Abstract
This study was to investigate whether a single dose of methylphenidate (MPH), a dopamine and noradrenaline enhancing drug for the treatment of attentional deficits, influences mismatch visual information processing in young healthy volunteers determined with N270. A randomized double-blinded placebo-controlled study was conducted, and each participant was tested on two sessions separated by two weeks. On each session, a matching task was given first, followed by taking an opaque capsule (20 mg MPH or placebo), and matching task was administered again after 90-min rest. There were two kinds of visually presented stimulus pairs in this task: in the matched condition, the second stimulus (S2) in a pair was identical to the first one (S1); in the mismatched condition, S2 differed from S1 in the color, global shape and direction of the figure. Subjects were asked to press a button in the matched condition and another button in the mismatched condition. Scalp event-related potential were recorded simultaneously. In the matched condition, P300 was elicited by S2; in the mismatched condition, N270 was also elicited by S2 before P300 stimulation. Results showed MPH shortened the latency of N270 and P300 suggesting that a single low dose of MPH promotes the information processing at several stages including conflict processing and working memory updating.
Keywords: Methylphenidate, event-related potential, N270, P300
Introduction
There is converging evidence from both animal and human studies that catecholamines including dopamine (DA) and noradrenaline (NA) are involved in a variety of cognitive functions related to prefrontal lobe. Brozoski and colleagues was for the first time demonstrated that catecholamines played a critical role in the modulation of spatial working memory of the prefrontal cortex (PFC) [1]. Depletion of DA and NE in the PFC may be produced by infusion of catecholamine neurotoxin, 6-hydroxy-DA (6-OHDA) into the dorsolateral PFC of monkeys. Animals with catecholamine depletion in the PFC show impaired impairment similar to those with PFC ablation, highlighting the critical role of catecholamine modulatory activity. Experimental and clinical observations support the fact that alterations of prefrontal cognitive function may also be present in diseases with presumed catecholamine dysfunction such as attention deficit hyperactivity disorder (ADHD) [2,3], schizophrenia [4] and depression [5]. Increasing evidence indicates that altered transmission of DA and NA contributes to the changes in cognitive function in both healthy individuals [6,7] and patients with ADHD, Pakinson’s disease (PD), schizophrenia or other diseases [8-10].
Methylphenidate (MPH) is the most commonly prescribed drug to treat ADHD. It is claimed that MPH is a stimulant drug that can block DA and norepinephrine transporter exerting therapeutic effects [11]. Particularly, MPH at therapeutic doses blocks more than 50% of dopamine transporters and significantly enhances extracellular DA in the human brain, suggesting its effect on DA transporters (DAT) [12,13]. It has been reported that MPH is effective to improve cognitive impairments such as deficient error evaluation and working memory in ADHD patient [14,15]. Nevertheless, little is known about the mechanism underlying its therapeutic effect.
With high resolution, event-related potential (ERP) is an important and sensitive method used for the investigation of neuronal basis of human sensory processing and cognition. Different components are related to different cognitive functions, and thus changes in these components can be used to evaluate the cognitive status in neurological disorders.
Previous studies showed the information from the second stimulus (S2) was a little bit different from that of the first one (S1) in matching tasks, and a negative ERP component N270 can be elicited at 270 ms after S2 onset. In such a task, the information from the first stimulus is first encoded into working memory system. When the information from the second stimulus is transmitted into some parts of the brain, the information from the first stimulus is retrieved and compared with that from the second one. The difference between information from two stimuli may reflect the mismatching or conflicting information [16]. N270 can be elicited by various stimuli, such as color [17,18], shape [19], orientation of pictures [20] and others [21,22], and can be recorded in a mental calculation task to a wrong answer [23]. In addition, N270 may also be elicited by a supra-modality conflict when the information from visual stimulus conflicts with that from auditory stimulus [24]. Based on above findings, N270 may represent the cerebral activity of processing mismatching information, otherwise known as conflict processing, in the human brain. Topographically, N270 is distributed mainly on the prefrontal and posterior areas [25]. A fMRI study using the same S1-S2 paradigm matching task revealed that greater activity was observed in the anterior cingulated cortex (ACC) and dorsolateral prefrontal cortex (DLPFC) on the mismatched pairs [26] The fMRI findings suggest that the activity of neurons in the ACC and DLPFC is a neural substrate for the processing of mismatching information reflected by N270. Changes in N270, such as a delay in the latency and a decrease in the amplitude are consistently found in patients with PD [27], major depression [28] and other neurological diseases. It has been found to be a more sensitive indicator in the evaluation of cognitive change [29,30].
In present study, a S1-S2 paradigm visual matching task was conducted in young healthy volunteers to investigate the effects of MPH on the conflicting information processing determined by N270. In addition, P300 was also evaluated given the potential effect of MPH on other aspects of information processing.
Subjects and methods
Subjects
Fourteen healthy adults (10 women) aged from 21 to 28 years participated in this study. All subjects were right-handed and had normal or corrected-to-normal vision. None had a history of neurological or psychiatric diseases, drug abuse and alcoholism. All subjects were asked to refrain from drinking caffeine or any other drugs on the day of test. Written informed consent was obtained from each participant. The protocol was approved by the Ethics Committee of Capital Medical University.
Procedures
A randomized double-blinded placebo-controlled design was employed into present study. Each participant was tested on two sessions separated by two weeks. On each session, ERP test was given first, followed by taking an opaque capsule (20 mg MPH or placebo). After 90-min rest, ERP test was performed again. The dose of MPH was determined based on a previous study which showed 20-mg MPH occupying 54% of DAT and 50% of DAT was required for its therapeutic effects on ADHD [31].
Stimuli
The order of visual stimuli used in this study was determined according to previously reported [32]. Stimuli consisted of 144 pairs of color figures. Each stimulus was defined by color (red, yellow, green or white), global shape (triangle, quadrate, hexagon or circle) and direction of a gap (upward, downward, leftward or rightward) (Figure 1). The visual stimuli subtended for a visual angle of 2.47° horizontally and vertically. In each trial, a warning tone (60 dB, 1000 Hz) lasting 30 ms was given first, then visual stimuli were administered sequentially in pairs (S1, S2) at 1500 ms after the onset of warning tone at the center of a computer-controlled video monitor (STIM, Neurosoft, Inc, Sterling, VA, USA) against a black background. S1 and S2 were present for 300 ms with an interval of 500 ms. The interval between S2 onset and following warning tone onset was 2500 ms.
Figure 1.
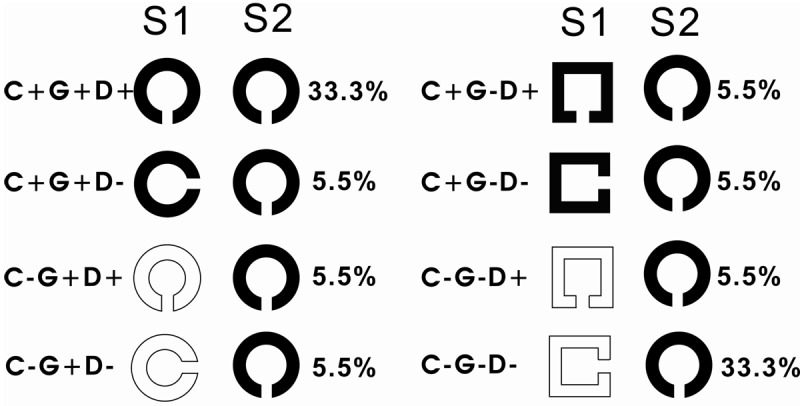
Examples of stimulus pairs (S1, S2) used in the detection of event related potential (ERP). In each task, there were eight conditions. The probability of eight conditions occurring is shown as percentages. C: color, G: global shape, D: shape direction. + indicates an attribute shared by the pair of stimuli; – indicates the attribute differing between two stimuli in a pair.
To indicate the various stimulus types, C represents color, G global shape and L local shape (direction of a gap) with the suffix ‘+’ indicating an attribute shared by the pair of stimuli, and suffix ‘-’ indicating an attribute differing between the two stimuli. In the matched condition, the attributes of S2 were the same as those of S1 (C+G+L+). In the mismatched condition, S2 was completely different from S1 in all three attributes (C-G-L-). The probability for the presence of each kind of stimulus pairs was 33.3%. Other kinds of stimulus pairs (including C+G+L-, C+G-L+, C-G+L+, C+G-L-, C-G–L+, C-G+L-) were presented at a probability rate of 5.5%. All kinds of stimulus pairs were presented randomly.
Tasks
Subjects were asked to press one button using one hand in the matched condition (C+G+L+) and another button using the other hand in the conflicting condition (C-G-L-). They did not have to respond in other conditions. The reaction time and the rate of their correct responses were recorded with the STIM system. Following 20 practice trials, the 144 test trials were presented. The task session was divided into two blocks, a short break was allowed between blocks to avoid fatigue in these subjects and the left and right button pressing was counterbalanced in each subject in these two blocks.
ERP recording and data analysis
Subjects were seated in a dimly lit room. Electroencephalogram (EEG) was recorded from 20 scalp electrodes according to the international 10-20 system, using Ag/AgCl electrodes with the impedance of <5 kΩ. All electrodes were referenced to the nose tip. Vertical electrooculogram (EOG) was recorded via electrodes situating above and below the left eye, and horizontal EOG was recorded via electrodes situating 2 cm away from the outer canthi of both eyes. EEG was amplified with a band pass of 0.05-100 Hz, sampled at 1000 Hz and later low-pass filtered down 24 dB at 30 Hz. For accurate analysis, we only averaged and analyzed ERPs obtained from the mismatched condition and the matched condition. The epoch averaged was 1200 ms, including 200 ms at baseline prior to the onset of S2. Trials with EOG, artifacts or incorrect behavioral responses were excluded. ERPs obtained in two conditions were averaged over 30 trials in each task.
Statistical analysis
Amplitudes were measured with respect to the averaged voltage over 200 ms epoch before the onset of S2. According to the visual inspection of the grand average waveform and our previous findings, two main components were analyzed. N270 was identified between 210 ms and 260 ms, and P300 between 290 ms and 350 ms. While EEG was recorded from 20 sites. ERP at the sites where the ERP components were predominant was analyzed, and thus frontal sites (F3, F4) were used for N270 and parietal sites (P3, P4) for P300. The mean amplitude and peak latency of N270 were measured in the mismatched condition, while those of P300 in the matched condition in order to avoid the superimposing of N270.
In order to avoid the influence of differences at baseline, changes in pre-/post-treatment data (subtracting data of pre-treatment tests from post-treatment tests) were compared using repeated measures analysis of variance (ANOVA). All statistical analyses were performed using STATISTICA 7.0 and a value of P<0.05 was considered statistically significant after Greenhouse-Geisser correction. Post-hoc test was done using Bonferroni method.
Results
ERP data
ERP waveforms are shown in Figure 2. In the matched condition, P300 was elicited by S2. In the mismatched condition, N270 was elicited by S2 before P300. Changes in ERP data were calculated by subtracting the data of pre-treatment tests from those of post-treatment tests and are showed in Table 1.
Figure 2.
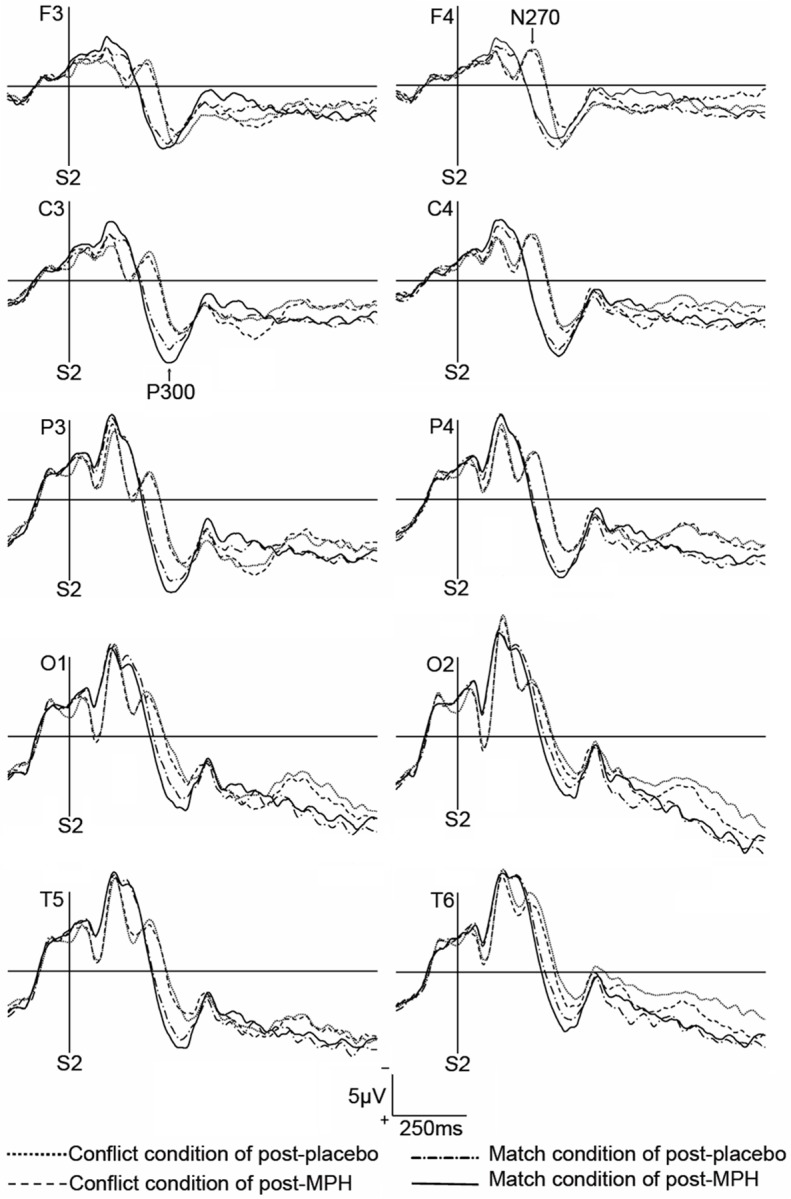
ERP waveforms of one subject in the matched condition under four drug conditions. The vertical solid lines represent the onset of S2. In the matched condition, P300 was elicited by S2. In the mismatched condition, N270 was elicited by S2 before P300.
Table 1.
The changes of amplitudes (μV) and latencies (ms) of N270 and P300 (x̅±SD)
| Component | Sites | Amplitude | Latency | ||
|---|---|---|---|---|---|
|
| |||||
| Placebo | MPH | Placebo | MPH | ||
| N270 | F3 | 0.70±2.11 | -0.40±2.93 | 5.93±13.46 | -12.07±22.73* |
| F4 | 0.99±1.88 | -0.39±3.51 | 9.08±18.34 | -14.93±23.57* | |
| P300 | P3 | 0.68±3.97 | 0.94±2.28 | 12.31±22.34 | -8.92±17.65* |
| P4 | 1.36±3.55 | 0.79±3.64 | 6.69±16.86 | -6.31±15.19 | |
Significantly different from placebo condition at the same electrode (P<0.05).
N270
A significant main effect of the drug (F1, 13=8.59, P=0.012) on the N270 latency was observed due to significantly shortened latency at F3 (P=0.001) and F4 (P=0.013) electrodes. Hemisphere had no main effect or interaction effect with drug. For N270 mean amplitude within 210 ms and 260 ms, no effects were found for drug and hemisphere.
P300
There was a main effect of the drug on P300 latency (F1, 13=8.60, P=0.013). Neither main effect nor interaction effect of the drug was found for hemisphere. Post-hoc test showed MPH significantly shortened P300 latency at P3 electrode (P=0.002). For P300 mean amplitude within 290 ms and 350 ms, no effect was found for drug and hemisphere.
Behavioral data
The mean reaction time and correct rate are shown in Table 2. Two-factor (drug condition) repeated ANOVA showed a main effect of stimulus condition on the reaction time (F1, 13=16.75, P=0.002) due to significant reduction in the reaction time in the matched condition. Drug showed neither main effect nor interaction effect in both conditions on the correct rate and reaction time.
Table 2.
The changes of reaction time and correct-rate (x̅ ±SD)
| Condition | Reaction time (ms) | Correct rate (%) | ||
|---|---|---|---|---|
|
| ||||
| Placebo (post-pre) | MPH (post-pre) | Placebo (post-pre) | MPH (post-pre) | |
| Match | -6.94±74.59 | -10.99±45.18 | 0.00±2.08 | 0.80±2.00 |
| Mismatched | -41.48±70.32 | -52.20±32.88 | 3.18±11.39 | 1.67±7.49 |
Discussion
In the present study, we investigated the effect of MPH on the conflicting visual information processing in young healthy volunteers. Our results showed MPH significantly shortened the latency of N270 without changing its amplitude. Botvinick et al. proposed that there was a conflicting processing system in the human brain [33]. Neuroimaging studies also reveal that the ACC is mainly associated with monitoring the presence of processing conflict, while the DLPFC involves in the implementation of control in conflicting situation [34-36]. As N270 is only evoked by the stimulus pairs with discrepancies or conflicts, and ACC and PFC are the most likely generators of N270 [26], we proposed that N270 reflects the neural activity of the conflict processing system It has been known that dopaminergic neurons of the ventral tegmental area projected to the ACC and PFC play an important role in regulating the activity of cells in these areas. Volkow et al. showed that oral MPH could significantly enhance the dopamine action in synapses by blocking DAT locating at the presynaptic terminal [37]. Our results suggested that the conflicting information processing was speeded because of enhanced activity of cells in the ACC and PFC which was regulated by MPH through regulating DA. In clinical studies, PD patients showed a delayed and smaller N270 as compared to controls [27]. Study in ADHD children also showed significantly increased latency of N270. After two-week MPH treatment, ADHD children showed decreased N270 latency resulting in no significant difference between ADHD children and controls (not published). These results provide additional support for the fact that there is a relation between changes in N270 and alterations in DA and NE transmission.
Although P300 is the most widely used ERP component used to evaluate the cognitive function, its role in the neural generation and neuropsychology was still unclear. Frontal and hippocampal/temporal-parietal lobe are the most possible generators revealed by fMRI [38,39]. It is generally accepted that P300 reflects the reprocessing of information which updates a cognitive model of the environment within working memory storage [40,41]. Despite inconsistencies in different studies, evidence shows that P300 may be influenced by MPH and modulated by catecholamines [42,43]. In the study of Cooper et al., MPH was found to reduce the latency of target P300 in healthy subjects in continuous performance task (CPT). Investigators speculated that MPH selectively accelerated the updating in working memory to task-relevant stimuli which require a fast response [43]. In S1-S2 paradigm used in this experiment, the probable cognitive processes included were acquisition, encoding of stimuli, retrieval and comparison of stimuli, and determination of left or right button pressing [24]. In addition, stimuli may be reprocessed in parallel with the response selection to update working memory. This stimulus reprocessing may modulate the strategy used for processing the next trial. Herein, we found that MPH shortened the latency of P300. Consistent with the findings from the study of Cooper et al., MPH speeded the working memory updating in the present study.
In present study, we failed to found an effect of MPH on the amplitude of any component, which was consistent with previous findings [43,44]. It is considered that the amplitude of ERP reflects the neural resources recruited to complete the information processing. Coons et al. found that MPH failed to affect the amplitude of late positive component (LPC) during an easy version of the CPT, but produced a significant increase in the amplitude of LPC during a difficult version. Coons et al. suggested that only in longer or more difficult tasks could subjects benefit from the extra attentional resources produced by MPH [44]. Considering the task employed in the present study, it was not difficult enough for the subjects to recruit more neural resources to process the task-related information. Thus, in future study, tasks with different difficulties should be employed for further investigations.
Behaviors were improved in the post-treatment tests demonstrated by the reduced reaction time and enhanced correct rate. Though significant difference was not observed, the reaction time tended to reduce after MPH treatment as compared to that after placebo treatment as. In addition, this lack of obvious behavioral effect of 20-mg MPH is consistent with the view that ERP is a more sensitive marker for drug effects on the cognitive function than the accuracy and reaction time.
In summary, our results show that MPH is able to shorten the latencies of N270 and P300. Although MPH at a low dose does not significantly affect the behavioral response in healthy subjects, it speeds the information processing in several stages including conflict processing and working memory updating.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (30800366).
Disclosure of conflict of interest
None.
References
- 1.Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- 2.Dramsdahl M, Westerhausen R, Haavik J, Hugdahl K, Plessen KJ. Cognitive control in adults with attention-deficit/hyperactivity disorder. Psychiatry Res. 2011;188:406–410. doi: 10.1016/j.psychres.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Alderson RM, Kasper LJ, Hudec KL, Patros CH. Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: a meta-analytic review. Neuropsychology. 2013;27:287–302. doi: 10.1037/a0032371. [DOI] [PubMed] [Google Scholar]
- 4.Kaller CP, Loosli SV, Rahm B, Gossel A, Schieting S, Hornig T, Hennig J, Tebartz van Elst L, Weiller C, Katzev M. Working memory in schizophrenia: behavioral and neural evidence for reduced susceptibility to item-specific proactive interference. Biol Psychiatry. 2014;76:486–494. doi: 10.1016/j.biopsych.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linssen AM, Vuurman EF, Sambeth A, Riedel WJ. Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology (Berl) 2012;221:611–619. doi: 10.1007/s00213-011-2605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linssen AM, Sambeth A, Vuurman EF, Riedel WJ. Cognitive effects of methylphenidate and levodopa in healthy volunteers. Eur Neuropsychopharmacol. 2014;24:200–206. doi: 10.1016/j.euroneuro.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Rubia K, Alegria AA, Cubillo AI, Smith AB, Brammer MJ, Radua J. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76:616–628. doi: 10.1016/j.biopsych.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. doi: 10.1093/schbul/sbq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulisevsky J. Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs Aging. 2000;16:365–379. doi: 10.2165/00002512-200016050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155:1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- 13.Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, Ding YS, Gatley SJ, Gifford A, Zhu W, Swanson JM. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- 14.Kobel M, Bechtel N, Weber P, Specht K, Klarhofer M, Scheffler K, Opwis K, Penner IK. Effects of methylphenidate on working memory functioning in children with attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2009;13:516–523. doi: 10.1016/j.ejpn.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Jonkman LM, van Melis JJ, Kemner C, Markus CR. Methylphenidate improves deficient error evaluation in children with ADHD: an event-related brain potential study. Biol Psychol. 2007;76:217–229. doi: 10.1016/j.biopsycho.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Cui L, Wang H, Tian S, Zhang X. The sequential processing of visual feature conjunction mismatches in the human brain. Psychophysiology. 2004;41:21–29. doi: 10.1111/j.1469-8986.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 17.Tian S, Wang Y, Wang H, Cui L. Interstimulus interval effect on event-related potential N270 in a color matching task. Clin Electroencephalogr. 2001;32:82–86. doi: 10.1177/155005940103200207. [DOI] [PubMed] [Google Scholar]
- 18.Kimura M, Katayama J, Murohashi H. Neural correlates of pre-attentive and attentive processing of visual changes. Neuroreport. 2005;16:2061–2064. doi: 10.1097/00001756-200512190-00019. [DOI] [PubMed] [Google Scholar]
- 19.Cui L, Wang Y, Wang H, Tian S, Kong J. Human brain sub-systems for discrimination of visual shapes. Neuroreport. 2000;11:2415–2418. doi: 10.1097/00001756-200008030-00015. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Tang X, Kong J, Zhuang D, Li S. Different systems in human brain are involved in presemantic discrimination of pictures as revealed by event-related potentials. Neurosci Lett. 1998;257:143–146. doi: 10.1016/s0304-3940(98)00828-3. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Zhang Y, Wang H, Cui L, Tian S. Brain potentials elicited by matching global and occluded 3-dimensional contours. Brain Cogn. 2003;53:28–33. doi: 10.1016/s0278-2626(03)00186-6. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Wang Y. Event-related potentials elicited by stimulus spatial discrepancy in humans. Neurosci Lett. 2002;326:73–76. doi: 10.1016/s0304-3940(02)00204-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Kong J, Tang X, Zhuang D, Li S. Event-related potential N270 is elicited by mental conflict processing in human brain. Neurosci Lett. 2000;293:17–20. doi: 10.1016/s0304-3940(00)01480-4. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Wang H, Cui L, Tian S, Zhang Y. The N270 component of the event-related potential reflects supramodal conflict processing in humans. Neurosci Lett. 2002;332:25–28. doi: 10.1016/s0304-3940(02)00906-0. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Wang Y, Li S, Wang L. Event-related potential N270, a negative component to identification of conflicting information following memory retrieval. Clin Neurophysiol. 2003;114:2461–2468. doi: 10.1016/s1388-2457(03)00251-7. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Ma L, Li S, Wang Y, Weng X, Wang L. A mismatch process in brief delayed matching-to-sample task: an fMRI study. Exp Brain Res. 2008;186:335–341. doi: 10.1007/s00221-008-1285-0. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Wang Y, Wang D, Cui L, Tian S, Zhang Y. Cognitive impairment in Parkinson’s disease revealed by event-related potential N270. J Neurol Sci. 2002;194:49–53. doi: 10.1016/s0022-510x(01)00674-8. [DOI] [PubMed] [Google Scholar]
- 28.Mao W, Wang Y, Wang D. Cognitive impairment in major depressive disorder revealed by event-related potential N270. Clin EEG Neurosci. 2005;36:9–14. doi: 10.1177/155005940503600104. [DOI] [PubMed] [Google Scholar]
- 29.Mao W, Yang J, Wang M, Wang Y, Wang D, Zhu L, Jia J. Event-related potential N270 in detecting cognitive impairment in patients with transient ischemic attack. J Clin Neurophysiol. 2006;23:559–564. doi: 10.1097/01.wnp.0000229942.22556.8a. [DOI] [PubMed] [Google Scholar]
- 30.Meng X, Mao W, Sun W, Zhang X, Han C, Lu C, Huang Z, Wang Y. Event-related potentials in adolescents with different cognitive styles: field dependence and field independence. Exp Brain Res. 2012;216:231–241. doi: 10.1007/s00221-011-2919-1. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Wang GJ, Fowler JS, Telang F, Maynard L, Logan J, Gatley SJ, Pappas N, Wong C, Vaska P, Zhu W, Swanson JM. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. Am J Psychiatry. 2004;161:1173–1180. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Tian S, Wang H, Cui L, Zhang Y, Zhang X. Event-related potentials evoked by multi-feature conflict under different attentive conditions. Exp Brain Res. 2003;148:451–457. doi: 10.1007/s00221-002-1319-y. [DOI] [PubMed] [Google Scholar]
- 33.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 35.Kerns JG, Cohen JD, MacDonald AW 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 36.Iannaccone R, Hauser TU, Staempfli P, Walitza S, Brandeis D, Brem S. Conflict monitoring and error processing: new insights from simultaneous EEG-fMRI. Neuroimage. 2015;105:395–407. doi: 10.1016/j.neuroimage.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford A, Franceschi D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He B, Lian J, Spencer KM, Dien J, Donchin E. A cortical potential imaging analysis of the P300 and novelty P3 components. Hum Brain Mapp. 2001;12:120–130. doi: 10.1002/1097-0193(200102)12:2<120::AID-HBM1009>3.0.CO;2-V. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF. Neural sources involved in auditory target detection and novelty processing: an event-related fMRI study. Psychophysiology. 2001;38:133–142. [PubMed] [Google Scholar]
- 40.Frodl-Bauch T, Bottlender R, Hegerl U. Neurochemical substrates and neuroanatomical generators of the event-related P300. Neuropsychobiology. 1999;40:86–94. doi: 10.1159/000026603. [DOI] [PubMed] [Google Scholar]
- 41.Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. Int J Psychophysiol. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 42.Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- 43.Cooper NJ, Keage H, Hermens D, Williams LM, Debrota D, Clark CR, Gordon E. The dose-dependent effect of methylphenidate on performance, cognition and psychophysiology. J Integr Neurosci. 2005;4:123–144. doi: 10.1142/s0219635205000744. [DOI] [PubMed] [Google Scholar]
- 44.Coons HW, Peloquin LJ, Klorman R, Bauer LO, Ryan RM, Perlmutter RA, Salzman LF. Effect of methylphenidate on young adult’s vigilance and event-related potentials. Electroencephalogr Clin Neurophysiol. 1981;51:373–387. doi: 10.1016/0013-4694(81)90101-2. [DOI] [PubMed] [Google Scholar]


