Abstract
Background: The aim of this experimental study was to evaluate the effect of intra-articular injection of Deoxycholic acid (DCA) on articular cartilage and subchondral bone following induction of knee Osteoarthritis (OA) in a rat model. Methods: Twenty-four Sprague Dawley rats were randomized divided into 4 groups (n = 6). Eighteen of the 24 rats underwent surgical destabilization of the medial meniscus on the right knee joints to induce OA, were divided into 3 groups: DCA 30 mg/kg group, DCA 120 mg/kg group and OA group. The rats in DCA-treated groups were given intra-articular injections of DCA (30 mg/kg or 120 mg/kg) in the operated knees once per 3 days for 42 days. The rats in OA group given intra-articular injections of vehicle alone in the operated knees under the same conditions. The remaining 6 rats (sham-operation group) received sham operations on the right knee joints. 45 days postoperatively, all of the animals were euthanized for macroscopic, histological and radiographic analysis to evaluate the effect of DCA on OA and to determine its potential mechanisms. Results: The results showed that DCA attenuated the severity of OA by reducing macroscopic observation sores for femoral condyles and histological sores for articular cartilage. DCA also significantly decreased bone destruction and erosion of joint evaluated by radiographic examination. Furthermore, DCA could markedly reduce the release of MMP-1, MMP-3 and IL-1β in serum. Conclusions: Intra-articular injection of DCA is beneficial for knee OA. It might repair and protect OA cartilage by delaying cartilage degeneration and impairing the function of inflammatory mediators. These findings highlight DCA might be a useful therapeutic agent for OA.
Keywords: Deoxycholic acid, osteoarthritis, intra-articular injection, treatment
Introduction
Osteoarthritis (OA) is the most prevalent form of joint disease and evolves from a local inflammatory response to a chronic process accompanied by progressive degeneration of articular cartilage [1,2]. The clinical manifestations of OA are pain and musculoskeletal disorder that may lead to disability, deterioration of quality of life and a heavy socioeconomic burden [3,4]. It is characterized mainly by the degeneration of articular cartilage and subsequent synovial inflammation, bone remodeling and muscle weakness [5]. Though much research has been performed, the concrete causes of OA remain unclear.
At present, OA progression is considered to be regulated largely by an excess of matrix metalloproteinases (MMPs), which contributes to the degradation of the extracellular matrix. Among the various MMPs, collagenases such as MMP-1 and MMP-3 play pivotal roles in OA progression by degrading the extracellular matrix [6-8]. In addition, inflammatory mediator IL-1β has been implicated in the synovial inflammation and cartilage degradation in OA [9]. Elevated IL-1β levels are present in the synovial fluid and cartilage of OA patients, implying a role for IL-1β in the pathogenesis of OA [10].
The currently available pharmacological treatments for OA are effective only temporarily and might result in undesirable cardiovascular and gastrointestinal side effects [11-13]. There is an increasing interest in the use of natural compounds extracted from Traditional Chinese Medicine (TCM) for the treatment of OA because they are reported to demonstrate satisfactory clinical efficacy with minimal side effects, compared to routine pharmacological strategies [14]. Deoxycholic acid (DCA) was an important active constituent from animal bile, which has been reported for the treatment of cancer, respiratory and immunologic system diseases [15-17]. However, little is known about its possible use in the treatment of OA. Our group has previously reported that animal bile constituents exhibited obviously inhibitory effect on MMPs in vitro [18]. In continuation of our previous study and development of a novel small molecule drug to treat OA, we elucidated the therapeutic effect of DCA in an experimental rat model of OA for the first time in this article.
Materials and methods
Experimental animal model and drug treatment
Twenty-four adult male Sprague Dawley rats (8 weeks old, 240-270 g in weight) were purchased from Shanghai SLAC Laboratory Animal Co. Ltd (Shanghai, China). All experiments were conducted with the approval of Soochow University Animal Care and Use Committee. Eighteen of the 24 rats underwent surgical destabilization of the medial meniscus (DMM) on the right knee joints to induce OA as described previously by Glasson et al [19] and were randomly divided into 3 groups (n = 6 per group). The remaining 6 rats (sham-operation group) received sham operations on the right knee joints, which involved opening the articular cavity and resuturing it without transecting the medial meniscotibial ligament. After surgery, all animals were returned to their cages, the limbs were not immobilized. Treatments began 4 weeks after surgery. Drugs were given once per 3 days for 42 days. The rats in DCA-treated (DCA, purity above 98%, Sigma-Aldrich, St. Louis, MO, USA) groups were given intra-articular injections of DCA (30 mg/kg or 120 mg/kg) in the operated knees once per 3 days for 42 days. The rats in OA group were given intra-articular injections of 50 μL vehicle alone in the operated knees under the same conditions. In sham-operation group, no other procedures were conducted. All rats were sacrificed 3 days after the last injection.
Macroscopic observation
After the treatment, the rats were sacrificed and femoral condyles were collected for macroscopic observation. The cartilage degradation on the surface of femoral condyles was observed under dissecting microscope and the degree of degradation was graded on a scale of 0-4 as follows: 0 = surface smooth with normal color; 1 = surface rough with minimal fibrillation or a slight yellowish discoloration; 2 = cartilage erosion extending into superficial or middle layers; 3 = cartilage ulceration extending into deep layers; 4 = cartilage depletion with subchondral bone exposed [20]. The examination was performed by two independent observers who were kept unaware of the treatment groups.
Histological examination
After macroscopic observation, isolated specimens were prepared for further histological analysis. The specimens were decalcified (10% EDTA), embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin (H&E). The histological evidence of articular cartilage was assessed according to the scoring system by Kikuchi et al and eight parameters [21], namely loss of superficial layer, erosion of cartilage, fibrillation and/or fissures, loss of proteoglycan, disorganization of chondrocytes, loss of chondrocytes, exposure of subchondral bone, and cluster formation. All sections were graded by two independent observers that were kept unaware of the treatment groups.
Radiographic evaluation
At the end of study, the right knee joints of experimental rats were subject to radiographic analysis using a mammographic imager (Siemens Medical Solutions, Germany). The joint destruction and bone erosion were scored on a scale of 0-4 in a blind manner: 0 = no osteophytes; 1 = doubtful osteophytes; 2 = minimal osteophytes, possibly with narrowing, cysts, and sclerosis; 3 = moderate or definite osteophytes with moderate joint space narrowing; 4 = severe with large osteophytes and definite joint space narrowing [22].
ELISA for measurement of MMP-1, MMP-3 and IL-1β in serum
Blood was obtained from the eyeball vein of rats before sacrificing, and centrifuged at 3000 rpm for 15 min. The serum was collected and stored at -70°C until assayed. The levels of MMP-1, MMP-3 and IL-1β in serum were measured by ELISA kits (R&D Systems Inc., USA) according to the manufacturer’s instructions. All samples from animals in each experimental group were assayed in duplicate.
Statistics
Data were presented as the mean ± SEM. Statistical analyses were performed using SPSS 16.0. Statistical comparisons were performed using the Mann-Whitney U test. P < 0.05 was considered significant.
Results
Effect of DCA at macroscopic observation in the OA rat model
As shown in Figure 1A, the cartilage on the femoral condyles in the sham-operation group was macroscopically normal, with a glistening, smooth surface, and no cartilage defect or osteophyte was observed. In the OA group, general characteristics of OA, including erosion and osteophyte formation, were seen on the side of the femoral condyles after surgery. In the DCA-treated groups, less bone wear was observed after DCA treatment, compared with the OA group. Accordingly, as scored by macroscopic observation, DCA treatment could significantly decrease the degree of cartilage degradation (Figure 1B).
Figure 1.

Effect of DCA at macroscopic observation in the OA rat model. A. Representative macroscopic observation images of femoral condyles are shown. B. Macroscopic observation sores for femoral condyles in the four groups of experimental rats. Each reported value is a mean ± SEM (n = 6); ##P < 0.01, compared with the sham-operation group; *P < 0.05 and **P < 0.01, compared with the OA group. Similar results were observed in two separated experiments.
Effect of DCA on histological evaluation in the OA rat model
As shown in Figure 2A, the sham-operation of rats revealed no significant histological changes in the articular cartilages, whereas the OA group and other treated groups developed different degrees of OA-like degenerative changes. A significant decrease in the severity of cartilage degradation was observed in DCA-treated groups, particularly in the 120 mg/kg group. Histological sores in the DCA-treated group were significantly lower than in the OA group (Figure 2B).
Figure 2.
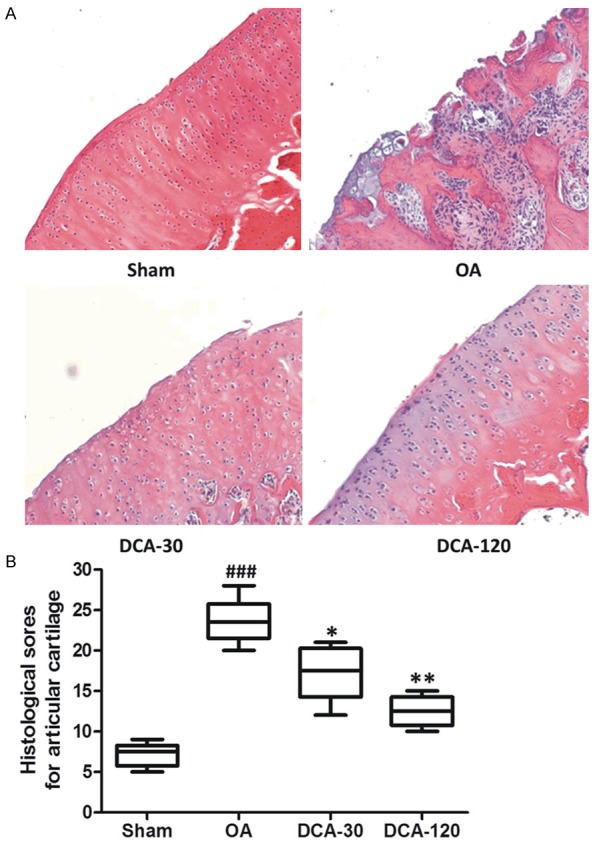
Effect of DCA on histological evaluation in the OA rat model. A. Representative Hematoxylin-eosin (HE) stained sections of articular cartilage are shown. B. Histological sores for articular cartilage in the four groups of experimental rats. Each reported value is a mean ± SEM (n = 6); ###P < 0.001, compared with the sham-operation group; *P < 0.05 and **P < 0.01, compared with the OA group. Similar results were observed in two separated experiments.
Effect of DCA at radiographic analysis in the OA rat model
In order to observe the bone changes of knee joints after DCA treatment, radiographic examination was performed on OA rat treated with DCA and vehicle in this study. As shown in Figure 3A, the surface of knee joints was smooth in the sham-operation group. In contrast, obvious osteophyte, incomplete and thickening articular surface with sclerosis and deformation were observed in the OA group. The DCA treated groups markedly prevented bone erosions at the operated knee joint, no obvious sclerosis and osteophyte formation were observed. Accordingly, radiological sores in the DCA-treated group were significantly lower than in the OA group (Figure 3B). The results suggested that DCA treatment was an effective therapeutic approach to reduced joint destruction in OA rats.
Figure 3.

Effect of DCA at radiographic analysis in the OA rat model. A. Representative radiographic images of rat knee joints are shown. B. Radiological scores for knee joints in the four groups of experimental rats. Each reported value is a mean ± SEM (n = 6); ##P < 0.01, compared with the sham-operation group; *P < 0.05 and **P < 0.01, compared with the OA group. Similar results were observed in two separated experiments.
Effects of DCA on the levels of MMP-1, MMP-3 and IL-1β in serum were measured by ELISA
Compared to sham-operation group, OA group showed higher level of MMP-1 and MMP-3 in serum (P < 0.01) (Figure 4A and 4B). The levels of MMP-1 and MMP-3 in serum were significantly decreased by DCA treatment in a dose-dependent manner compared with those in OA group. Moreover, inflammatory mediator IL-1β involved in cartilage destruction was also significantly inhibited by DCA treatment (Figure 4C).
Figure 4.

Effects of DCA on the levels of MMP-1 (A), MMP-3 (B) and IL-1β (C) in serum were measured by ELISA. Each reported value is a mean ± SEM (n = 6); ##P < 0.01, compared with the sham-operation group; *P < 0.05 and **P < 0.01, compared with the OA group. Similar results were observed in two separated experiments.
Discussion
Osteoarthritis (OA) is the most prevalent form of joint disease and evolves from a local inflammatory response to a chronic process accompanied by progressive degeneration of articular cartilage [1,2]. The clinical manifestations of OA are pain and functional disability that can limit the normal activities of daily life and lead to depression and isolation [3,4]. At present, Nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and hyaluronan have been clinically used for the treatment of OA in the clinic. However, they fail to reverse cartilage damage, always result in undesirable cardiovascular and gastrointestinal side effects [11]. Thus, there is a continuing need for novel better agents with which to treat OA.
DCA was an important active constituent from animal bile, which has been reported for the treatment of cancer, respiratory and immunologic system diseases [15-17]. However, the effect of DCA on OA severity in vivo has not yet been studied at present. Our group has previously reported that animal bile constituents exhibited obviously inhibitory effect on MMPs in vitro [18]. In continuation of our previous study and search for a novel small molecule drug to treat OA, we elucidated the beneficial effect of DCA on an OA model in rats for the first time in this article.
In our study, we investigated the protective effect of DCA against cartilage degradation in a rat OA model induced by DMM, which is a commonly used method of identifying OA disease-modifying therapies by intra-articular administration. The results showed that DCA attenuated the severity of OA by reducing macroscopic observation sores for femoral condyles and histological sores for articular cartilage (Figures 1, 2). DCA also significantly decreased bone destruction and erosion of joint evaluated by radiographic examination (Figure 3). Notably, DCA significantly reduced the degree of OA-like lesions at a dose of 120 mg/kg.
It is well established that MMPs, especially MMP-1 and MMP-3, play vital roles in the progression of cartilage degradation in OA due to their ability to cleave diverse components of the extracellular matrix [6-8]. There are many research groups have confirmed that inhibition of MMPs delayed the progression of cartilage degradation in vitro and in vivo [23]. MMP-1 breaks down fibrillar collagens in an irreversible way, while MMP-3 degrades a variety of matrix substrates, including type II collagen and aggrecan [24]. Many studies have reported that the MMP-1 level is upregulated in the synovium, synovial fluid and cartilage of OA patients, indicating that MMP-1 has a vital role in the pathological degradation of articular cartilage [25,26]. MMP-3 may have utility as a prognostic biomarker of OA progression. Patients with higher MMP-3 levels are more likely to suffer from progression of OA over a 30-month period than patients in the lower tertile [27]. Therefore, effects of DCA on the levels of MMP-1 and MMP-3 in serum were measured by ELISA in this paper. As shown in Figure 4A and 4B, DCA significantly decreased the levels of MMP-1 and MMP-3 in serum in a dose-dependent manner.
The role of inflammatory mediators IL-1β in the pathogenesis of osteoarthritis has drawn more and more attention in recent years. IL-1β has been implicated in the synovial inflammation and cartilage degradation in OA [9]. Elevated IL-1β levels are present in the synovial fluid and cartilage of OA patients, implying a role for IL-1β in the pathogenesis of OA [10]. In this study, the levels of IL-1β in serum were also measured. Our results indicate that the levels of IL-1β in serum were significantly inhibited in the DCA-treated group (Figure 4C).
Taken together, these results confirm the therapeutic potential of DCA for treatment of OA. However, the exact mechanism of action of DCA needs to be further investigated.
Conclusions
Intra-articular injection of DCA is beneficial for knee OA. It might repair and protect OA cartilage by delaying cartilage degeneration and impairing the function of inflammatory mediators. These findings highlight DCA might be a useful therapeutic agent for OA.
Acknowledgements
This work was supported by the grants from the Medical Research Projects of Health Department of Jiangsu Province (No. Z201304), National Natural Science Foundation of China (No. 81202348 and No. 81202394) and Jiangsu Province National Natural Science Funds (No. BK2012171), and Medical Research Projects of Health Department of Guangxi Zhuang Autonomous Region (No. Z2011291).
Disclosure of conflict of interest
None.
References
- 1.Gore M, Tai KS, Sadosky A, Leslie D, Stacey BR. Clinical comorbidities, treatment patterns, and direct medical costs of patients with osteoarthritis in usual care: a retrospective claims database analysis. J Med Econ. 2011;14:497–507. doi: 10.3111/13696998.2011.594347. [DOI] [PubMed] [Google Scholar]
- 2.Suri S, Walsh DA. Osteochondral alterations in osteoarthritis. Bone. 2012;51:204–211. doi: 10.1016/j.bone.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Baker-LePain JC, Lane NE. Role of bone architecture and anatomy in osteoarthritis. Bone. 2012;51:197–120. doi: 10.1016/j.bone.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creamer P. Osteoarthritis pain and its treatment. Curr Opin Rheumatol. 2000;12:450–5. doi: 10.1097/00002281-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Tubach F, Ravaud P, Baron G, Falissard B, Loqeart I, Bellamy N, Bombardier C, Felson D, Hochberq M, Heijde van der, Douqados M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 7.Ding QH, Zhong HM, Qi YY, Cheng WL, Yan S, Wang X. Anti-arthriticeffects of crocin in interleukin-1β-treated articular chondrocytes and cartilage in a rat osteoarthritic model. Inflamm Res. 2013;62:17–25. doi: 10.1007/s00011-012-0546-3. [DOI] [PubMed] [Google Scholar]
- 8.Tchetverikov I, Ronday HK, Van EI B, Kiers GH, Verzijl N, TeKoppele JM, Huizinqa TW, DeGroot J, Hanemaaijer R. MMP profile in paired serum and synovial fluid samples of patients with rheumatoid arthritis. Ann Rheum Dis. 2004;63:881–883. doi: 10.1136/ard.2003.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 10.Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D694–703. doi: 10.2741/martel. [DOI] [PubMed] [Google Scholar]
- 11.Farkouh ME, Greenberg JD, Jeger RV, Ramanathan K, Verheugt FW, Chesebro JH, Kirshner H, Hochman JS, Lay CL, Ruland S, Mellein B, Matchaba PT, Fuster V, Abramson SB. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66:764–770. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, Gitton X, Krammer G, Mellein B, Gimona A, Matchaba P, Hawkey CJ, Chesebro JH. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364:675–684. doi: 10.1016/S0140-6736(04)16894-3. [DOI] [PubMed] [Google Scholar]
- 13.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberq MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. [DOI] [PubMed] [Google Scholar]
- 14.Ye Y, Li XQ, Tang CP, Yao S. Natural products chemistry research 2010’s progress in China. Chin J Nat Med. 2012;10:1–12. doi: 10.1016/S1875-5364(12)60001-6. [DOI] [PubMed] [Google Scholar]
- 15.Sievänen E. Exploitation of bile acid transport systems in prodrug design. Molecules. 2007;12:1859–1889. doi: 10.3390/12081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins GJ, Harries K, Doak SH, Wilmes A, Griffiths AP, Baxter JN, Parry JM. The bile acid deoxycholic acid (DCA) at neutral pH activates NF-kB and induces IL-8 expression in oesophageal cells in vitro. Carcinogenesis. 2004;25:317–323. doi: 10.1093/carcin/bgh032. [DOI] [PubMed] [Google Scholar]
- 17.Wu MM, Guo K, Dong HW, Zeng R, Tu M, Zhao JH. In vitro drug release and biological evaluation of biomimetic polymeric micelles self-assembled from amphiphilic deoxycholic acid-phosphorylcholine-chitosan conjugate. Mat Sci Eng C-mater. 2014;45:162–169. doi: 10.1016/j.msec.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Yan ZW, Liu JP, Lu D, Xue JF, Chen S, Li PY. Chemical constituents from the bile of Anser anser and their anti-MMP activity. Nat Prod Res Dev. 2008;20:960–963. [Google Scholar]
- 19.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Wu HB, Du JY, Zheng QX. Expression of MMP-1 in cartilage and synovium of experimentally induced rat ACLT traumatic osteoarthritis: immunohistochemical study. Rheumatol Int. 2008;29:31–36. doi: 10.1007/s00296-008-0636-2. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi T, Yamada H, Shimmei M. Effect of high molecular weight hyaluronan on cartilage degeneration in a rat model of osteoarthritis. Osteoarthr Cartil. 1996;4:99–110. doi: 10.1016/s1063-4584(05)80319-x. [DOI] [PubMed] [Google Scholar]
- 22.Mainil-Varlet P, Schiavinato A, Ganster MM. Efficacy evaluation of a new hyaluronan derivative HYADD® 4-G to maintain cartilage integrity in a rabbit model of osteoarthritis. Cartilage. 2013;4:28–41. doi: 10.1177/1947603512455193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salminen HJ, Säämänen AM, Vankemmelbeke MN, Auho PK, Perälä MP, Vuorio EI. Differential Expression patterns of matrix metalloproteinases and their inhibitors during development of osteoarthritis in a transgenic mouse model. Ann Rheum Dis. 2002;61:591–597. doi: 10.1136/ard.61.7.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jo H, Park JS, Kim EM, Jung MY, Lee SH, Seong SC, Park SC, Kim HJ, Lee MC. The in vitro effects of dehydroepiandrosterone on human osteoarthritic chondrocytes. Osteoarthr Cartil. 2003;11:585–594. doi: 10.1016/s1063-4584(03)00094-3. [DOI] [PubMed] [Google Scholar]
- 25.Ishiguro N, Ito T, Ito H, Iwata H, Jugessur H, Ionescu M, Poole AR. Relationship of matrix metalloproteinases and their inhibitors to cartilage proteoglycan and collagen turnover: analyses of synovial fluid from patients with osteoarthritis. Arthritis Rheum. 1999;42:129–136. doi: 10.1002/1529-0131(199901)42:1<129::AID-ANR16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Tetlow LC, Adlam DJ, Woolley DE. Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum. 2001;44:585–594. doi: 10.1002/1529-0131(200103)44:3<585::AID-ANR107>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 27.Lohmander LS, Brandt KD, Mazzuca SA, Katz BP, Larsson S, Struglics A, Lane KA. Use of the plasma stromelysin (matrix metalloproteinase 3) concentration to predict joint space narrowing in knee osteoarthritis. Arthritis Rheum. 2005;52:3160–3167. doi: 10.1002/art.21345. [DOI] [PubMed] [Google Scholar]


