Abstract
Objective: To determine the potential role of intraoperative carbon nanoparticles (CN) injections for identification and preservation of parathyroid glands, thereby reducing the postoperative hypocalcaemia. Methods: 100 patients with thyroid cancer who underwent total thyroidectomy and central compartment node dissection (CCND) were randomly assigned to receive intraoperative injection of (CN) or not for identifying and preserving normal parathyroid glands. Results: There was no significantly difference for preoperative and postoperative parathyroid hormone (PTH) levels between the CN and control group (P>0.05). The levels of albumin-adjusted serum calcium (AASC) before surgery and at day 1 and 1 month after surgery did not reach the significant difference between the two groups (P>0.05). However, the patients in CN group had the higher level of AASC at day 3 after surgery than those in control group (P=0.044). Transient postoperative hypoparathyroidism occurred in 24 (48%) patients in CN group and 28 (56%) in control groups, respectively (P=0.423). The incidence of transient postoperative hypocalcemia was 20% (10/50) in CN group and 24% (12/50) in control groups, respectively (P=0.629). Conclusions: Carbon nanoparticles can make the thyroid gland and the central lymph node black-stained, but no-stained for parathyroid glands. After rapidly identifying parathyroid and distinguishing it from thyroid and lymph nodes by carbon nanoparticles, complete lymph node dissection and preservation of parathyroid glands become feasible during total thyroidectomy with neck lymph node dissection. After identification, strict adherence to capsular dissection remains essential for safe preservation in situ of the parathyroid glands and their blood supply.
Keywords: Parathyroid glands, identification, preservation, carbon nanoparticle, lymph node excision, thyroid neoplasms
Introduction
During thyroid surgery, the inadvertent injury of parathyroid glands and laryngeal nerves may result in a profound influence in patient’s quality of life. With meticulous surgical techniques and intraoperative monitoring the nerve, the recurrent laryngeal nerve palsy has been reduced to a minimum rate, which could result in hoarseness, voice fatigue and even respiratory paralysis for tracheotomy [1,2]. However, how to prevent and reduce the incidence of postoperative hypocalcaemia remains obscure. Intraoperative identification of the parathyroid glands can be a challenge even for experienced surgeons and unintentional parathyroidectomy may be as high as 9% [3]. Estimates for transient hypoparathyroidism after total thyroidectomy range from 5% to 60% [4,5] and for permanent hypoparathyroidism from 0.5% to 2% [6]. Patients who developed into hypocalcemia will eventually need to take oral calcium and vitamin D for a long time, which causes them significant discomfort. The most common factor for post-surgical hypocalcaemia was parathyroid gland injury, including an accidental resection or, most commonly, parathyroid “devascularization”. To minimize parathyroid injury, a variety of techniques have been used for localization and identification of parathyroid glands, such as preoperative ultrasound, Sestamibi scintigraphy, computed tomography (CT), magnetic resonance imaging, and intraoperative intact parathyroid hormone assay [7]. Moreover, intraoperative methylene blue infusion [8], technetium-99 m-sestamibi [Tc-MIBI] localization using a hand-held gamma probe [9,10] and optical coherence tomography [11,12] may help maximize parathyroid preservation. These existing methods for identifying parathyroid glands are limited in their applicability and sensitivity, rendering them inadequate to prevent surgical complications [13]. Therefore, there remains a need for a way to accurately identify parathyroid glands during thyroid surgery, especially total thyroidectomy with central lymph node dissection for thyroid cancer.
Anatomical evidence shows rich lymphatics and lymphatic capillaries in the thyroid but almost none in the parathyroid glands. In addition, there are anatomically independent external capsules for the thyroid and parathyroid glands [14,15]. Based on these findings, after injection into the thyroid, a carbon nanoparticle (CN) suspension can stain the thyroid lymphatics and capillary lymphatics completely black but not the parathyroid glands [16]. Thus, CN suspension injections could be used to identify the parathyroid glands during surgery.
We conducted this study to compare the levels of parathyroid hormone (PTH) and albumin-adjusted serum calcium (AASC) after total thyroidectomy and neck lymph node dissection with visualization alone and with CN suspension injections. The aim of the study is to determine the potential role of intraoperative CN injections for identification and preservation of parathyroid glands, thereby reducing the postoperative hypocalcaemia.
Material and methods
Materials
Carbon nanoparticles (Chongqing LUMMY Pharmaceutical Co., Chongqing, China) were applied in the form of a standard carbon nanoparticles suspension injection (1 ml: 50 mg). This product, which is a stable suspension of carbon pellets of 150 nm in diameter, does not enter the blood circulation and has no toxic side effects on the human body. A small amount of tiny carbon particles may be captured by macrophages, and they are excreted through the lungs and intestines after a few months. Carbon nanoparticles also do not cause acute systemic toxicity.
Patients
Between June 2012 and August 2014, a total of 100 patients with thyroid carcinoma were randomly allocated to CN suspension group (n=50) or direct visualization group (n=50) for the identification of parathyroid during the surgery at the department of head and neck surgery in Zhejiang Cancer Hospital. All patients were performed with total thyroidectomy and ipsilateral central lymph nodes dissection by the same surgeon. If there was an existing suspicion of lateral neck lymph node metastasis, the therapeutic lateral neck dissection would be performed as the same time. Exclusion criteria included non-thyroid cancer, previous thyroid or parathyroid surgery, preoperative hypoparathyroidism or hypocalcemia and pregnancy or lactation. Ethics approval for this study was obtained from our hospital’s research ethics board and informed consent was obtained before these procedures.
Medical records were reviewed and the following data were collected: demographic information, such as sex and age at diagnosis; extent of surgery; various histological parameters, including primary tumor size, the number of tumor foci, extrathyroidal invasion, and cervical lymph node involvement; radioactive iodine (RAI) therapy and accumulated RAI doses; and clinical outcome at the last follow-up. Preoperative and postoperative AASC levels and (PTH) levels were also collected.
Surgical procedures
The surgical procedures were as follows: After general anesthesia, a standard approach was employed with an incision two finger breadths above the clavicle. Subplatysmal flaps were raised, the strap muscles retracted and the thyroid gland exposed. The CN suspension was injected into the lower and upper points of the thyroid gland by avoiding lesions, with 0.1 to 0.15 mL administered for each area, and the total amount injected would be less than 0.5 mL per lobe (Figure 1A). In addition, when injecting or withdrawing from the thyroid, the syringe was pumped back to avoid mistakenly injecting into any blood vessels. After injection, the sites of injection should be pressed lightly for 3-5 minutes, to prevent the overflow of CN suspension and pollution to surgical field. The thyroid and surrounding lymph tissue were stained black by CN suspension, whereas the parathyroid glands negative stained (Figure 1B-E). The middle thyroid vein was ligated and the lobes of the thyroid were retracted medially. The superior and inferior thyroid arteries were identified together with recurrent laryngeal nerve (RLN). In order to preserve the blood supply of parathyroid glands, inferior thyroid artery was ligated distal to branches to inferior parathyroid gland and superior thyroid artery ligated adjacent to the thyroid capsule. Then the lobe was resected from the trachea. The contralateral lobe was managed in a similar manner. All tumor resections were pathologically confirmed via intraoperative frozen biopsy. Then all patients were underwent ipsilateral central compartment node dissection (CCND). The extent of dissection involved clearance of nodal tissue from the hyoid bone superiorly to the innominate vein inferiorly and the medial border of the carotid artery laterally. The radical lateral lymph node dissection was performed for patients with preoperative evidence of lateral neck nodal disease by physical examination, ultrasonography and fine-needle biopsy.
Figure 1.
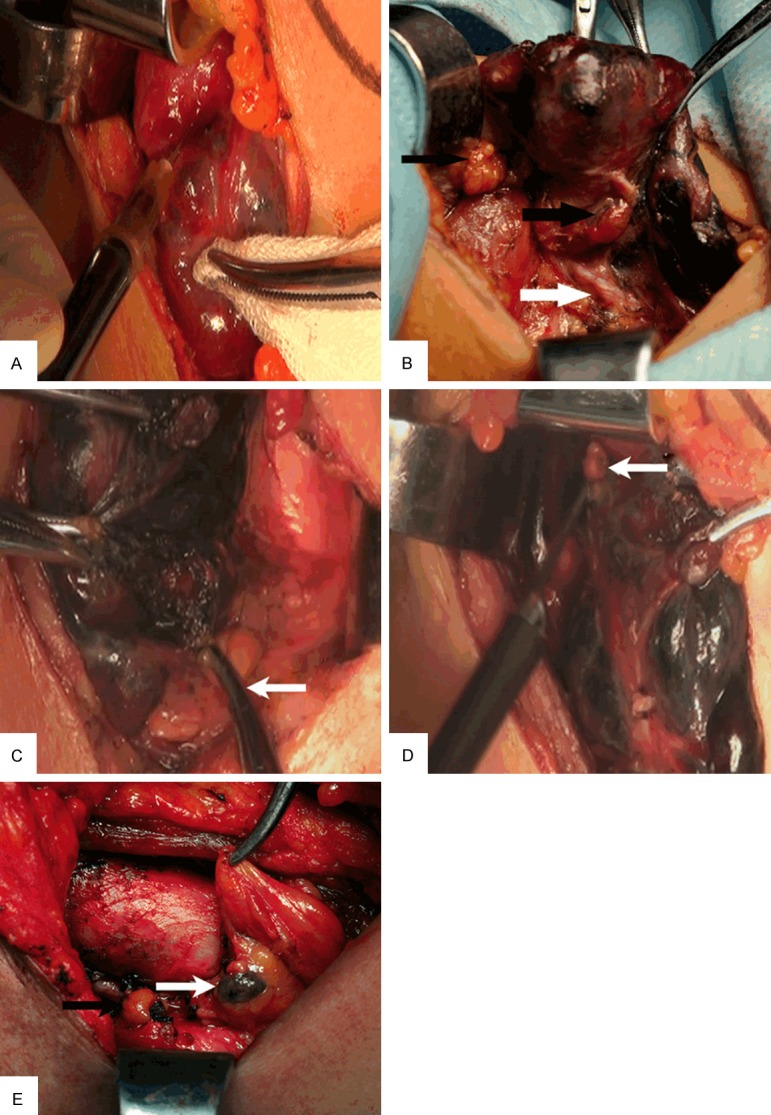
A. Injection of CN suspension into thyroid gland; B. The parathyroid gland (black arrow) and the recurrent laryngeal nerve (white arrow) at the dorsal thyroid; C. The right inferior parathyroid gland (white arrow); D. The right superior parathyroid gland (white arrow); E. The black-stained lymph node (white arrow), and the unstained parathyroid gland (black arrow).
Postoperative evaluation and follow-ups
Calcium supplementation was not routinely administered to patients undergoing thyroid surgery. The AASC and PTH levels are both measured at day 1, 3, and 1 month after thyroid surgery. Hypoparathyroidism was defined as any drop in serum PTH below the normal limit (normal range, 15 to 65 pg/mL), regardless of hypocalcemic symptoms. Hypocalcemia was defined as AASC levels <1.90 mmol/L and/or any signs or symptoms of hypocalcemia (e.g., perioral numbness, digital paresthesia, or positive Trousseau’s sign). Patients with symptomatic hypocalcemia received oral calcium supplements and vitamin D, depending on the severity of the clinical symptoms. Intravenous substitution of calcium therapy was necessary to serious symptomatic hypocalcemia such as muscle cramps, tetany, or seizures. If the serum PTH at 3 months after surgery was still lower than normal standard, the patient was considered to have permanent hypoparathyroidism.
Statistical method
The t-test and chi-square test were used for the measurement and count data, respectively. SPSS 16.0 package was used for all statistical analyses. The case with a P-value of less than 0.05 was considered to be statistically significant.
Results
One hundred patients underwent total thyroidectomy and node dissection for thyroid cancer at the surgical department of head and neck of Zhejiang Cancer Hospital between February 2012 and July 2014. Among them, 50 patients were identified parathyroid glands with the aid of intraoperative CN suspension injections (CN group) and 50 patients by visualization alone (control group). Patients’ demographics, operative details, histological findings, and postoperative events are reported in Table 1. The CN group consisted of 10 (20%) males and 40 (80%) females, ranging in age from 25 to 63 years, with a mean of 46.98 years. The control group consisted of 6 (12%) males and 44 (88%) females, ranging in age from 10 to 77 years, with a mean of 47.76 years. All the patients underwent total thyroidectomy, 17 (34%) with unilateral CCND, and 33 (66%) with bilateral CCND in both two groups. Fourteen (28%) patients underwent lateral neck dissection together with CCND in CN group, and 19 (38%) cases in control group. The pathological types of specimen consisted of papillary (47:48, CN vs. control group), follicular (1:1) and medullary (2:1) carcinomas. The mean of tumor diameter in CN and control group was 1.36±0.94 and 1.48±1.25 cm, respectively (P=0.09). The mean number of lymph nodes removed by CCND in CN and control group was 5.78±4.55 and 6.62±5.07, respectively (P=0.138). The mean number of central lymph nodes metastasis in CN and control group was 1.66±2.353 and 3.06±4.501, respectively (P=0.002). Sixteen (32%) patients received postoperative RAI therapy in CN group, and 20 (40%) in control group. Parathyroid autotransplantation was performed for 3 (6%) patients in CN group and for 13 (26%) in control group.
Table 1.
Clinicopathologic characteristics of the patients in CN and control group
| Characteristic | CN group (n=50) | Control group (n=50) | P value |
|---|---|---|---|
| Age (yr) | 46.98±9.027 | 47.76±13.912 | 0.013a |
| Sex | 0.275b | ||
| Male | 10 (20%) | 6 (12%) | |
| Female | 40 (80%) | 44 (88%) | |
| Central compartment node dissection | 1.000b | ||
| Unilateral | 17 (34%) | 17 (34%) | |
| Bilateral | 33 (66%) | 33 (66%) | |
| Lateral neck dissection | 0.288b | ||
| Yes | 14 (28%) | 19 (38%) | |
| No | 36 (72%) | 31 (62%) | |
| Size of tumor (cm) | 1.36±0.94 | 1.48±1.25 | 0.090a |
| Pathology | - | ||
| Papillary | 47 (94%) | 48 (96%) | |
| Follicular | 1 (2%) | 1 (2%) | |
| Medullary | 2 (4%) | 1 (2%) | |
| Removed central lymph node | 5.78±4.55 | 6.62±5.07 | 0.138a |
| No. of central lymph node metastasis | 1.66±2.353 | 3.06±4.501 | 0.002a |
| Parathyroid autotransplantation | 0.006b | ||
| Yes | 3 (6%) | 13 (26%) | |
| No | 47 (94%) | 37 (74%) | |
| Postoperative RAI therapy | 0.405b | ||
| Yes | 16 (32%) | 20 (40%) | |
| No | 34 (68%) | 30 (60%) | |
| Transient hypoparathyroidism | 0.423b | ||
| Yes | 24 (48%) | 28 (56%) | |
| No | 26 (52%) | 22 (44%) | |
| Transient hypocalcemia | 0.629b | ||
| Yes | 10 (20%) | 12 (24%) | |
| No | 40 (80%) | 38 (76%) | |
Footnotes: Values are presented as number (%) or mean ± SD. CN: carbon nanoparticle; RAI: radioactive iodine;
t-test;
χ 2 test.
Table 2 lists the AASC and PTH levels before and after surgery in CN and control group. There was no significantly difference for preoperative and postoperative PTH levels between the CN and control group (P>0.05). The levels of AASC before surgery and at day 1 and 1 month after surgery did not reach the significant difference between the two groups (P>0.05). However, the patients in CN group had the higher level of AASC at day 3 after surgery than those in control group (2.14±0.36: 1.93±0.21 mmol/L, P=0.044). Transient postoperative hypoparathyroidism occurred in 24 (48%) patients in CN group and 28 (56%) in control groups, respectively (P=0.423). The incidence of transient postoperative hypocalcemia was 20% (10/50) in CN group and 24% (12/50) in control groups, respectively (P=0.629). Eleven (22%) patients in CN group have experienced symptoms of hypocalcemia (eg, perioral numbness, digital paresthesia) and 15 (30%) in control group (P=0.362).
Table 2.
The PTH and AASC levels before and after surgery in the two groups
| Characteristic | CN group (n=50) | Control group (n=50) | P value* |
|---|---|---|---|
| PTH (pg/ml) | |||
| Preoperative | 46.46±16.19 | 46.71±19.63 | 0.400 |
| Postoperative | |||
| Day 1 | 18.01±12.00 | 16.67±13.62 | 0.410 |
| Day 3 | 15.90±11.04 | 16.64±14.37 | 0.083 |
| One month | 29.56±13.67 | 31.61±18.02 | 0.352 |
| AASC (mmol/L) | |||
| Preoperative | 2.21±0.09 | 2.22±0.10 | 0.685 |
| Postoperative | |||
| Day 1 | 2.08±0.21 | 2.07±0.44 | 0.551 |
| Day 3 | 2.14±0.36 | 1.93±0.21 | 0.004 |
| One month | 2.16±0.20, | 2.10±0.18 | 0.815 |
Footnotes: Values are presented as mean ± SD; CN: carbon nanoparticle; PTH: parathyroid hormone; AASC: albumin-adjusted serum calcium;
t-test.
Discussion
The incidence of thyroid carcinoma has increased in recent years due to the widespread use of ultrasonographic screening procedures. Thyroidectomy has been performed safely with technical improvement and better knowledge of thyroid anatomy [17], however, postoperative complications can still occur even with experienced surgeons. The most common complication after total thyroidectomy (TT) is hypocalcemia, which can be either transient or permanent. The causes for hypocalcemia are attributed to devascularization, injury, or removal of the parathyroid glands during the surgery [18,19]. Preservation of the parathyroid glands in situ by meticulous dissection and preservation of their blood supply [20-22] is a recommended surgical strategy in thyroid surgery to decrease the rate of postoperative hypoparathyroidism. Whereas removal of a single parathyroid gland is not associated with postoperative hypocalcemia, resection of at least 2 parathyroid glands increases the risk of transient and permanent hypoparathyroidism [23]. If the parathyroid gland was mistakenly removed during the surgery, a small piece of the sample should be sent for intraoperative frozen section, and the remaining tissues should be transplanted into sternocleidomastoid when parathyroid gland was proved. However, the incidences of permanent hypoparathyroidism were 1.4% and 21.4% after autotransplantation of 1 or 2 parathyroid glands, respectively [24]. Thus, autotransplantation of parathyroid glands did not completely restore the normal function. So it is essential to identify and preserve parathyroid glands in situ during thyroid surgery and surgeons should have a good knowledge of its anatomical location and relations with other cervical structures.
There are typically four parathyroid glands; however, supernumerary glands and less than four glands have been reported [25,26]. The color of each gland varies from yellow to reddish brown, measuring about 3-8 mm and are usually oval shaped [15]. The superior glands were usually located on the posterior aspect of the thyroid gland within a circumscribed area 2 cm in diameter about 1 cm above the crossing point of the recurrent laryngeal nerve and inferior thyroid artery [26]. The inferior glands were evenly distributed between the lower pole of the thyroid and isthmus [27]. However, there existed ectopic positions of the superior and inferior parathyroid glands. As parathyroid glands with small size, variable color, supernumerary number and ectopic positions, it was difficult to identify and preserve them only by surgeons’ naked eyes. Therefore, various procedures have been developed and tested in order to facilitate the identification and preservation of normal parathyroid glands both before and during surgery.
In recent years, staining agents such as methylene blue or 5-aminolevulinic acid (5-ALA) have been applied to visualize parathyroid glands intraoperatively. However, adverse effects have been reported and their effectiveness has yet to be shown [28-31]. Intravenous application of methylene blue has the staining rate close to 100% for the localization of enlarged parathyroid glands. Meanwhile, methylene blue may falsely stain the normal parathyroid glands, lymph nodes, thyroid tissue and fat. There have been several reports of the development of postoperative toxic metabolic encephalopathy in patients taking serotonin reuptake inhibitors and serotonergic medication [28,31]. Intraoperatively, parathyroid glands would show red fluorescence under the blue light with a wavelength of 380-440 nm after the administration of 5-Aminolevulinic acid(5-ALA), which converted into protoporphyrin IX (PpIX) and accumulates in mitochondria. It may be unaccustomed and uncomfortable that patients should be shielded from direct light exposure within 48 hours after surgery. Protoporphyria constitutes a clear contraindication for this technique [29,30]. Intraoperative sesta MIBI scintigraphy using a gamma probe is probably the most established in localizing enlarged parathyroid glands, especially in ectopic sites, with a sensitivity of 93%, a positive predictive value of 88% and an accuracy of 83% [32]. Optical coherence tomography (OCT) imaging technique has shown promising potential to identify parathyroid tissue and discriminate it from other tissues in the operating field. Further advances in OCT miniaturization and development of sterile intraoperative probe formats may allow OCT to offer an intraoperative “optical biopsy” without fixation, staining, or tissue resection [11,12]. However, there were no randomized clinical trials available to determine the effectiveness of aforementioned methods for the identification of parathyroid glands yet.
With the development of nanotechnology, nanocarbon has been widely used as a lymph node tracer in breast, thyroid and colorectal cancer [33-35]. An injection of CN suspension comprises nanosized carbon particles with an average diameter of 150 nm. Particles could pass through the lymphatic vessels rather than the blood capillaries mainly due to the difference in permeability. Upon injection into the tissues around the tumor, through penetration, diffusion and macrophage pinocytosis, carbon nanoparticles rapidly moved into the drainage capillary lymphatic strand and accumulated in the corresponding lymph nodes to result in black staining. This unique selective biodistribution has been applied in sentinel lymph node (SLN) staining, drug carriers and thermotherapy, in recent years [36-38].
During sentinel lymph node biopsy (SLNB) using carbon nanoparticles for patients with thyroid cancer, Hao et al found that the parathyroid glands were not dyed black and clearly shown during the dissection of SLNs in the patients with thyroid cancer [34]. Recent studies have reported that CN suspension was a new method to allow a complete dissection of the lymph nodes in cervical level VI without injury to the parathyroid during thyroid surgery [16,39]. At present, we conducted this research to further determine the potential role of CN suspension for identifying and protecting the parathyroid during thyroid surgery. AASC and PTH levels were taken as the measurements to predict the risk of transient or permanent postoperative hypoparathyroidism or hypocalcemia. After injection into thyroid, CN suspension rapidly stained the thyroid completely black and gradually dyed the central compartment lymph nodes but not the parathyroid glands behind the thyroid. The unstained parathyroid glands were identified and preserved in situ by meticulous dissection in CN group. The parathyroid was identified and protected with visual inspection and surgeon’s experience in control group. It was unexpected that the comparison of postoperative levels of AASC and PTH between the two groups had no significant difference. Moreover, the incidence of postoperative hypoparathyroidism and hypocalcemia were not significantly different between the two groups. Several explanations for this result are as follow: 1) the patient population enrolled in our study was limited; 2) the CN suspension effusion affected the surgical field in experiment group; 3) the operation in two groups was performed by the same experienced surgeon. Anyhow, CN suspension certainly could help the identification of parathyroid glands. It was the first step to preserve parathyroid in situ during thyroid surgery. Next, strict adherence to capsular dissection represents the optimal method for safe preservation of the parathyroid glands and their blood supply. Distal ligation of all terminal branches of the superior and inferior thyroid arteries, close to the thyroid capsule, enables reliable separation of all tissues carrying the parathyroid gland away from the thyroid surface.
Through the identification of parathyroid glands by CN suspension injections, parathyroid preservation and subsequent function is available during thyroid surgery. After identification, capsular dissection remains essential for parathyroid glands preservation in situ. The combination between new technology to identify parathyroid and conventional capsular dissection to preserve it in situ could decrease the incidence of symptomatic postoperative hypocalcemia. Application of CN suspension in total thyroidectomy and central lymph node dissection is a new and effective method to identify and protect the parathyroid glands and deserves clinical application for young surgeons, especially. However, further investigation of this new technology should be considered for surgical management of thyroid cancer.
Acknowledgements
The study was supported by Backbone Talent Fund in Medical Science Platform of Zhejiang Province (2012RCA010).
Disclosure of conflict of interest
None.
References
- 1.Thomusch O, Sekulla C, Walls G, Machens A, Dralle H. Intraoperative neuromonitoring of surgery for benign goiter. Am J Surg. 2002;183:673–678. doi: 10.1016/s0002-9610(02)00856-5. [DOI] [PubMed] [Google Scholar]
- 2.Timmermann W, Dralle H, Hamelmann W, Thomusch O, Sekulla C, Meyer T, Timm S, Thiede A. [Does intraoperative nerve monitoring reduce the rate of recurrent nerve palsies during thyroid surgery?] . Zentralbl Chir. 2002;127:395–399. doi: 10.1055/s-2002-31979. [DOI] [PubMed] [Google Scholar]
- 3.Lin DT, Patel SG, Shaha AR, Singh B, Shah JP. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope. 2002;112:608–611. doi: 10.1097/00005537-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Page C, Strunski V. Parathyroid risk in total thyroidectomy for bilateral, benign, multinodular goitre: report of 351 surgical cases. J Laryngol Otol. 2007;121:237–241. doi: 10.1017/S0022215106003501. [DOI] [PubMed] [Google Scholar]
- 5.Rosato L, Avenia N, Bernante P, De Palma M, Gulino G, Nasi PG, Pelizzo MR, Pezzullo L. Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28:271–276. doi: 10.1007/s00268-003-6903-1. [DOI] [PubMed] [Google Scholar]
- 6.Costanzo M, Marziani A, Condorelli F, Migliore M, Cannizzaro MA. Post-thyroidectomy hypocalcemic syndrome: predictive value of early PTH. Preliminary results. Ann Ital Chir. 2010;81:301–305. [PubMed] [Google Scholar]
- 7.Mohebati A, Shaha AR. Imaging techniques in parathyroid surgery for primary hyperparathyroidism. Am J Otolaryngol. 2012;33:457–468. doi: 10.1016/j.amjoto.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuriloff DB, Sanborn KV. Rapid intraoperative localization of parathyroid glands utilizing methylene blue infusion. Otolaryngol Head Neck Surg. 2004;131:616–622. doi: 10.1016/j.otohns.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Pederson LC, Shapiro SE, Fritsche HA Jr, Delpassand ES, Gagel RF, Sherman SI, Vassilopoulou-Sellin R, Evans DB, Lee JE. Potential role for intraoperative gamma probe identification of normal parathyroid glands. Am J Surg. 2003;186:711–717. doi: 10.1016/j.amjsurg.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Grubbs EG, Mittendorf EA, Perrier ND, Lee JE. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am J Surg. 2008;196:931–935. doi: 10.1016/j.amjsurg.2008.07.026. discussion 935-936. [DOI] [PubMed] [Google Scholar]
- 11.Conti de Freitas LC, Phelan E, Liu L, Gardecki J, Namati E, Warger WC, Tearney GJ, Randolph GW. Optical coherence tomography imaging during thyroid and parathyroid surgery: a novel system of tissue identification and differentiation to obviate tissue resection and frozen section. Head Neck. 2014;36:1329–1334. doi: 10.1002/hed.23452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ladurner R, Hallfeldt KK, Al Arabi N, Stepp H, Mueller S, Gallwas JK. Optical coherence tomography as a method to identify parathyroid glands. Lasers Surg Med. 2013;45:654–659. doi: 10.1002/lsm.22195. [DOI] [PubMed] [Google Scholar]
- 13.Prosst RL, Gahlen J, Schnuelle P, Post S, Willeke F. Fluorescence-guided minimally invasive parathyroidectomy: a novel surgical therapy for secondary hyperparathyroidism. Am J Kidney Dis. 2006;48:327–331. doi: 10.1053/j.ajkd.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Gray SW, Skandalakis JE, Akin JT Jr. Embryological considerations of thyroid surgery: developmental anatomy of the thyroid, parathyroids and the recurrent laryngeal nerve. Am Surg. 1976;42:621–628. [PubMed] [Google Scholar]
- 15.Fancy T, Gallagher D 3rd, Hornig JD. Surgical anatomy of the thyroid and parathyroid glands. Otolaryngol Clin North Am. 2010;43:221–7. vii. doi: 10.1016/j.otc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Huang K, Luo D, Huang M, Long M, Peng X, Li H. Protection of parathyroid function using carbon nanoparticles during thyroid surgery. Otolaryngol Head Neck Surg. 2013;149:845–850. doi: 10.1177/0194599813509779. [DOI] [PubMed] [Google Scholar]
- 17.Attie JN, Khafif RA. Preservation of parathyroid glands during total thyroidectomy. Improved technic utilizing microsurgery. Am J Surg. 1975;130:399–404. doi: 10.1016/0002-9610(75)90472-9. [DOI] [PubMed] [Google Scholar]
- 18.Goodman WG, Frazao JM, Goodkin DA, Turner SA, Liu W, Coburn JW. A calcimimetic agent lowers plasma parathyroid hormone levels in patients with secondary hyperparathyroidism. Kidney Int. 2000;58:436–445. doi: 10.1046/j.1523-1755.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- 19.Bourrel C, Uzzan B, Tison P, Despreaux G, Frachet B, Modigliani E, Perret GY. Transient hypocalcemia after thyroidectomy. Ann Otol Rhinol Laryngol. 1993;102:496–501. doi: 10.1177/000348949310200702. [DOI] [PubMed] [Google Scholar]
- 20.Attie JN, Moskowitz GW, Margouleff D, Levy LM. Feasibility of total thyroidectomy in the treatment of thyroid carcinoma: postoperative radioactive iodine evaluation of 140 cases. Am J Surg. 1979;138:555–560. doi: 10.1016/0002-9610(79)90418-5. [DOI] [PubMed] [Google Scholar]
- 21.Thompson NW, Olsen WR, Hoffman GL. The continuing development of the technique of thyroidectomy. Surgery. 1973;73:913–927. [PubMed] [Google Scholar]
- 22.Clark OH. Total thyroidectomy: the treatment of choice for patients with differentiated thyroid cancer. Ann Surg. 1982;196:361–370. doi: 10.1097/00000658-198209000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingert DJ, Friesen SR, Iliopoulos JI, Pierce GE, Thomas JH, Hermreck AS. Post-thyroidectomy hypocalcemia. Incidence and risk factors. Am J Surg. 1986;152:606–610. doi: 10.1016/0002-9610(86)90435-6. [DOI] [PubMed] [Google Scholar]
- 24.Kihara M, Miyauchi A, Kontani K, Yamauchi A, Yokomise H. Recovery of parathyroid function after total thyroidectomy: long-term follow-up study. ANZ J Surg. 2005;75:532–536. doi: 10.1111/j.1445-2197.2005.03435.x. [DOI] [PubMed] [Google Scholar]
- 25.Alveryd A. Parathyroid glands in thyroid surgery. I. Anatomy of parathyroid glands. II. Postoperative hypoparathyroidism--identification and autotransplantation of parathyroid glands. Acta Chir Scand. 1968;389:1–120. [PubMed] [Google Scholar]
- 26.Akerstrom G, Malmaeus J, Bergstrom R. Surgical anatomy of human parathyroid glands. Surgery. 1984;95:14–21. [PubMed] [Google Scholar]
- 27.Wang C. The anatomic basis of parathyroid surgery. Ann Surg. 1976;183:271–275. doi: 10.1097/00000658-197603000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han N, Bumpous JM, Goldstein RE, Fleming MM, Flynn MB. Intra-operative parathyroid identification using methylene blue in parathyroid surgery. Am Surg. 2007;73:820–823. [PubMed] [Google Scholar]
- 29.Prosst RL, Weiss J, Hupp L, Willeke F, Post S. Fluorescence-guided minimally invasive parathyroidectomy: clinical experience with a novel intraoperative detection technique for parathyroid glands. World J Surg. 2010;34:2217–2222. doi: 10.1007/s00268-010-0621-2. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki T, Numata T, Shibuya M. Intraoperative photodynamic detection of normal parathyroid glands using 5-aminolevulinic acid. Laryngoscope. 2011;121:1462–1466. doi: 10.1002/lary.21857. [DOI] [PubMed] [Google Scholar]
- 31.Patel HP, Chadwick DR, Harrison BJ, Balasubramanian SP. Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg. 2012;99:1345–1351. doi: 10.1002/bjs.8814. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Mack E, Starling JR. Radioguided parathyroidectomy is equally effective for both adenomatous and hyperplastic glands. Ann Surg. 2003;238:332–7. doi: 10.1097/01.sla.0000086546.68794.9a. discussion 337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Q, Wang XD, Chen J, Tian CX, Li HJ, Chen YJ, Lv Q. A clinical study on regional lymphatic chemotherapy using an activated carbon nanoparticle-epirubicin in patients with breast cancer. Tumour Biol. 2012;33:2341–2348. doi: 10.1007/s13277-012-0496-y. [DOI] [PubMed] [Google Scholar]
- 34.Hao RT, Chen J, Zhao LH, Liu C, Wang OC, Huang GL, Zhang XH, Zhao J. Sentinel lymph node biopsy using carbon nanoparticles for Chinese patients with papillary thyroid microcarcinoma. Eur J Surg Oncol. 2012;38:718–724. doi: 10.1016/j.ejso.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 35.Yan J, Xue F, Chen H, Wu X, Zhang H, Chen G, Lu J, Cai L, Xiang G, Deng Z, Zheng Y, Zheng X, Li G. A multi-center study of using carbon nanoparticles to track lymph node metastasis in T1-2 colorectal cancer. Surg Endosc. 2014;28:3315–3321. doi: 10.1007/s00464-014-3608-5. [DOI] [PubMed] [Google Scholar]
- 36.Madru R, Kjellman P, Olsson F, Wingardh K, Ingvar C, Stahlberg F, Olsrud J, Latt J, Fredriksson S, Knutsson L, Strand SE. 99mTc-labeled superparamagnetic iron oxide nanoparticles for multimodality SPECT/MRI of sentinel lymph nodes. J Nucl Med. 2012;53:459–463. doi: 10.2967/jnumed.111.092437. [DOI] [PubMed] [Google Scholar]
- 37.Lian HY, Hu M, Liu CH, Yamauchi Y, Wu KC. Highly biocompatible, hollow coordination polymer nanoparticles as cisplatin carriers for efficient intracellular drug delivery. Chem Commun (Camb) 2012;48:5151–5153. doi: 10.1039/c2cc31708g. [DOI] [PubMed] [Google Scholar]
- 38.Asin L, Ibarra MR, Tres A, Goya GF. Controlled cell death by magnetic hyperthermia: effects of exposure time, field amplitude and nanoparticle concentration. Pharm Res. 2012;29:1319–1327. doi: 10.1007/s11095-012-0710-z. [DOI] [PubMed] [Google Scholar]
- 39.Tian W, Jiang Y, Gao B, Zhang X, Zhang S, Zhao J, He Y, Luo D. Application of nano-carbon in lymph node dissection for thyroid cancer and protection of parathyroid glands. Med Sci Monit. 2014;20:1925–1930. doi: 10.12659/MSM.890721. [DOI] [PMC free article] [PubMed] [Google Scholar]


