Abstract
The interleukin-6 (IL-6) C-572G gene polymorphism has been suggested to be associated with the increased coronary artery disease (CAD) risk, but the study results are still debatable. To explore the association between IL-6 C-572G gene polymorphism and CAD in the Asian population, the current meta-analysis involving 2511 subjects from 7 separate studies was conducted. The combined odds ratio (ORs) for the association between IL-6 C-572G gene polymorphism and CAD and their corresponding 95% confidence intervals (95% CIs) were assessed by random or fixed effect model. A significant association between IL-6 C-572G gene polymorphism and CAD was found in the Asian population under an allelic (OR: 1.50, 95% CI: 1.30-1.71, P<0.00001), recessive (OR: 2.221, 95% CI: 1.444-3.417, P=1.0×10-10) dominant (OR: 1.313, 95% CI: 1.188-1.451, P=1.0×10-10), homozygous (OR: 2.454, 95% CI: 1.606-3.751, P=1.0×10-10), heterozygous (OR: 3.01, 95% CI:1.99-4.55, P<0.00001) and additive genetic models (OR: 1.372, 95% CI: 1.231-1.528, P=1.0×10-10). In the Asian population, the IL-6 C-572G gene polymorphism was indicated to be correlated with CAD susceptibility. The carriers of -572G allele might be predisposed to CAD risk.
Keywords: Interleukin-6, C-572G, polymorphism, coronary artery disease, Asian
Introduction
Coronary artery disease (CAD), also namely ischemic heart disease (IHD), is caused by the coronary artery organic stenosis or occlusion which leads to myocardium ischemia (angina pectoris) or myocardial infarction. CAD is the premier reason contributing to death and deformity worldwide [1,2]. The World Health Organization has prospected that the CAD death toll would account for 13.1% of the total population death [3]. The Chinese cardiovascular report has pointed out that the CAD mortality is 57.1 per 100 thousand in the cities and 33.7 per 100 thousand in the countries in 2007 which was increased by 23.3% and 43.8% respectively than that in 2004 [4].
The epidemiological survey has shown that CAD was a polygenic disease. The hereditary and environmental factors participate in the CAD progression jointly. The pedigree and twin researches have shown that the CAD heredity grade is up to 50% [5,6]. To our knowledge, the inflammation reaction effect has been much emphasized on the CAD pathogenesis recently [7]. Interleukin-6 (IL-6) is an important inflammatory cytokine produced by various cells as adipocytes, endothelial cells, fibroblasts, myocytes and white blood cells [8]. IL-6 is the main mediator for the hepar reaction protein in the acute stage including C reaction protein and fibrinogen. IL-6 plays a significant role in the immune response and inflammation reaction regulation. IL-6 could promote the occurrence and development of atherosclerosis which was the basic pathology of CAD [9].
IL-6 gene, located in 7p21, spans 5kb and contains 4 introns and 5 exons. IL-6 C-572G mutation in the promoter region is that cytosine (C) being substituted by guanine (G). The IL-6 C-572G gene polymorphism might influence the IL-6 gene transcription and expression level by changing the gene combining sequence, thereby resulted in the different susceptibility to CAD.
Although the studies on the association between IL-6 C-572G gene polymorphism and CAD have been carried out extensively in Asia, the results were still inconsistent. In 2007, Park et al found that there was not a significant association between IL-6 C-572G gene polymorphism and CAD in Korea [10]. On the contrast, in 2010, Liang et al found an association between IL-6 C-572G gene polymorphism and CAD in a Chinese population and they reported that IL-6 -572G allele carriers might be predisposed to CAD susceptibility [11]. Similarly, in 2011, Liu et al also found the same association between IL-6 C-572G gene polymorphism and CAD in another Chinese population [12].
Therefore, in an attempt to gain a credible conclusion on the association of IL-6 C-572G gene polymorphism with CAD in the Asian population, the current meta-analysis including 2511 subjects was conducted.
Material and methods
Publication search and inclusion criteria
The words as ‘interleukin-6’, ‘coronary artery disease’, ‘coronary heart disease’, and ‘polymorphism’ were adopted to search the electronic databases as Web of Science, PubMed, Embase, China Biological Medicine Database and China National Knowledge Infrastructure. The obtained studies were published between 2006 and 2011 (last research updated on February 10, 2015).
The included studies had to be in accordance with the major criteria as follows: a) Assessment of the IL-6 C-572G gene polymorphism and CAD in the Asian population. b) The diagnosis of CAD was in the light of the examination results of coronary arteriography, clinical symptoms combined with electrocardiogram, treadmill exercise test, echocardiography, and myocardial perfusion imaging in Emission Computed Tomography. The major coronary artery minimal diameter stenosis was no less than 50%. c) The study should be a case-control or cohort study published in an official journal.
Data extraction
In the current meta-analysis, the repeated publications, studies against the main inclusion criteria or providing insufficient data were eliminated. If same data came out of different studies, the result was only once used. The extracted data was composed of the follows: the first author’s name, publication year, region, number of genotypes, genotyping, study design, matching criteria, total number of cases and controls.
Statistical analysis
The association between IL-6 C-572G gene polymorphism and CAD was compared by using the odds ratio (OR) corresponding to 95% confidence interval (CI). In the current meta-analysis, the presence of between-studies heterogeneity was calculated by using Chi-square-based Q-test and significance was set at P<0.05 level [13]. Meanwhile, the inconsistency index I2 was calculated to assess the variation caused by heterogeneity. If heterogeneity existed among the individual studies in the present meta-analysis, the pooled OR was assessed by the random-effects model (the DerSimonian and Laird method) [14]. Otherwise, the fixed-effects model was used to assess the ORs (the Mantel-Haenszel method) [15]. The Z test was used to determine the combined OR and significance was also set at P<0.05.
In the current study, the Hardy-Weinberg equilibrium (HWE) was assessed by Fisher’s exact test and significance was set as P<0.05. The potential publication bias was estimated by the funnel plot. The funnel plot asymmetry was assessed by Egger’s linear regression test on the natural logarithm scale of the OR (significance was set at P<0.05 level) [16]. The statistical analysis was conducted by STATA 11.0 software (StataCorp, College Station, TX) and Review Manager.
Results
Studies and populations
Fifteen papers were obtained by the literature search, of which seven papers accorded with the inclusion criteria. Of the eight excluded studies, one paper was double publication, three papers were of review character and two studies were not involved with the IL-6 C-572G gene polymorphism or CAD. Two studies deviating from HWE were excluded. The total data were gathered from 1283 CAD patients and 1228 controls (Table 1) [10-12,17-20]. The two surveyed countries were consisted of China and Korea. According to the Newcastle-Ottawa Quality Assessment Scale, all of the included individual studies score more than 6 stars which suggested that the individual studies quality were good [21].
Table 1.
Characteristics of the investigated studies of the association between the interleukin-6 C-572G polymorphism and coronary artery disease in the Asian population
| Author | Year | Region | Country | CAD | Control | geno-typing | Study design | Matching criteria | sample size (CAD/control) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| CC | CG | GG | CC | CG | GG | ||||||||
| Wei YS [17] | 2006 | Gunagxi | China | 89 | 67 | 9 | 113 | 55 | 2 | PCR-RFLP | Case-control | Age, sex, ethnicity | 165/170 |
| Liu YS [18] | 2007 | Jilin | China | 49 | 39 | 2 | 68 | 26 | 0 | PCR-RFLP | Case-control | Ethnicity | 90/94 |
| Park S [10] | 2007 | Seoul | Korea | 92 | 62 | 12 | 97 | 62 | 9 | PCR-RFLP | Case-control | Age, sex, BMI, ethnicity | 166/168 |
| Gao CX [19] | 2008 | Jiangsu | China | 65 | 51 | 10 | 72 | 32 | 4 | PCR-RFLP | Case-control | Age, sex, ethnicity | 126/108 |
| Fan WH [20] | 2009 | Guangdong | China | 97 | 72 | 7 | 86 | 33 | 2 | PCR-RFLP | Case-control | sex, ethnicity | 176/121 |
| Liang ZY [11] | 2010 | Shanxi | China | 259 | 161 | 14 | 283 | 126 | 8 | PCR-RFLP | Case-control | Age, sex, ethnicity | 434/417 |
| Liu YC [12] | 2011 | Shanxi | China | 63 | 52 | 11 | 92 | 55 | 3 | PCR-RFLP | Case-control | Age, sex, ethnicity | 126/150 |
Abbreviations: CAD, coronary artery disease; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism; BMI, body mass index.
Combined analyses
A significant association between IL-6 C-572G gene polymorphism and CAD was found in the Asian population under an allelic (OR: 1.50, 95% CI: 1.30-1.71, P<0.00001), recessive (OR: 2.221, 95% CI: 1.444-3.417, P=1.0×10-10), dominant (OR: 1.313, 95% CI: 1.188-1.451, P=1.0×10-10), homozygous (OR: 2.454, 95% CI: 1.606-3.751, P=1.0×10-10), heterozygous (OR: 3.01, 95% CI: 1.99-4.55, P<0.00001) and additive genetic models (OR: 1.372, 95% CI: 1.231-1.528, P=1.0×10-10) (Table 2; Figures 1, 2).
Table 2.
Summary of meta-analysis of association of interleukin-6 C-572G polymorphism and coronary artery disease in the Asian population
| Genetic model | Pooled OR (95% CI) | P value | Literature number | CAD size | control size | P heterogeneity (I2%) |
|---|---|---|---|---|---|---|
| Allelic genetic model | 1.50 (1.30-1.71) | <0.00001* | 7 | 1283 | 1228 | 0.45 (0%) |
| Recessive genetic model | 2.221 (1.444-3.417) | 1.0×10-10 * | 7 | 1283 | 1228 | 0.663 (0%) |
| Dominant genetic model | 1.313 (1.188-1.451) | 1.0×10-10 * | 7 | 1283 | 1228 | 0.414 (1.4%) |
| Homo genetic model | 2.454 (1.606-3.751) | 1.0×10-10 * | 7 | 1283 | 1228 | 0.554 (0%) |
| Hetero genetic model | 3.01 (1.99-4.55) | <0.00001* | 7 | 1283 | 1228 | 0.004 (68.3%)* |
| Subgroup 1: CG1<60 | 4.41 (2.80-6.96) | <0.00001* | 3 | 342 | 352 | 0.50 (0%) |
| Subgroup 2: CG1>60 | 2.40 (1.44-4.02) | 0.0008* | 4 | 941 | 876 | 0.01 (73.3%)* |
| Additive genetic model | 1.372 (1.231-1.528) | 1.0×10-10 * | 7 | 1283 | 1228 | 0.426 (0%) |
P<0.05.
Abbreviations: CAD, coronary artery disease; CI, confidence interval; OR, odds ratio; CAD size, the total number of CAD cases; control size, the total number of control group; homo genetic model, homozygous genetic model; hetero genetic model, heterozygous genetic model.
Figure 1.
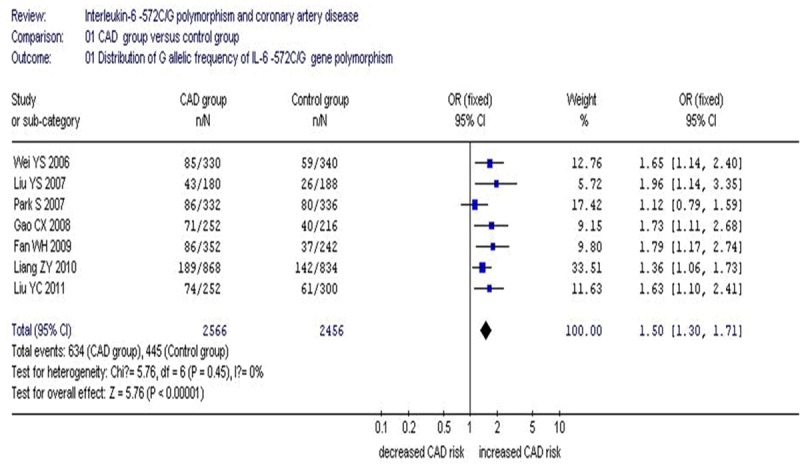
Forest plot of coronary artery disease associated with IL-6 C-572G gene polymorphism under an allelic genetic model (distribution of G allelic frequency of IL-6 gene).
Figure 2.
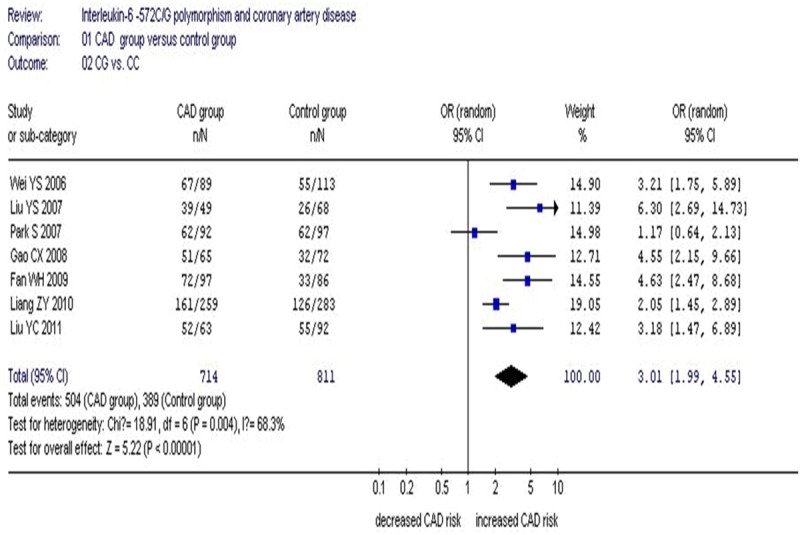
Forest plot of coronary artery disease associated with IL-6 C-572G gene polymorphism under a heterozygous genetic model (CG vs. CC).
Under the heterozygous genetic model, there was significant heterogeneity (P=0.004, I2=68.3%). The following meta-regression was conducted to explore the heterogeneity source. Among the confounding factors, the heterogeneity could be explained by CC sample size of CAD group (CC1, P=0.019) and CG sample size of CAD group (CG1, P=0.012). The whole population was divided into two subgroups according to CG1. The studies with CG1<60 were grouped into subgroup 1 and the remained studies with CG1>60 grouped into subgroup 2.
In the subgroup analysis stratified by CG1, under the heterozygous genetic model, significantly increased CAD risk was observed in both subgroups (subgroup 1: OR: 4.41, 95% CI: 2.80-6.96, P<0.00001; subgroup 2: OR: 2.40, 95% CI: 1.44-4.02, P=0.0008). No significant heterogeneity was detected in subgroup 1 any more (Pheterogeneity=0.50, I2=0%), but there was still significant heterogeneity in the subgroup 2 (Pheterogeneity=0.01, I2=73.3%) which suggested that CG1 was the main confounding factor to explain the heterogeneity source (Tables 2, 3; Figure 3).
Table 3.
The meta-regression results among 7 studies in Asian population under a heterozygous genetic model for interleukin-6 C-572G gene polymorphism
| Coefficient | Standard Error | T value | P value | 95% Confidence Interval | |
|---|---|---|---|---|---|
| CC sample size of CAD group | 0.0481114 | 0.012674 | 3.80 | 0.019* | 0.0129228~0.0833 |
| CG sample size of CAD group | -0.0964588 | 0.0222123 | -4.34 | 0.012* | -0.15813~-0.0347876 |
| cons | 3.192847 | 0.3264789 | 9.78 | 0.001* | 2.286396~4.099298 |
P<0.05.
Coefficient: regression coefficient. The regression coefficients are the estimated increase in the lnOR per unit increase in the covariates. cons: constant item.
Figure 3.
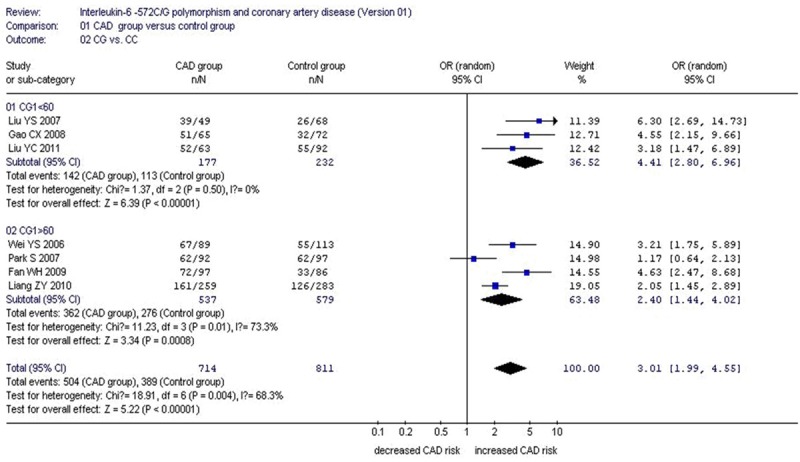
Forest plot of coronary artery disease associated with IL-6 C-572G gene polymorphism under a heterozygous genetic model stratified by CG1 (CG vs. CC).
Among all of the individual studies, only the Park S Korean study showed no significant association between the IL-6 C-572G gene polymorphism and CAD. The sensitivity analysis has been performed. After the Park S Korean study was excluded from the current meta-analysis, the association was more stronger than before under the allelic genetic model (OR: 1.57, 95% CI: 1.36-1.83) which suggested that there was a more significant association between them in Chinese population than in the whole Asian population.
Bias diagnostics
The funnel plot and Egger’s test were used to evaluate the publication bias of the studies. No visual publication bias was detected in the funnel plot (Figure 4). No statistically significant difference was observed in the Egger’s test which suggested that there was low publication bias in the present meta-analysis by using heterozygous genetic model (T=-2.21, P=0.078).
Figure 4.
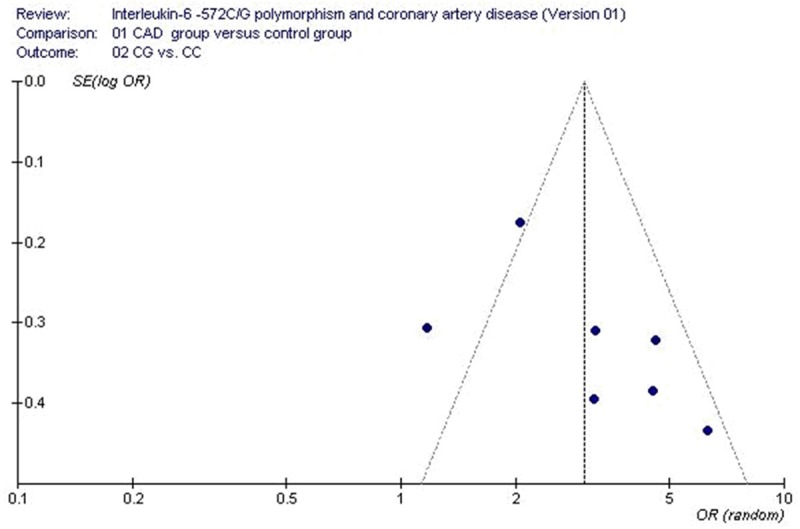
Funnel plot for studies of the association of coronary artery disease with IL-6 C-572G gene polymorphism under a heterozygous genetic model stratified by CG1 (CG vs. CC). The horizontal and vertical axis correspond to the OR and confidence limits. OR: odds ratio; SE: standard error.
Discussion
In the current meta-analysis, a significant association between IL-6 C-572G gene polymorphism and CAD was found in the Asian population under an allelic (OR: 1.50), recessive (OR: 2.221), dominant (OR: 1.313), homozygous (OR: 2.454), heterozygous (OR: 3.01) and additive genetic models (OR: 1.372). Hence, it has been concluded that in Asian population, the IL-6 C-572G gene polymorphism was indicated to be correlated with CAD susceptibility. The carriers of -572G allele might be predisposed to CAD risk.
As the heterogeneity existed under the heterozygous genetic model (Pheterogeneity<0.05), the subsequent meta-regression has been conducted to explore the heterogeneity source. In the heterogeneity source analysis, CG1 was possibly indicated to be the main heterogeneity source (P=0.012). Although the subgroup analysis stratified by CG1 demonstrated that significantly increased CAD risk was observed in both subgroups, the heterogeneity disappeared in subgroup 1 (P=0.50) and still existed in subgroup 2 (P=0.01). Therefore, CG1 was actually the main confounding factor leading to the heterogeneity.
CAD is considered to be an inflammation disease. Researches have shown that the inflammation factors regulation network was constituted by a variety of proinflammatory, anti-inflammatory factors as tumor necrosis factor. IL-6 possibly plays an immune regulation role in the occurrence and development of CAD [7]. Recently the studies on the association of inflammation factor gene polymorphism and CAD have been performed widely.
IL-6 is a 27KD glycoprotein constituted by 184 amino acids. IL-6 is a proinflammatory factor, mainly secreted by the activated inflammation cells as the lymphocyte and macrophage cells. IL-6 is an important mediator closely associated with the immune and endocrine systems. As the end differentiation factor of B lymphocyte and the end aid factor of T lymphocyte, the most primary biological function of IL-6 is to enhance the body immune function and further produce a series of corresponding biological alternations [22].
IL-6 might play the following roles in the development of CAD. (a) IL-6 promotes the chemotaxis and activation of the leukocytes and monocytes. (b) IL-6 promotes the vascular endothelial cells to express the adhesion molecules and other inflammation transmitter, and augment the local inflammation response; (c) IL-6 induces the hepatocytes to synthesize the acute stage proteins as fibrinogen, C reactive protein; (d) IL-6 facilitates the vascular endothelial cells to release the coagulation factors as coagulating factor III and launch the coagulating process; (e) IL-6 stimulates the synthesis of matrix degradation enzyme, erodes the plaque matrix and leads to the unstable plaque rupture. Hence, IL-6 was considered to be the prediction index for the early CAD basic pathological changes and serious complications. The higher the IL-6 level, the higher was the severe CAD risk [23-26].
The mechanism of IL-6 C-572G gene mutation leading to CAD was still unclear. Theoretically, any gene mutation in the promoter region might change the IL-6 gene combining sequence, enhance or weaken the gene transcription and expression level, and influence the susceptibility to CAD. Some studies have shown that there was no significant difference between IL-6 C-572G gene polymorphism and serum IL-6 level [27]. Hence, IL-6 C-572G gene polymorphism promotes the CAD development not through the circulating IL-6 level, but the IL-6 level from the local tissue as the vascular wall. The IL-6 concentration from the local tissue might be more important than the circulating IL-6 concentration to the increased CAD risk [28].
In 2012, Zheng et al performed a meta-analysis on the association of the IL-6 C-572G gene polymorphism and CAD and they concluded that the IL-6 C-572G gene polymorphism might contribute to the CAD development [29]. By contrast, in 2012, Yang et al also carried out a meta-analysis on the relationship between them and they found no statistically significant differences between controls and CAD cases for the G allele contrasts of the -174 G/C and -572 G/C polymorphisms [30]. However, in both of the two meta-analyses, the Asian population and Caucasus populations were mixed to analyze the association. Moreover, the individual studies deviating from HWE were included. In addition, only three genetic models as additive, recessive and dominant were adopted. While in the current meta-analysis, besides the above three genetic models, other three genetic models as allelic, homozygous and heterozygous genetic models are also used. Hence, our results should be more objective and credible than that of Zheng’s work.
Some limitations still existed in the present research. The large-scale studies on the association of IL-6 C-572G gene polymorphism and CAD were still insufficient. The IL-6 level was influenced not only by the IL-6 C-572G gene polymorphism, but also by other environmental factors as smoke and inflammation. Additionally, other IL-6 gene polymorphisms as G-174C, C-634G and G-597A also influence the IL-6 level.
In conclusion, the present meta-analysis indicated that the IL-6 -572G allele might increase the CAD risk in the Asian population. The conclusion had potential guidance significance in formulating CAD individual therapy strategy. In consideration of the above limitations, the conclusion was required to be confirmed by more researches in the future.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (NSFC 81100073 to Dr Yan-Yan Li), Excellent Young and Middle-Aged Teachers Assistance Program of Nanjing Medical University for Dr Yan-Yan Li (2013-2015, JX2161015034), Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents (2014), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). Thank all our colleagues working in the Department of geriatrics, the First Affiliated Hospital of Nanjing Medical University. No conflict of interest existed.
Disclosure of conflict of interest
None.
References
- 1.Zimmer RJ, Lee MS. Transplant coronary artery disease. JACC Cardiovasc Interv. 2010;3:367–377. doi: 10.1016/j.jcin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Lu XF. Advances in genome-wide association study of coronary heart disease. Yi Chuan. 2010;32:97–104. doi: 10.3724/sp.j.1005.2010.00097. [DOI] [PubMed] [Google Scholar]
- 5.Mayer B, Erdmann J, Schunkert H. Genetics and heritability of coronary artery disease and myocardial infarction. Clin Res Cardiol. 2007;96:1–7. doi: 10.1007/s00392-006-0447-y. [DOI] [PubMed] [Google Scholar]
- 6.Evans A, Van Baal GC, McCarron P, DeLange M, Soerensen TI, De Geus EJ, Kyvik K, Pedersen NL, Spector TD, Andrew T, Patterson C, Whitfield JB, Zhu G, Martin NG, Kaprio J, Boomsma DI. The genetics of coronary heart disease: the contribution of twin studies. Twin Res. 2003;6:432–441. doi: 10.1375/136905203770326439. [DOI] [PubMed] [Google Scholar]
- 7.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 8.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 9.Dandona S, Stewart AF, Roberts R. Genomics in coronary artery disease: past, present and future. Can J Cardiol. 2010;26(Suppl A):56A–59A. doi: 10.1016/s0828-282x(10)71064-3. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Youn JC, Shin DJ, Park CM, Kim JS, Ko YG, Choi D, Ha JW, Jang Y, Chung N. Genetic polymorphism in the pregnancy-associated plasma protein-A associated with acute myocardial infarction. Coron Artery Dis. 2007;18:417–422. doi: 10.1097/MCA.0b013e328241d967. [DOI] [PubMed] [Google Scholar]
- 11.Liang ZY, Zhang XL, Sun Y, Feng XY, Bai J. Association of interleukin-6 gene -572C/G single nucleotide polymorphism with coronary artery disease in Han population of north China. Progress in Modern Biomedicine. 2010;10:4675–4678. [Google Scholar]
- 12.Liu YC, Duan ZM, Zhang MJ. Association between polymorphisms in interleukin 6 gene promoter region and coronary artery disease. Clinical Focus. 2011;26:1200–1203. [Google Scholar]
- 13.Cochran WG. The effectiveness of adjustment by subclassification in removing bias in observational studies. Biometrics. 1968;24:295–313. [PubMed] [Google Scholar]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 16.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei YS, Lan Y, Liu YG, Tang RG, Lan JS. Relationship between interleukin-6 gene polymorphism and coronary artery disease and its effect on plasma lipid levels. Chin Crit Care Med. 2006;18:233–236. [PubMed] [Google Scholar]
- 18.Liu YS, Wang JF, Yu LH, Liu HP, Meng FC. Association of polymorphism in interleukin 6 (IL-6) gene promoter region with morbidity of coronary artery disease. Chinese Journal of Cardiovascular Review. 2007;5:264–266. [Google Scholar]
- 19.Gao CX, Wang YL. Interleukin-6 gene polymorphism in elderly patients with coronary heart disease. J Fourth Mil Med Univ. 2008;29:2183–2185. [Google Scholar]
- 20.Fan WH, Liu DL, Zhang JC, Xiao LM, Xie CJ, et al. Polymorphisms of the interleukin-6 -174G/C, -572C/G gene and the association between chronic peridontitis and coronary heart disease. J Modern Stomatol. 2009;23:415–418. [Google Scholar]
- 21.Newcastle-Ottawa Quality Assessment Scale: Case control studies. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford. htm Exit Disclaimer. Accessed January 2011.
- 22.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 23.Versaci F, Reimers B, Prati F, Gaspardone A, Del Giudice C, Pacchioni A, Mauriello A, Cortese C, Nardi P, De Fazio A, Chiariello GA, Proietti I, Chiariello L. Prediction of cardiovascular events by inflammatory markers in patients undergoing carotid stenting. Mayo Clin Proc. 2012;87:50–58. doi: 10.1016/j.mayocp.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McPherson R, Davies RW. Inflammation and coronary artery disease: insights from genetic studies. Can J Cardiol. 2012;28:662–666. doi: 10.1016/j.cjca.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Scheller J, Rose-John S. The interleukin 6 pathway and atherosclerosis. Lancet. 2012;380:338. doi: 10.1016/S0140-6736(12)61246-X. [DOI] [PubMed] [Google Scholar]
- 26.Panichi V, Scatena A, Migliori M, Marchetti V, Paoletti S, Beati S. Biomarkers of chronic inflammatory state in uremia and cardiovascular disease. Int J Inflam. 2012;2012:360147. doi: 10.1155/2012/360147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu HX, Li GS, Li Y, Xu JL, Zhang JY. Interleukin-6 -597G/A and -572C/G polymorphisms and risk of coronary heart disease. Chin J Cardiol. 2006;34:519–522. [PubMed] [Google Scholar]
- 28.Humphries SE, Luong LA, Ogg MS, Hawe E, Miller GJ. The interleukin-6 -174 G/C promoter polymorphism is associated with risk of coronary heart disease and systolic blood pressure in healthy men. Eur Heart J. 2001;22:2243–2252. doi: 10.1053/euhj.2001.2678. [DOI] [PubMed] [Google Scholar]
- 29.Zheng GH, Chen HY, Xiong SQ. Polymorphisms of -174G>C and -572G>C in the interleukin 6 (IL-6) gene and coronary heart disease risk: a meta-analysis of 27 research studies. PLoS One. 2012;7:e34839. doi: 10.1371/journal.pone.0034839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Zhang F, Skrip L, Lei H, Wang Y, Hu D, Ding R. IL-6 gene polymorphisms and CAD risk: a meta-analysis. Mol Biol Rep. 2013;40:2589–98. doi: 10.1007/s11033-012-2345-x. [DOI] [PubMed] [Google Scholar]


