Abstract
Human cervical cancer oncogene (HCCR) was identified by differential display RT-PCR by screened abnormally expressed genes in cervical human cancers. The overexpressed gene is not only identified in cervical tissues, but also in various human cancers as leukemia/lymphoma, breast, stomach, colon, liver, kidney and ovarian cancer. For its special sensitivities and specificities in human breast cancer and hepatocellular carcinoma, it is expected to be a new biomarker to replace or combine with the existing biomarkers in the diagnose. The HCCR manifests as a negative regulator of the p53 tumor suppressor gene, and its expression is regulated by the PI3K/Akt signaling pathway, modulated by TCF/β-catenin, it also participates in induction of the c-kit proto-oncogene, in activation of PKC and telomerase activities, but the accurate biochemical mechanisms of how HCCR contributes to the malignancies is still unknown. The aim of this review is to summarize the roles of HCCR in cancer progression and the molecular mechanisms involved.
Keywords: HCCR, p53, PI3K/Akt signaling pathway, TCF/β-catenin, PKC
Introduction
The role of oncogenes and tumor suppressor genes are the hotspot in the tumor initiation and progression. Oncogenes derived from highly conserved proto-oncogenes that are altered by chromosomal point mutations, gene amplifications, or gene rearrangements [1,2]. The structure alteration leads to quantitative or qualitative changes in the corresponding protein, and the oncoproteins participate in the signaling pathways which govern fundamental cell functions, such as proliferation, cell cycle regulation and apoptosis [3].
Human cervical cancer oncogene (HCCR) was identified by differential display RT-PCR by screened abnormally expressed genes in normal human cervical tissues and cervical cancer tissues. The special fragment was strongly expressed in cervical cancer tissue and metastatic lymph node tissues but not in normal cervical tissues. There was little similarity between HCCR and other cDNAs already in the database. Using the partial cDNA as a probe to identify the full length cDNA, two clones were isolated. The longer one, named human cervical cancer -1 oncogene (HCCR-1), encodes 360 amino acids (~42 KDa). The shorter one, named human cervical cancer-2 oncogene (HCCR-2), encodes 304 amino acids (~36 KDa) molecules. HCCR-2 lacks exon 1 of HCCR-1. The gene location was identified in the chromosome 12q by FISH analysis [4]. Their coding proteins are alternative splicing variants [5], there are two potential N-myristylation sites, two potential phosphorylation sites for protein kinase C, N-glycosylation site, and hydrophobic transmembrane domain comprising 20 amino acids [6], and are expected to be type II membrane proteins [4].
HCCR in cancer progression as a potential biomarker
Both HCCR-1 and HCCR-2 mRNA were examined and found overexpressed in human cervical cancer cell line Hela, leukemia/lymphoma cell lines, including chronic myelogenous leukemia cell line K-562, Burkitt’s lymphoma cell line Raji, lymphoblastic leukemia cell line MOLT-4, promyelocytic leukemia cell line HL-60, but there is low expression in the colon cancer cell line SW480, the lung cancer line A549, and the melanoma line G361. Increased expression of HCCR-2 compared with normal tissue was detected in multiple tumor tissue, including breast, kidney, ovary, stomach, colon carcinomas and leukemia, lymphoma samples [4].
Although the HCCR-1 was first identified in cervical cancer [7], in several reports, the HCCR oncoprotein in serum was expected to be used as a new biomarker to replace or combine with the existing biomarkers in the diagnose of human breast cancer and hepatocellular carcinoma [7-9].
Breast cancer is one of the most frequent malignancies among female, and the decrease of its mortality benefits from the use of screening technology for the early disease and development of the biomarkers [10]. In the Jing’s research [7], serum HCCR concentration were detected from 299 subjects by using ELASA method, including 129 breast cancer patients, 24 patients with benign disease, and 158 normal volunteers, comparisons were also made between HCCR and the existing biomarker CA15-3. Serological research revealed an 86.8% sensitivity for HCCR in breast cancer, compared to 21.0% for CA15-3. 86 of 98 (87.8%) patients with breast cancers that were negative for CA15-3 were positive for HCCR-1. The results clearly showed that the HCCR assay had an advantage over CA15-3 in diagnose of breast cancer and detection of early stages of the disease.
HCCR-1 is also well correlated with known breast cancer prognostic markers ER, PR, p53 mutation and high HER2 overexpression [11], in the breast cancer cell lines BT-474, MCF-7, SK-BR-3, MDA-MB-231, and breast cancer tissues. An increasing expression level of HCCR-1 was observed, but HCCR-1 was not detected in the ER-negative, PR-negative, p53 negative and low HER2 cell lines and breast cancer tissue. Therefore, the HCCR level may also be used in the improvement of breast cancer prognosis.
Serum alpha fetoprotein (AFP) and hepatic imageology are the only available methods for hepatocellular carcinoma (HCC) surveillance [12], even serum AFP is still the golden standard in the HCC diagnostic [13]. However, the sensitivity and specificity of AFP are not satisfied and the imageology is an operator dependent technology which is limited in the ability to distinguish HCC from non-neoplastic lesions as regenerative nodules. The recent researches revealed that HCCR-1 might be used in combination with AFP in detection [8]. 25 normal subjects, 32 liver cirrhosis, 116 tissue were tested by immunohistochemical method, 120 normal 524 liver disease patients were evaluate AFP and HCCR-1 simultaneously. The sensitivities of AFP (20 ng/mL) and HCCR-1(10 ng/mL) in HCC were 55.8% (164/294) and 44.2% (130/294) respectively. Neither of them has satisfactory accuracy in detecting HCC or predicting the prognosis when used alone. However, when AFP was combined with HCCR-1, sensitivities for HCC increased to 77.2%, the combination use of AFP and HCCR-1 improved the diagnostic rate to 70.8% in small HCC (< 2 cm) and 81.6% in large HCC (≥ 2 cm), respectively [9]. Despite the fact that there was no significant difference in the diagnostic rate for HCC between AFP and HCCR-1, many cases for AFP-negative HCC were positive for HCCR-1 and vice versa.
The study [14] showed that the diagnostic rate of HCC can be improved by the simultaneous detection of both HCCR-1 and AFP. In the diagnosis of HCC, HCCR-1 could be used as a supplementary to AFP. In consideration of the high heterogeneity of HCC, an optimal serological test for HCC will be based on the simultaneous measurement of two or three highly specific serological markers. The combination use of AFP and HCCR-1 increases the diagnostic rate compared with AFP alone particularly in small HCC and may improve diagnostic rate of the recurrence of HCC in earlier period.
HCCR molecular mechanisms involved
The alteration in oncogenes as chromosomal point mutations, gene amplifications, or gene rearrangements lead to quantitative or qualitative changes in the corresponding protein product, and the oncoproteins participate in the signaling pathways that govern fundamental cell functions, finally contribute to the changes in cell proliferation, cell cycle regulation and apoptosis [15]. As reported, the HCCR manifests as a negative regulator of the p53 tumor suppressor gene and its expression is regulated by the PI3K/Akt signaling pathway, modulated by TCF/β-catenin, and it also participates in induction of the c-kit proto-oncogene, in activation of PKC and telomerase activities (Figure 1).
Figure 1.
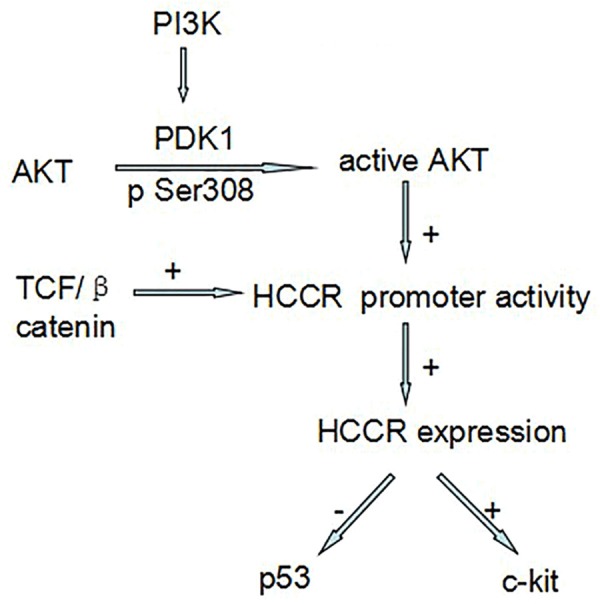
HCCR molecular mechanisms involved.
The HCCR manifests as a negative regulator of the p53 tumor suppressor gene
As a key tumor suppressor, p53 of which product is short lived triggers cell cycle arrest, senescence, or apoptosis in response to cellular stress [16]. In most cases, the alterations within the coding sequences of the gene affect the DNA binding ability of p53. Stress signaling induces p53 protein stabilization through phosphorylation and other post-translational modifications [17]. All the alterations related to p53 frequently contribute to the cell cycle change and finally imply the occurrence of malignancies. Many reports manifested that HCCR participated in the negative regulation of p53 transcriptional activity through p53 stabilization.
After HCCR was identified in cervical cancers and cervical cancer cell lines, transgenic mice with oncogene HCCR-2 were generated for the mechanisms research [18]. 198 of 360 (55%) female nude mice injected with NIH/3I3 stably transfected with HCCR-2 developed breast cancer and metastasis with a mean latency period of 4 months. The level of p53 in HCCR-2 transgenic mice was analyzed, result from western blot analyses indicated p53 expression level markedly increased compared with non-transgenic mice, but the p53 responsive genes including p21, MDM2, and bax were in low level.
A similar assay was also done in the HCCR-2 transfected RKO cells and NCI-H460 cells [4], the level of p53 were markedly elevated in both of the transfected cells, compared to cells transfected with the vector alone, but p21, MDM2, and bax were decreased in HCCR-2 transfected NCI-H460 and RKO cells. The increase of p53 may be attribute to the presence of mutated p53 protein or an increase in protein stability, a RNA mismatch detection assay manifested no genetic mutation occurred. Hence, the half-life of p53 was detected: the 293 cells transfected with the vector alone was approximately 30mins, HCCR-2 transfected 293 cells was more than 4h, and the half-life of p53 in most normal primary cells ranges between 20 min and 1h, which varies in different cell types [19]. Exposure to DNA damaging agents induces accumulation of the p53 protein and a lengthened half-life of four to five fold without significant changes in the mRNA level [20]. Post-translational regulation of p53 is likely largely dependent on a regulatory mechanism for ubiquitination and proteasomal degradation as p53 is highly sensitive to ubiquitination dependent proteasomal degradation [21], therefore the stabilization of p53 in HCCR-2 transfected NCI-H460 and RKO cells indicated that HCCR-2 was probably involved in the modification of p53 through the interference of ubiquitination-dependent proteasomal degradation. The decrease of p21, MDM2, and bax indicated that the increased level of p53 did not induce expression of these proteins, but did downregulate these proteins. The result showed that HCCR-2 possibly participated in the ubiquitin-dependent proteasomal degradation of p53 and played a role in negative regulation of p53 transcriptional activity.
PI3K regulates HCCR-1 gene expression
The expression of HCCR is also investigated in the PI3K/Akt signaling pathway which also is the major activated pathway involved in the oncogenesis of various types of cancers [22]. This phosphorylated activation of the pathway results in phosphorylation of other proteins that affect cell cycle entry, cell proliferation, and anti-apoptosis [23,24]. Approximately 50% of patients with breast cancer have a mutation or loss of at least one copy of the PTEN gene, resulting in activation of PI3K signaling which becomes a fundamental pathway for tumor proliferation and survival [25,26], and interestingly HCCR is detected to be overexpressed in the breast cancer.
The HCCR-1 gene consists of 9 exons and conserved exon/intron boundaries, the translation start and stop codons are located in the first and the last exon [4]. The putative promoter region is identified at position -515 to + 153, which contains a TATA box and a CAAT box, and is identified as putative DNA binding sites for TCF/LEF-1. Elk-1, E2F, GATA-1, estrogen, receptors, and several homeodomain protein. To determine the function of PI3K on HCCR promoter, the vector containing pCDNA3-PI3K was transfected in NIH/3T3 cells, the promoter activity was 1.72-fold in PI3K-transfected cells compared with those cells transfected with vector alone. Also vector with wild type Akt and dominant negative Akt cDNA were transfected in K562 cells. The result suggested that wild type Akt enhanced the HCCR-1 promoter activity. For further research, PI3K inhibitor LY294002 which is a synthetic compound based on the flavonoid quercetin was tested time and dose dependent inhibition in K562 cell and NIH/3T3 cell which was transfected with vector containing HCCR promoter constructs, the promoter activities in NIH/3T3 cells and HCCR-1 expression in K562 cells were decreased in a dose dependent manner [5]. In pancreatic cancer cell line PANC-1, the EGF-induced overexpression of HCCR-1 was also modulated by the PI3K/Akt pathway [27]. All the experiment data portrayed that the alterations in the PI3K/Akt signaling pathway influenced HCCR expression and that HCCR was one of the downstream components of the PI3K/Akt pathway (Figure 1).
TCF/β-catenin modulate HCCR
Wnts are a large family of secreted glycoprotein involved in cell proliferation, differentiation, and oncogenesis [28], TCF and β-catenin are major molecular in the pathway. A report indicated that Tcf1 site which located in HCCR-1 5’flanking region at position -26 to -4 played an important role in HCCR-1 promoter activity, a super-shift assay manifested that the transcription factor, TCF, and its cofactor, β-catenin were observed bounded to the site [29]. Treated with GSK-3β inhibitor, acting as Wnt signal activator, LiCl or AR-A014418, endogenous HCCR-1 expression was also increased in HEK/293 and K562 cells. These findings suggested that the Tcf1 site on the HCCR-1 promoter is a major element regulating HCCR-1 expression and the Wnt pathway stimulation of this site may induce human cancers (Figure 1).
HCCR participates in induction of the c-kit proto-oncogene, in activation of PKC and telomerase activities
C-Kit (CD117) is a type III tyrosine kinase receptor controlling in cell signal transduction [30], normally it is activated by binding with its ligand, the stem cell factor (SCF) [31,32]. This leads to a phosphorylation cascade ultimately activating various transcription factors that regulate apoptosis, cell differentiation, proliferation, chemotaxis, and cell adhesion in different cell types. Western blot results showed that NIH/3T3 cells stably transfected with HCCR-1 and nude mice derived tumors injected with NIH/3T3 cells transfected with HCCR-1 both overexpressed the c-kit protein compared with NIH/3T3 parental cells or cells transfected with vector alone. The results showed that HCCR-1 derived tumor cells express c-kit, HCCR-1 was related to the c-kit signaling pathway.
HCCR-1 gene transfection increased telomerase activity up to about 7-fold when compared with wild-type cells, and literatures showed that PKC could induce a marked increase in telomerase activity [33], so that a kinase assay was performed to determine whether the increased telomerase activity in HCCR-1 transfected cells was caused by PKC, and the PKC activity of HCCR-1 transfected cells was increased by about 10-fold when compared with wild-type cells [34]. The reports suggested that HCCR participated in induction of the c-kit proto-oncogene, in activation of PKC and telomerase activities.
Concluding remarks
HCCR, for its special sensitivities and specificities in human breast cancer and hepatocellular carcinoma, it is expected to be a new effective biomarker to replace or combine with the older biomarkers in diagnose. The molecule is involved in several important signaling pathway, as known it manifests as a negative regulator of the p53 tumor suppressor gene, and its expression is regulated by the PI3K/Akt signaling pathway, modulated by TCF/β-catenin, participates in induction of the c-kit proto-oncogene and in activation of PKC and telomerase activities. As far as the molecular mechanism of how HCCR contributes to the malignancy is explicit, it could be a potential therapeutic target in clinical therapy.
Acknowledgements
This study was partly supported by: National Natural Science Foundation (No. 81270598 and No. 81473486), National Public Health Grand Research Foundation (No. 201202017), Natural Science Foundations of Shandong Province (No. 2009ZRB14176 and No. ZR2012HZ003), Technology Development Projects of Shandong Province (No. 2008GG2NS02018, No. 2010GSF10250, and No. 2014GSF118021), Program of Shandong Medical Leading Talent, and Taishan Scholar Foundation of Shandong Province.
Disclosure of conflict of interest
None.
References
- 1.Bishop JM. Molecular themes in oncogenesis. Cell. 1991;64:235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- 2.Cheng G, Zhang L, Lv W, Dong C, Wang Y, Zhang J. Overexpression of cyclin D1 in meningioma is associated with malignancy grade and causes abnormalities in apoptosis, invasion and cell cycle progression. Med Oncol. 2015;32:439. doi: 10.1007/s12032-014-0439-0. [DOI] [PubMed] [Google Scholar]
- 3.Chirnomas SD, Kupfer GM. The inherited bone marrow failure syndromes. Pediatr Clin North Am. 2013;60:1291–1310. doi: 10.1016/j.pcl.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ko J, Lee YH, Hwang SY, Lee YS, Shin SM, Hwang JH, Kim J, Kim YW, Jang SW, Ryoo ZY, Kim IK, Namkoong SE, Kim JW. Identification and differential expression of novel human cervical cancer oncogene HCCR-2 in human cancers and its involvement in p53 stabilization. Oncogene. 2003;22:4679–4689. doi: 10.1038/sj.onc.1206624. [DOI] [PubMed] [Google Scholar]
- 5.Cho GW, Shin SM, Namkoong H, Kim HK, Ha SA, Hur SY, Kim TE, Chai YG, Kim JW. The phosphatidylinositol 3-kinase/Akt pathway regulates the HCCR-1 oncogene expression. Gene. 2006;384:18–26. doi: 10.1016/j.gene.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Chung YJ, Kim JW. Novel oncogene HCCR: its diagnostic and therapeutic implications for cancer. Histol Histopathol. 2005;20:999–1003. doi: 10.14670/HH-20.999. [DOI] [PubMed] [Google Scholar]
- 7.Jung SS, Park HS, Lee IJ, Namkoong H, Shin SM, Cho GW, Ha SA, Park YG, Lee YS, Ko J, Kim JW. The HCCR oncoprotein as a biomarker for human breast cancer. Clin Cancer Res. 2005;11:7700–7708. doi: 10.1158/1078-0432.CCR-04-2609. [DOI] [PubMed] [Google Scholar]
- 8.Jirun P, Zhang G, Kim HK, Ha SA, Zhongtian J, Shishi Q, Zhuqingqing C, Lei G, Yoo J, Kim S, Park YG, Wang J, Yang Y, Xu Z, Huang Z, Lee YK, Song EY, Kim JW. Clinical utility of alpha fetoprotein and HCCR-1, alone or in combination, in patients with chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. Dis Markers. 2011;30:307–315. doi: 10.3233/DMA-2011-0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang G, Ha SA, Kim HK, Yoo J, Kim S, Lee YS, Hur SY, Kim YW, Kim TE, Park YG, Wang J, Yang Y, Xu Z, Song EY, Huang Z, Jirun P, Zhongtian J, Shishi Q, Zhuqingqing C, Lei G, Kim JW. Combined analysis of AFP and HCCR-1 as an useful serological marker for small hepatocellular carcinoma: a prospective cohort study. Dis Markers. 2012;32:265–271. doi: 10.3233/DMA-2011-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels CC, Ruberta F, de Kruijf EM, van Pelt GW, Smit VT, Liefers GJ, Matsushima T, Shibayama M, Ishihara H, van de Velde CJ, Kuppen PJ. The prognostic value of apoptotic and proliferative markers in breast cancer. Breast Cancer Res Treat. 2013;142:323–339. doi: 10.1007/s10549-013-2748-y. [DOI] [PubMed] [Google Scholar]
- 11.Ha SA, Lee YS, Shin SM, Kim HK, Kim S, Namkoong H, Kim HJ, Jung SM, Lee YS, Chung YJ, Jung SS, Kim JW. Oncoprotein HCCR-1 expression in breast cancer is well correlated with known breast cancer prognostic factors including the HER2 overexpression, p53 mutation and ER/PR status. BMC Cancer. 2009;9:51. doi: 10.1186/1471-2407-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodes J EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 13.Wong GL, Chan HL, Tse YK, Chan HY, Tse CH, Lo AO, Wong VW. On-treatment alpha-fetoprotein is a specific tumor marker for hepatocellular carcinoma in patients with chronic hepatitis B receiving entecavir. Hepatology. 2014;59:986–995. doi: 10.1002/hep.26739. [DOI] [PubMed] [Google Scholar]
- 14.Dong A, Yu H, Wang Y, Dong H, Zuo C. FDG PET/CT and enhanced CT imaging of tumor heterogeneity in hepatocellular carcinoma: imaging-pathologic correlation. Clinical Nucl Med. 2014;39:808–810. doi: 10.1097/RLU.0b013e3182a75812. [DOI] [PubMed] [Google Scholar]
- 15.Brunet E, Simsek D, Tomishima M, DeKelver R, Choi VM, Gregory P, Urnov F, Weinstock DM, Jasin M. Chromosomal translocations induced at specified loci in human stem cells. Proc Natl Acad Sci U S A. 2009;106:10620–10625. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Ørntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- 17.Gosslau A, En Jao DL, Huang MT, Ho CT, Evans D, Rawson NE, Chen KY. Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol Nutr Food Res. 2011;55:198–208. doi: 10.1002/mnfr.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko J, Shin SM, Oh YM, Lee YS, Ryoo ZY, Lee YH, Na DS, Kim JW. Transgenic mouse model for breast cancer: induction of breast cancer in novel oncogene HCCR-2 transgenic mice. Oncogene. 2004;23:1950–1953. doi: 10.1038/sj.onc.1207356. [DOI] [PubMed] [Google Scholar]
- 19.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405–408. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 20.Lui K, An J, Montalbano J, Shi J, Corcoran C, He Q, Sun H, Sheikh MS, Huang Y. Negative regulation of p53 by Ras superfamily protein RBEL1A. J Cell Sci. 2013;126:2436–2445. doi: 10.1242/jcs.118117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis JR, Mossalam M, Lim CS. Controlled access of p53 to the nucleus regulates its proteasomal degradation by MDM2. Mol Pharm. 2013;10:1340–1349. doi: 10.1021/mp300543t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon SH, Kim DK, Cha Y, Jeon I, Song J, Park KS. PI3K/Akt and Stat3 signaling regulated by PTEN control of the cancer stem cell population, proliferation and senescence in a glioblastoma cell line. Int J Oncol. 2013;42:921–928. doi: 10.3892/ijo.2013.1765. [DOI] [PubMed] [Google Scholar]
- 24.Santo EE, Stroeken P, Sluis PV, Koster J, Versteeg R, Westerhout EM. FOXO3a is a major target of inactivation by PI3K/AKT signaling in aggressive neuroblastoma. Cancer Res. 2013;73:2189–2198. doi: 10.1158/0008-5472.CAN-12-3767. [DOI] [PubMed] [Google Scholar]
- 25.Pandolfi PP. Breast cancer--loss of PTEN predicts resistance to treatment. N Engl J Med. 2004;351:2337–2338. doi: 10.1056/NEJMcibr043143. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Romigh T, He X, Tan MH, Orloff MS, Silverman RH, Heston WD, Eng C. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene. 2011;30:4327–4338. doi: 10.1038/onc.2011.144. [DOI] [PubMed] [Google Scholar]
- 27.Xu Z, Zhang Y, Jiang J, Yang Y, Shi R, Hao B, Zhang Z, Huang Z, Kim JW, Zhang G. Epidermal growth factor induces HCCR expression via PI3K/Akt/mTOR signaling in PANC-1 pancreatic cancer cells. BMC Cancer. 2010;10:161. doi: 10.1186/1471-2407-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtin JC. Novel drug discovery opportunities for colorectal cancer. Expert Opin Drug Discov. 2013;8:1153–1164. doi: 10.1517/17460441.2013.807249. [DOI] [PubMed] [Google Scholar]
- 29.Cho GW, Kim MH, Kim SH, Ha SA, Kim HK, Kim S, Kim JW. TCF/beta-catenin plays an important role in HCCR-1 oncogene expression. BMC Mol Biol. 2009;10:42. doi: 10.1186/1471-2199-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phung B, Sun J, Schepsky A, Steingrimsson E, Ronnstrand L. C-KIT signaling depends on microphthalmia-associated transcription factor for effects on cell proliferation. PLoS One. 2011;6:e24064. doi: 10.1371/journal.pone.0024064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasaki T, Mizuochi C, Horio Y, Nakao K, Akashi K, Sugiyama D. Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse. Development. 2010;137:3941–3952. doi: 10.1242/dev.051359. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann UB, Kauczok-Vetter CS, Houben R, Becker JC. Overexpression of the KIT/SCF in uveal melanoma does not translate into clinical efficacy of imatinib mesylate. Clin Cancer Res. 2009;15:324–329. doi: 10.1158/1078-0432.CCR-08-2243. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Zhao L, Yang Z, Funder JW, Liu JP. Telomerase is controlled by protein kinase Calpha in human breast cancer cells. J Biol Chem. 1998;273:33436–33442. doi: 10.1074/jbc.273.50.33436. [DOI] [PubMed] [Google Scholar]
- 34.Ha SA, Kim HK, Yoo J, Kim S, Shin SM, Lee YS, Hur SY, Kim YW, Kim TE, Chung YJ, Jeun SS, Kim DW, Park YG, Kim J, Shin SY, Lee YH, Kim JW. Transdifferentiation-inducing HCCR-1 oncogene. BMC Cell Biol. 2010;11:49. doi: 10.1186/1471-2121-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]


