in the past two years. With more studies included, we were able to examine the effect of acid-suppressive drugs on the risk of fracture at various fracture sites (any, hip, spine and wrist fracture) and evaluate the association among postmenopausal women. Additionally, large prospective studies with relatively long follow-up enabled us to explore whether duration of exposure affected the risk of fracture.
Methods
Study selection
We systematically searched the Pubmed and Embase database for relevant publications. Following key words were used: “proton pump inhibitor”, “PPI”, histamine receptor antagonist”, “H2RA”, “cimetidine”, “lansoprazole”, “omeprazole”, “pantoprazole” and “fracture”, “bone density”, “osteoporosis”.
Studies were included if they satisfied the following criteria: 1. Cohort study or case-control study reported association between acid-suppressive medications and fracture risk. 2. Human studies. 3. Providing a measure of RR, OR or HR and 95%CI. 4. The risk estimates should be at least controlled for one cofounder. 5. Risk of fracture was an outcome.
Case-reports or reviews without providing risk estimates were excluded. One study was removed because the results were reported in the earlier publication. We did not include studies that reported bone mineral density alterations as outcome.
Data extraction
Following information from each included study was extracted: first authors’ name, study year, country in which study conducted, study design, duration of follow-up, age of participants, sample size of cohorts, number of cases and controls, RR or OR with 95% CI, adjustment of confounders.
When multiple risk estimates were provided, we included the one with most covariates adjusted. 3 studies were all based on the General Practice Research Database. Despite the same database used, they have different study design, duration of follow-up and sample size, showing discrepant findings [18]. Yang et al and De Varies concluded that PPI, especially long-term exposure was associated with high risk of fracture [19,20]. However, Kaye and colleagues conducted a nested case-control study and found no association between PPI use and fracture. We included these 3 studies in our meta-analysis [21]. One study provided results stratified by duration of exposure; we included the relative risk for more than one year, since most studies reported estimation for fracture risk associated with more than 1 year of PPI use [22]. Another study reported risk estimation stratified by dosage, we combined relative risk for different dosage categories in fixed-effects model, and included the summary results [23]. Yu’s study contain two cohorts, we pooled them separately in the present meta-analysis [24].
Data extraction was independently conducted by 2 investigators. Inconsistencies were resolved by discussion and referring back to the original citation.
Statistical analysis
We extracted odds ratios in case-control studies or relative risk in cohort studies. Due to relative rare incidence of fracture, RRs were considered equivalent to ORs [25]. Given inconsistence in the study design, exposure definition and duration of follow-up, random-effects model was used to calculate summary relative risk and 95% CIs. I2 statistic was used to evaluate the study heterogeneity. We performed stratified analysis by geographic location (Europe, North America) and study design (cohort study and case-control study). Sensitivity analysis was undertaken to evaluate the contribution of individual study to the summary results. We excluded each study in turn and calculated the risk estimation of remaining studies. We evaluated publication bias by inspection of funnel plot; we also conducted Egger’s test and Begger’s test. All statistical analysis was performed using STATA version 12.0. P<0.05 was considered significant.
Results
Study characteristics
We identified 11 studies through manually searching the reference lists of previous reviews [19-24,26-30]. After systematical literature search, we identified 7 additional studies [12-17,31]. Finally, we included 18 observational studies which fulfilled our selection criteria, of which 8 were cohort studies, 10 were case-control studies. The case-control studies contained 199511 cases of fracture and matched with 687500 controls. The participants of cohort studies ranged from 1211 to 400933, including a total of 102504 fracture cases.
Of 18 included studies, 8 were conducted in North America, 1 from Asian and 9 from Europe. 5 studies were conducted among postmenopausal women and 3 studies consisted entirely of men. All the included studies published in English.
Among 18 studies, some reported risk of fracture for both PPI and H2RA, we pooled them separately. 17 studies provided data on the relationship between PPI use and fracture risk, 10 studies provided relative risk for H2RA use. Main characteristics of included studies were presented in Table 1.
Table 1.
Case-control and cohort studies on the association between acid-suppressing medication and fracture risk
| source | country | Study design | Gender (% female) | Study population | Mean age (year) | Duration of follow-up (year) | Fracture site |
|---|---|---|---|---|---|---|---|
| Yang 2006 | UK | Nested | 79.9% | 13556 cases | 77 | 1987-2003 | hip |
| Case-control | 135386 controls | ||||||
| Corley 2010 | USA | Case-control | 65% | 33752 cases | 69.4% older | 1995-2007 | hip |
| 130471 controls | Than 70 | ||||||
| Reyes 2013 | Spain | Case-control | 76.9% | 358 cases | 82 | 2007-2010 | hip |
| 698 controls | |||||||
| Adam 2014 | USA | Case-control | 0% | 6774 cases | 69% older | 1997-2006 | hip |
| 6774 controls | Than 70 | ||||||
| Gray 2010 | USA | cohort | 100% | 161806 women | 50-79 | 7.8 | Spine |
| 119084 nonusers | Hip | ||||||
| 2731 PPI users | wrist | ||||||
| 7952 H2RA users | |||||||
| Pouwels 2011 | Netherland | Case-control | 72.7% | 6763 cases | 75 | 1991-2002 | hip |
| 26341 controls | |||||||
| Targownik 2008 | Canada | cohort | 70.2% | 15792 cases | More than | 1996-2004 | Hip |
| 47289 controls | 50 | Vertebra | |||||
| wrist | |||||||
| Vestergaard 2006 | Denmark | Case-control | 51.8% | 124655 cases | 43.44 | NA | Hip |
| 373962 controls | Spine | ||||||
| wrist | |||||||
| Kaye 2008 | UK | Nested | 71.6% | 1098 cases | 50.79 | 1995-2005 | hip |
| case-control | 10923 controls | ||||||
| Yu 2008 | USA | cohort | SOF: 100% | F: 234 PPI users | F: 79 | NA | Hip |
| MrOS: 0% | 519 H2RA users | M: 74 | Non-spine | ||||
| 4574 controls | |||||||
| M: 487 PPI users | |||||||
| 335 H2RA users | |||||||
| 4920 controls | |||||||
| Chiu 2010 | Taiwan | Case-control | 57.94% | 1241 cases | 74 | NA | hip |
| 1241 controls | |||||||
| Raux 2008 | Europe | cohort | 100% | 61 PPI users> | 55-79 | Patients received | spine |
| 1150 nonusers | |||||||
| Fraser 2013 | Canada | cohort | 69.39% | 261 PPI users | 1999-2001 | ||
| De Varies 2009 | UK | cohort | 55.8% | 234144 PPI users | PPI users 62 | PPI: 3.5 | Hip |
| 0 non-users | H2RA 61 | H2ra: 6.3 | Spine | ||||
| 166798 H2RA users | Any | ||||||
| 0 nonusers | |||||||
| Moberg 2014 | Sweden | cohort | 100% | 47 users | 56.4 | 1995-2012 | any |
| 2724 nonusers | |||||||
| Khalili 2012 | USA | cohort | 100% | 74558 nonusers | 67 | 2000-2008 | hip |
| 5341 user | |||||||
| Soriano 2014 | USA | Case-control | 10958 cases | 40-89 | 2000-2008 | hip | |
| 20000 controls | |||||||
| Grisso 1997 | USA | Case-control | 0% | 356 cases | Older than 45 | 1991-1993 | hip |
| 402 controls |
PPI and risk of fracture
As shown in Figure 1, the pooled relative risk (RR) was 1.18 (95% CI, 1.077-1.294) for any fracture, 1.216 (1.134-1.304) for hip fracture, 1.504 (1.317-1.717) for spine fracture and 1.085 (0.946-1.244) for wrist fracture. PPI users were associated with increased risk of hip fracture and spine fracture except for wrist fracture. Substantial heterogeneity was found in the stratified analysis by fracture sites, but no heterogeneity was observed for spine fracture (Table 2).
Figure 1.
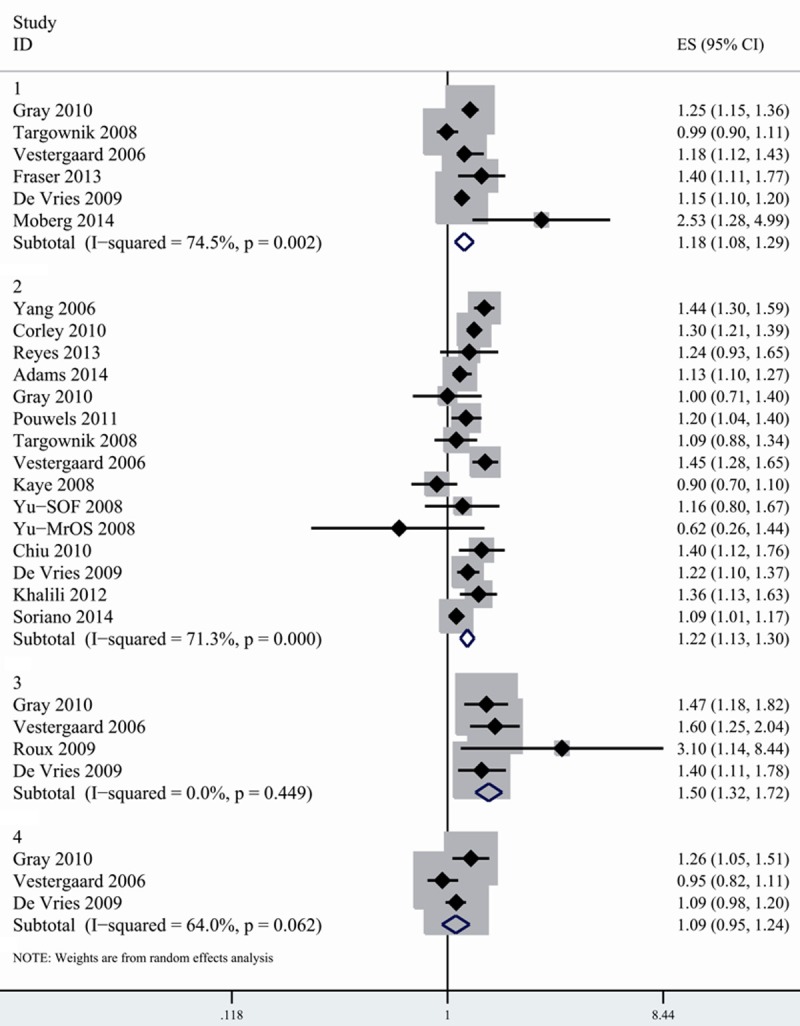
Combined random-effects estimates of RR and 95% CI for the association between fracture risk and PPI use. 1. Any fracture. 2. Hip fracture. 3. Spine fracture. 4. Wrist fracture.
Table 2.
Summary relative risks and 95% confidence intervals (CI) of fracture for the acid-suppressive medication users, according to the characteristics of included studies
| Fracture site | NO. of studies | RR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
|
| ||||
| I% | p | |||
| PPI and fracture | ||||
| any | 6 | 1.181 (1.077-1.294) | 74.5% | 0.0079 |
| spine | 4 | 1.504 (1.317-1.717) | 0.0% | 0.0000 |
| wrist | 3 | 1.085 (0.946-1.244) | 64.0% | 0.0093 |
| hip | 15 | 1.216 (1.134-1.304) | 71.3% | 0.0106 |
| PPI and hip fracture (study design) | ||||
| cohort | 6 | 1.19 (1.077-1.316) | 18.2% | 0.296 |
| Case-control | 9 | 1.233 (1.128-1.347) | 81.2% | 0.0000 |
| Location | ||||
| Europe | 7 | 1.220 (1.087-1.396) | 82.1% | 0.0183 |
| North America | 7 | 1.190 (1.081-1.308) | 56.2% | 0.0068 |
| H2RA and fracture | ||||
| any | 3 | 0.996 (0.896-1.107) | 89.8% | 0.0078 |
| spine | 3 | 1.045 (0.919-1.188) | 0.0% | 0.0000 |
| wrist | 3 | 1.002 (0.926-1.083) | 0.0% | 0.0000 |
| hip | 11 | 1.128 (1.022-1.245) | 72.1% | 0.0167 |
| H2RA and hip fracture (study design) | ||||
| cohort | 5 | 1.186 (1.089-1.292) | 0.0% | 0.850 |
| Case-control | 6 | 1.096 (0.937-1.283) | 85.1% | 0.000 |
| Location | ||||
| Europe | 5 | 1.059 (0.894-1.255) | 86.8% | 0.000 |
| North America | 6 | 1.187 (1.105-1.275) | 0.0% | 0.550 |
Of 17 studies evaluating associations between PPI and fracture risk, 15 reported risk estimation for hip fracture. The pooled results showed significant heterogeneity (I2=71.3%). We conducted subgroup analysis by study design. Although similar association was detected in both cohort and case-control studies, the pooled estimates were slightly higher in case-control studies (1.233, 1.128-1.347). Considerable heterogeneity was observed among case-control studies (I2=81.2%), but not in cohort studies (I2=18.2%). When stratified by geographic locations, we found the association between hip fracture and PPI exposure was consistent across study location. However, more considerable heterogeneity was detected in the study conducted in Europe (I2=82.1%) compared with North American studies (I2=56.2%).
No suggestion of publication bias was observed on the funnel plot or by Egger’s test (P=0.276) and Begger’s test (P=0.703). We also carried out sensitivity analysis by removing each study in turn and evaluating the remaining studies. After excluding each study in turn, the relationship between PPI and hip fracture risk did not materially altered. The pooled relative risk ranged from 1.197 (1.12-1.28) (when excluding Gray’s study [27]) to 1.237 (1.15-1.32) (when excluding Kaye’s study [21]).
H2RA and fracture of risk
10 studies reporting on the association between H2RA and risk of fracture were included [16,17,19,20,24,27-31]. When stratified by facture sites, we found that H2RA use was not associated with increased risk of any fracture; spine fracture and wrist fracture (Figure 2). However, there existed a statistically significant increase in hip fracture in H2RA users (1.128, 1.022-1.245) with high heterogeneity detected (I2=72.1%). To further analyze the association, we conducted subgroup analysis by study design and found inconsistent results. No association between hip fracture and H2RA use was observed in case-control studies, but there was a significant increased risk of hip fracture in cohort studies with no heterogeneity detected (1.186, 1.089-1.292, I2=0%). When stratified by study location, the combined RR between hip fracture and H2RA was 1.059 (0.894-1.255) in European studies, suggesting no overall association. However, summary RR for studies conducted in North America indicated positive association (1.187, 1.105-1.275), with no evidence of heterogeneity (I2=0%) (Table 2). No asymmetry of funnel plot was detected. Both Egger’s test (P=0.115) and Begger’s test (P=0.119) indicated absence of publication bias.
Figure 2.
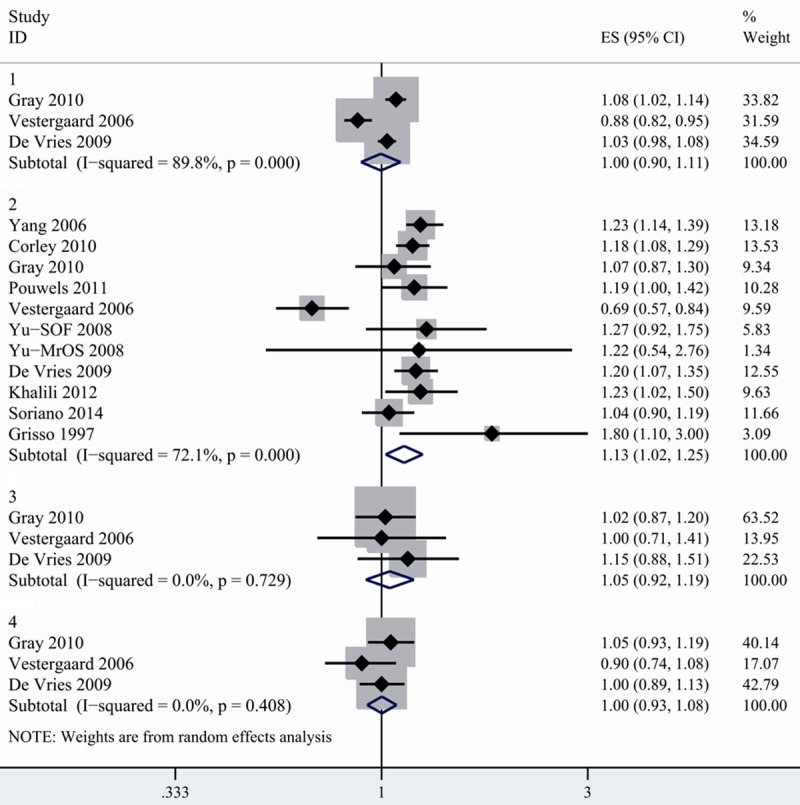
Summary random-effects estimate of RR and 95% CI for the association between fracture risk and H2RA use. 1. Any fracture. 2. Hip fracture. 3. Spine fracture. 4. Wrist fracture.
The positive association was not consistent when excluding each study in turn. When removing the study conducted by Yang [19], Corley [30] or De Varies [20], the association between H2RA use and hip fracture lost its significance.
Duration of PPI use and fracture risk
9 studies provided data on the associations between duration of PPI use and risk of hip fracture. The studies divided duration of drug exposure as different intervals and cutoffs. Some used a 1-year cutoff, some used a 2-year cutoff and other studies provided RR for 30 days, 30-365 day and more than 365 days respectively. To pool data, we divided exposure time as <1 year, 1-3 year, >3 year and >5 year. For studies that used days and months as cutoff, we converted it into corresponding year. For studies that provided RR for several categories of duration, we combined the separate relative risk according to appropriate interval and used the combine RRs in our analysis. For example, the original study provided risk estimates for 1-2 year, 3 year and 4 year separately. We combined the RR of 1-2 year and 3 year in the fixed effects model. We considered the pooled data as risk estimates of 1-3 year duration. We found that 1-3 year’s exposure of PPI was more strongly related to hip fracture risk (1.225, 1.137-1.319) than less than 1 year (1.191, 1.111-1.278). Exposure of more than 5 years was related to even higher risk of fracture. However, the risk of hip fracture for >3 year of PPI use was slightly lower than those for 1-3 year of exposure (Figure 3; Table 3).
Figure 3.
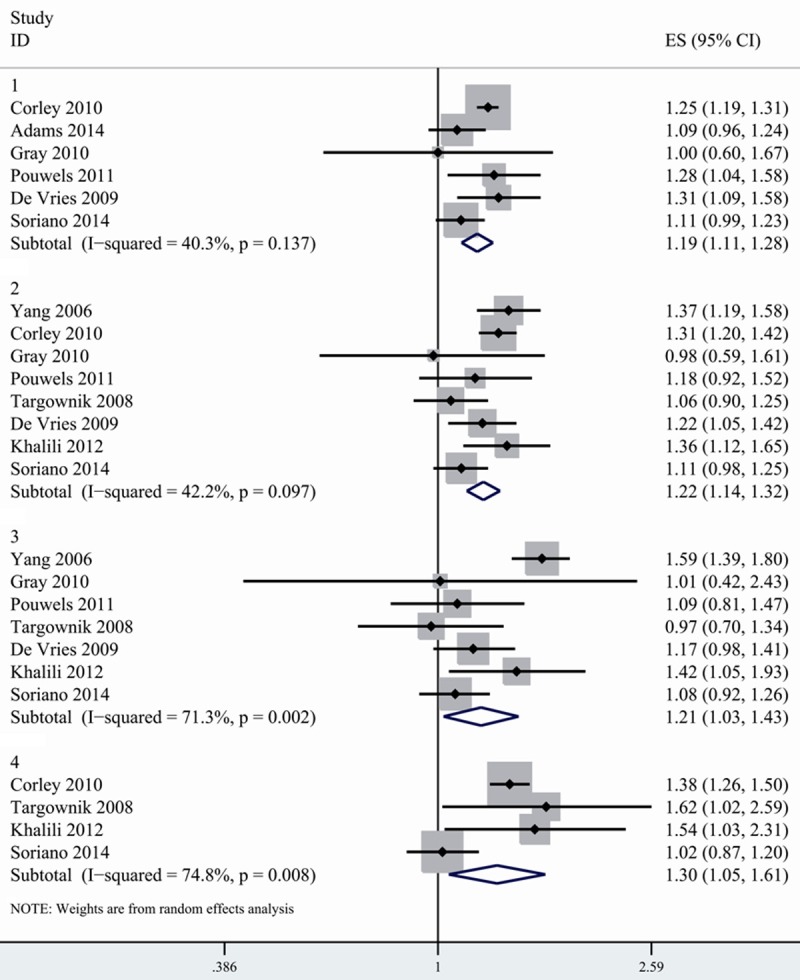
Association between PPI use and risk of hip fracture according to duration of PPI exposure. 1. Duration < 1 year 2. Duration: 1-3 years. 3. Duration >3 years. 4. Duration >5 years.
Table 3.
Summary relative risk for the relationship between PPI use and fracture according to the exposure duration
| Duration of PPI (year) | NO. of studies | RR (95% CI) | Heterogeneity | |
|---|---|---|---|---|
|
| ||||
| I% | P | |||
| <1 | 6 | 1.191 (1.111-1.278) | 40.3% | 0.137 |
| 1-3 | 8 | 1.225 (1.137-1.319) | 42.2% | 0.097 |
| >3 | 7 | 1.212 (1.025-1.433) | 71.3% | 0.002 |
| >5 | 4 | 1.302 (1.050-1.614) | 74.8% | 0.008 |
PPI and risk of fracture in postmenopausal women
Postmenopausal women are among high risk population of developing osteoporosis-related fracture [32]. 5 included studies provided data on PPI use in relation to risk of fracture among postmenopausal women [15,16,24,26,27]. 3 studies showed that postmenopausal women with PPI use were associated with higher risk of hip fracture, whereas the remaining 2 studies reported no association on this issue. On meta-analysis of the 5 studies, we found that postmenopausal women with PPI use increased risk of hip fracture (1.376, 1.043-1.816) with modest heterogeneity (I2=57.7%) (Figure 4).
Figure 4.
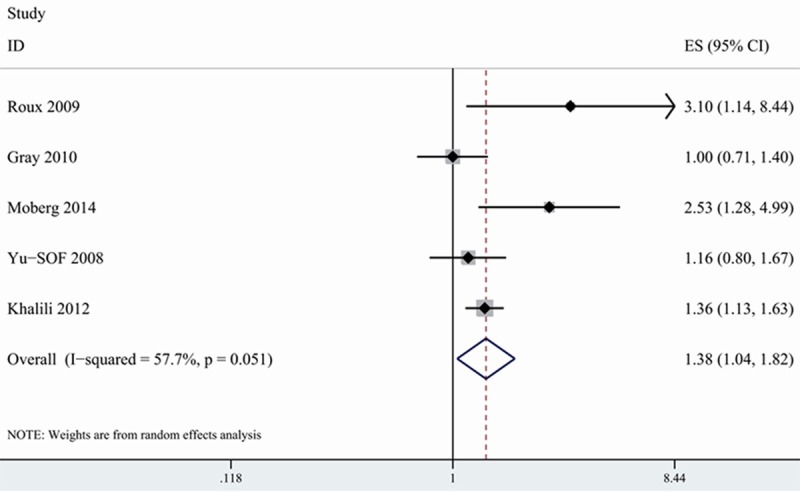
Association between PPI use and risk of hip fracture among postmenopausal women.
Discussion
Given the worldwide use of antacid medication and significant decline in life quality associated with fracture [33,42]. It is of great clinical importance to clarify the association. Although FDA and previous reviews suggested a link between PPI and fracture risk, this issue remains much debated [34]. Several additional studies have been published recently, again yielding conflicting results. Adam et al conducted a case-control study with over 10-year period and found that PPI use increased risk of hip fracture, especially long-term exposure and more recent use [13]. Another multicenter cohort studies with 9423 participants suggested a modest risk of fracture in PPI users [14]. However, a case-control study carried out in a Mediterranean region reported no association [12].
Our meta-analysis of 10 case-control studies and 8 cohort studies involved a total of 302015 cases and found that both PPI and H2RA were associated with increased risk of hip fracture. Additionally, PPI but not H2RA was also related to increased risk of any fracture and spine fracture. Our findings were in agreement to some extent with previous meta-analysis, which showed positive association between PPI and hip fracture. However, different with prior studies, we also observed increased risk of hip fracture in H2RA users. The inconsistence in the results can be partially explained by different sample size. Prior meta-analysis included 7 studies evaluating the risk for H2RA [11]; our analysis included 10 eligible studies. With more studies included, we were able to provide more precise estimation of acid-suppressive medication’s effects on fracture.
Excess risk of wrist fracture was not observed in both PPI and H2RA exposure, although this result was based on 3 studies. Due to limited statistical power and small sample size, this finding should be interpreted carefully. Further studies focus on the relationship between antacid drugs and risk of wrist fracture are warranted.
For PPI use, the results were stable across study design, location and sensitivity analysis. However, the association between H2RA use and hip fracture risk was inconsistent across subgroups. In contrast to the positive association observed in cohort studies, limiting analysis to the case-control studies showed no association. Moreover, increased risk of hip fracture was observed among H2RA user from North America, but not Europe. Sensitivity analysis also suggested unstable association. When excluding studies conducted by Yang et al [19], Corley et al [30], and De Vries et al [20], the analysis lost statistical significance.
With respect to the effect of PPI dosage on the fracture risk, Yang and colleagues’ study found that PPI use, especially at high dose, was related to increased fracture risk. A case-control studies performed by Chiu et al also reported that PPI prescription was related to an increased fracture risk in a dose-response way. It seems that the strength of relationship increased with greater dosage of PPI exposure. However, considering limited studies reported the dose-response relationship and the calculation of dosage varied between studies, we were unable to explore the impact of increasing dose of PPI on the fracture risk.
The mechanism that linked acid-suppressive medication and fracture risk remains largely unknown [43]. PPI has been associated with calcium malabsorption and bone mineral density loss, thus resulting in increased risk of fracture [30]. However, studies evaluating the association between acid inhibition and bone mineral density have demonstrated different conclusions. Some studies found that mildly reduced bone density was related to PPI users [24], but there was no significant difference in the levels of bone mineral density between PPI users and controls in other studies [24,35].
In addition, acid-suppressive medication was reported to affect bone modeling, which plays a role in promoting development of fracture [36]. Additionally, intake of omeprazole had been reported to inhibit absorption of calcium and reduce bone mineral density in rat models [37]. It was also reported that calcium malabsorption would cause hyperparathyroidism, thus leading to reduction of bone mineral density [38,39]. However, no conclusive evidence existed to support these possible mechanisms.
The major advantage of our analysis is the big sample size. With inclusion of recent publications, our analysis was based on 18 observational studies. The large sample size allowed for accurate estimation with greater statistical power. With more studies conducted in different countries and populations, we were able to carry out additional subgroup analysis by geographic location and gender. Another advantage is the rigorous inclusion and exclusion criteria for the study selection. Most included studies controlled for the main risk factors which may alter the association [44]. When evaluating the duration-response relationship, the cutoff points of duration varied between studies. We addressed it by dividing duration of exposure into appropriate categories.
Several limitations of our study should not be ignored when interpreting the findings of our analysis. First, no RCT on this issue was available. Our study consisted entirely of observational studies, which inherited limitations of such studies. Although potential cofounders were adjusted, there existed unknown or unmeasured covariates which may affect the associations. Few studies adjusted for dietary and lifestyle factors, such as, physical excise, hormone therapy, which are closely associated with fracture risk. Second, most of included studies did not provide information on the dosage of acid-suppressive medication, which precluded us from evaluating the dose-response relationship. Third, half of included studies were case-control studies, which were subject to recall and selection biases and contributed to the considerable heterogeneity among studies [45]. In our analysis, excluding case-control studies resulted in relatively low heterogeneity.
Prescription adherence should be considered when we examined the risk estimates, because low adherence rate would lead to underestimation of the association. One recent study conducted by Adams et al evaluated whether medication adherence affected the association between PPI use and fracture risk. They categorized adherence as 80% or more or less than 80% and found that greater medication adherence was associated with increased risk of hip fracture, whereas no significant association was observed among PPI user with adherence less than 80% [13]. However, the information about the adherence of acid-suppressive medication was not available in most other studies.
Substantial heterogeneity should be noted in present study. However, the association between PPI use and hip fracture risk remained consistent across subgroup studies and sensitivity analysis. Taking into account the significant heterogeneity, we used random-effect model to calculate the risk estimates. With regard to H2RA use, the significant heterogeneity was mainly due to case-control studies and location differences. When excluding European studies or case-control studies, no heterogeneity were detected (Table 2).
Although no evidence of publication bias was observed, we were unable to exclude the possibility of publication bias. It is known that studies with small sample size and negative results are not prone to be published. No attempts were made to search unpublished studies. If the publication bias exists, this will resulted in overestimation of the association between acid-suppressive drugs and risk of fracture.
Conclusion
In summary, the results of our meta-analysis of cohort and case-control studies found that PPI and H2RA use was associated with increased risk of hip fracture. Higher risk of any fracture and spine fracture was also observed in PPI users, but not H2RA users. Considering chronic use of PPI and few studies evaluated the effect of exposure duration on fracture risk, further studies with long duration PPI treatment were encouraged. Since no conclusive mechanism proposed to explain the association, the research should explore the mechanism involved. The clinicians should keep in mind the risk of fracture when balancing the safety and efficacy of prescribing these antacid medications.
Disclosure of conflict of interest
None.
References
- 1.Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122:896–903. doi: 10.1016/j.amjmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Thomson AB, Sauve MD, Kassam N, Kamitakahara H. Safety of the long-term use of proton pump inhibitors. World J Gastroenterol. 2010;16:2323. doi: 10.3748/wjg.v16.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimer C. Safety of long-term PPI therapy. Best Practice & Research Clinical Gastroenterology. 2013;27:443–454. doi: 10.1016/j.bpg.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–521. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis J. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15:897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 6.Johnell O, Kanis J. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 7.Ngamruengphong S, Leontiadis GI, Radhi S, Dentino A, Nugent K. Proton pump inhibitors and risk of fracture: a systematic review and meta-analysis of observational studies. Am J Gastroenterol. 2011;06:1209–1218. doi: 10.1038/ajg.2011.113. [DOI] [PubMed] [Google Scholar]
- 8.Ye X, Liu H, Wu C, Qin Y, Zang J, Gao Q, Zhang X, He J. Proton pump inhibitors therapy and risk of hip fracture: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:794–800. doi: 10.1097/MEG.0b013e328348a56a. [DOI] [PubMed] [Google Scholar]
- 9.Kwok CS, Yeong JK, Loke YK. Meta-analysis: risk of fractures with acid-suppressing medication. Bone. 2011;48:768–776. doi: 10.1016/j.bone.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Eom CS, Park SM, Myung SK, Yun JM, Ahn JS. Use of acid-suppressive drugs and risk of fracture: a meta-analysis of observational studies. Ann Fam Med. 2011;9:257–267. doi: 10.1370/afm.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reyes C, Formiga F, Coderch M, Hoyo J, Ferriz G, Casanovas J, Monteserín R, Brotons C, Rojas M, Moral I. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone. 2013;52:557–561. doi: 10.1016/j.bone.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Adams AL, Black MH, Zhang JL, Shi JM, Jacobsen SJ. Proton-pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol. 2014;24:286–290. doi: 10.1016/j.annepidem.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Fraser L, Leslie W, Targownik L, Papaioannou A, Adachi J. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporos Int. 2013;24:1161–1168. doi: 10.1007/s00198-012-2112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moberg LM, Nilsson PM, Samsioe G, Borgfeldt C. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The WHILA Study. Maturitas. 2014;78:310–5. doi: 10.1016/j.maturitas.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Khalili H, Huang ES, Jacobson BC, Camargo CA, Feskanich D, Chan AT. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMJ. 2012;30:344. doi: 10.1136/bmj.e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cea Soriano L, Ruigómez A, Johansson S, García Rodríguez LA. Study of the Association Between Hip Fracture and Acid-Suppressive Drug Use in a UK Primary Care Setting. Pharmacotherapy. 2014;34:570–81. doi: 10.1002/phar.1410. [DOI] [PubMed] [Google Scholar]
- 18.De Vries F, van Staa TP, Leufkens HG. Proton pump inhibitors, fracture risk and selection bias: three studies, same database, two answers. Osteoporos Int. 2011;22:1641–1642. doi: 10.1007/s00198-010-1323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 20.De Vries F, Cooper A, Cockle S, van Staa TP, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int. 2009;20:1989–1998. doi: 10.1007/s00198-009-0891-4. [DOI] [PubMed] [Google Scholar]
- 21.Kaye JA, Jick H. Proton pump inhibitor use and risk of hip fractures in patients without major risk factors. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2008;28:951–959. doi: 10.1592/phco.28.8.951. [DOI] [PubMed] [Google Scholar]
- 22.Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. Can Med Assoc J. 2008;179:319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu HF, Huang YW, Chang CC, Yang CY. Use of proton pump inhibitors increased the risk of hip fracture: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2010;19:1131–1136. doi: 10.1002/pds.2026. [DOI] [PubMed] [Google Scholar]
- 24.Yu EW, Blackwell T, Ensrud KE, Hillier TA, Lane NE, Orwoll E, Bauer DC. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83:251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Garg SK, Singh PP, Iyer PG, El-Serag HB. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett’s oesophagus: a systematic review and meta-analysis. Gut. 2014;63:1229–37. doi: 10.1136/gutjnl-2013-305997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roux C, Briot K, Gossec L, Kolta S, Blenk T, Felsenberg D, Reid DM, Eastell R, Glüer CC. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcif Tissue Int. 2009;84:13–19. doi: 10.1007/s00223-008-9188-4. [DOI] [PubMed] [Google Scholar]
- 27.Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, Chen Z. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79:76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 29.Pouwels S, Lalmohamed A, Souverein P, Cooper C, Veldt B, Leufkens H, de Boer A, Van Staa T, De Vries F. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporos Int. 2011;22:903–910. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grisso JA, Kelsey JL, O’Brien LA, Miles CG, Sidney S, Maislin G, LaPann K, Moritz D, Peters B. Risk factors for hip fracture in men. Am J Epidemiol. 1997;145:786–793. doi: 10.1093/oxfordjournals.aje.a009171. [DOI] [PubMed] [Google Scholar]
- 32.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. The Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 33.Silverman S, Viswanathan H, Yang YC, Wang A, Boonen S, Ragi-Eis S, Fardellone P, Gilchrist N, Lips P, Nevitt M. Impact of clinical fractures on health-related quality of life is dependent on time of assessment since fracture: results from the FREEDOM trial. Osteoporos Int. 2012;23:1361–1369. doi: 10.1007/s00198-011-1720-0. [DOI] [PubMed] [Google Scholar]
- 34.Food U, Administration D. FDA drug safety communication: possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors. Online documentat:wwwfdagov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ ucm213206 htm Accessed August 7. 2011
- 35.Targownik LE, Lix LM, Leung S, Leslie WD. Protonpump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138:896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Mizunashi K, Furukawa Y, Katano K, Abe K. Effect of omeprazole, an inhibitor of H+, K+-ATPase, on bone resorption in humans. Calcif Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 37.Chonan O, Takahashi R, Yasui H, Watanuki M. Effect of L-lactic acid on calcium absorption in rats fed omeprazole. J Nutr Sci Vitaminol (Tokyo) 1998;44:473–481. doi: 10.3177/jnsv.44.473. [DOI] [PubMed] [Google Scholar]
- 38.Insogna KL. The effect of proton pump-inhibiting drugs on mineral metabolism. Am J Gastroenterol. 2009;104:S2–S4. doi: 10.1038/ajg.2009.44. [DOI] [PubMed] [Google Scholar]
- 39.Yang YX. Proton pump inhibitor therapy and osteoporosis. Curr Drug Saf. 2008;3:204–209. doi: 10.2174/157488608785699414. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Yuan YC, Leontiadis GI, Howden CW. Recent safety concerns with proton pump inhibitors. J Clin Gastroenterol. 2012;46:93–114. doi: 10.1097/MCG.0b013e3182333820. [DOI] [PubMed] [Google Scholar]
- 41.Vestergaard P. Systematic review of observational studies finds increased risk of fracture among older adults taking a proton pump inhibitor. Evid Based Med. 2012;17:39–40. doi: 10.1136/ebm.2011.100114. [DOI] [PubMed] [Google Scholar]
- 42.Shen C, Chen F, Zhang Y, Guo Y, Ding M. Association between use of antiepileptic drugs and fracture risk: a systematic review and meta-analysis. Bone. 2014;64:246–253. doi: 10.1016/j.bone.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Cea-Soriano L, Johansson S, García Rodríguez LA. Risk factors for falls with use of acid-suppressive drugs. Epidemiology. 2013;24:600–607. doi: 10.1097/EDE.0b013e318294bec6. [DOI] [PubMed] [Google Scholar]
- 44.de Vries F, Cooper AL, Cockle SM, van Staa TP, Cooper C. Fracture risk in patients receiving acid-suppressant medication alone and in combination with bisphosphonates. Osteoporos Int. 2009;20:1989–1998. doi: 10.1007/s00198-009-0891-4. [DOI] [PubMed] [Google Scholar]
- 45.Liu H, Wang XC, Hu GH, Guo ZF, Lai P, Xu L, Huang TB, Xu YF. Fruit and vegetable consumption and risk of bladder cancer: an updated meta-analysis of observational studies. Eur J Cancer Prev. 2015 doi: 10.1097/CEJ.0000000000000119. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


