Abstract
It is reported that gossypol acetate (GAA) has obvious effects on inhibiting the growth of tumors, by inhibiting the activity of enzymes. Ultrastructural study showed that GAA can cause morphological changes of mitochondria which leads to the apoptosis of tumors. However, little is known about the pathways that how the GAA triggers apoptosis of tumors and what kind of the molecular events have happened when GAA is added. The aim of the study is try to know if GAA have some functions on pituitary tumor cell. And if there are any changes after GAA treatment, we try to understand the mechanisms that how GAA regulate the growth of pituitary tumor cell. The study was carried out on rat lactotroph cell lines, GH3 and MMQ. Q-PCR and western blot (WB) assay are used to determine the expression level of genes and the protein level. Both the miR-15a (mimics) overexpression cell line and miR-15a knock out (inhibitor) cell line were obtained in GH3 and MMQ. Apoptosis rate was determined by flow cytometry (FCM). Our study revealed that: 1) GAA inhibits the proliferation of pituitary tumor cells of GH3, MMQ. 2) GAA upregulate the expression of miR15a in GH3 and MMQ. 3) Overexpressed miR-15a (mimics) downregulates the expression level of Bcl-2. 4) Knock down miR-15a (inhibitor) upregulates Bcl-2 and reverse the apoptosis induced by GAA. Our study indicates that GAA-induced decrease in cell proliferation is associated with decreased expression of Bcl-2 and increased miR-15a. Based on this, we propose developing GAA as a novel therapeutic tool in the management of pituitary tumor.
Keywords: Gossypol acetate, apoptosis, hypophysoma, pituitary tumor, microRNA miR-15a
Introduction
Gossypol is the active component extracted from cotton roots and seeds. It accounts for about 1.5% of the seeds in weight, mostly used as medicine in the form of Gollypol Acetate (GAA) [1]. It is the natural male contraceptive and is also used for treatment of female gynecological disorders. In the late 1990s, researchers find that GAA has obvious effect on tumors, caused a great sensation throughout the world [2-5]. After decades of clinic studies have indicated that it has potent activity of antitumor and antivirus and is used for treating the cancer of adrenal cortex and bladder cancer so far. Ultrastructural study also shows that GAA can cause morphological changes such as swollen mitochondria, crest fracture and so on; which will activate the endogenous pathways of apoptosis, namely the mitochondrial [6] pathway. It is currently believed that gossypol in itself will not kill cancerous cells; however, it changes the chemistry within the cancer cell and makes it more susceptible to traditional chemotherapy drugs. Phased trials have been done on resistant prostate and lung cancer. Few results have been published to date, so no conclusions can be drawn [2,7].
Prolactinomas, the most commonly diagnosed pituitary tumor, is considered to comprising almost twenty seven percent of all pituitary tumors. All though, many researches have been done about the pituitary tumors, ones with common occurrence, little is known about the molecular events that lead to the development of pituitary tumors. Recently, a lot of researchers have paid attentions on the molecular mechanisms of cell proliferation, invasion and migration of pituitary tumors. Meanwhile, they also want to find some chemicals with high efficiency to inhibit the cell proliferation of pituitary tumor and less toxicity to the cells. According to the literatures from other groups, we choose GAA as the chemicals and studied have been down to explore the functions of GAA on cell proliferation and also the mechanisms.
It is also reported that Bcl2 family proteins regulate apoptosis through the intrinsic mitochondrial apoptosis pathway that can be triggered by numerous stress stimuli, such as growth-factor deprivation, calcium-flux or DNA-damage [8]. Cimmino et al. reported that B cell lymphoma 2 (BCL2) is a central player in the genetic program of eukaryotic cells favoring survival by inhibiting in cell death [8-10]. Overexpression of Bcl2 protein has been reported in many types of human cancers, including leukemias, lymphomas, and carcinomas. Their work indicated that actually Bcl2 is the targets of microRNA 15 and microR 16, the two miRs can induce apoptosis of tumor cell.
Previous data has also shown that microRNAs (miRNAs) are a class of genes involved in human tumor genesis. The microRNAs (miRNAs) are a group of small RNAs present in a wide variety of organisms, such as plants, Drosophila, and humans. They regulate the expression of target genes by binding to the 3’-untranslated region (3’UTR) of target messenger RNAs (mRNAs), resulting in either their degradation or inhibition of their translation, depending on the degree of complementary base pairing. It has been shown that miRNAs are involved in cancer initiation and progression, and their expression patterns serve as phenotypic signatures of various cancers [11,12]. In animals, single-stranded miRNA binds specific mRNA through sequences that are imperfectly complementary to the target mRNA. Two miRNAs, miR-15a and miR-16-1, so far, are found in around 65% of B cell CLL patients. The absence of miR-15a/miR-16-1 has a great effect on tumor growth which may indicate their important roles in regulating the formation of tumor, as the suppressor genes of tumor. And as we all know that GAA also has some functions on oncotherapy. Since there is a lot of work on miR-15a, miR-16-1 and Bcl2 has been down, little is known about the mechanisms. According our previous data and the information of many literatures from other groups, by using the two cell lines GH3 and MMQ, we want to further understand the regulatory mechanism from genetic level to molecular level to see how GAA can induce the apoptosis of pituitary tumor cell.
Material and methods
Cell culture
GH3 cells were maintained in high-glucose DMEM (Sigma), supplemented with 10% (v/v) fetal calf serum (FCS; Sigma), 100 U penicillin/ml and 100 μg streptomycin sulfate per ml (Sigma) in 5% CO2 humidified air at 37°C, as described previously (Fowkes and Burrin 2003). These growth hormone and prolactin-secreting cells were originally derived from Wistar-Furth rats with pituitary tumors [13,14]. MMQ cells were maintained in DMEM containing 15% horse serum, 2.5% FBS, and 5 U/ml penicillin/5 g/ml streptomycin.
Analysis of apoptosis
GH3 and MMQ cells were treated with different concentration of GAA (0, 1, 5, 10, 50, 100, and 200) μM for 36 h. After treatment, apoptosis was assessed using the propidium iodide (PI) solution (Sigma). The cells were washed twice with PBS, suspended in binding buffer and stained with PI, all the survival cells are calculated manually under microscope. Cells undergoing apoptosis were detected by flow cytometry (FCM).
Western blotting
After treatments, ice-cold PBS solution was used two times to rinse cells. Subsequently lysis buffer [Tris (pH 7.5), 50 mm; EGTA 5 mm; NaCl 120 mm; α-glycerophosphate 20 mm; Nonidet P-40 1%; Na pyrophosphate 15 mm; Na fluoride 50 mm; Na orthovanadate 10 mm; phenylmethylsulfonyl fluoride 0.5 mm; aprotinin 10 μg/ml; leupeptin 10 μg/ml; glycerol 20%] was added (100-180 μl) and dishes incubated for 10-30 min at 4°C. Cells were scraped into lysis buffer, and lysates were clarified by centrifugation (12,000 rpm, 15 min at 4°C). The protein content in the supernatant was determined by the BCA protein assay (Pierce Chemical Co., Rockford, IL). For Western blotting equal amounts of proteins were used. Briefly, 7.5-90 μg of cell lysate were subjected to electrophoresis on 8-12% SDS-PAGE gels, and the separated proteins were electrophoretically transferred onto polyvinyl difluoride membranes. Membranes were rinsed twice with PBS and Tween 20 (PBST)/Tris-buffered saline and Tween 20 (TBST) and then incubated with a blocking buffer (4-5% nonfat milk in PBST/TBST) for 1-2 h at room temperature. Overnight incubation of membranes with primary antibodies [anti-Bcl-2 antibody (Ab), 1:5,000-12,500; anti-rat GADPH Ab, 1:1,000,] were done at 4°C, followed by three 10-min washes with PBST/TBST. Membranes were incubated with secondary antibodies at room temperature for 1 h, washed three times with PBST/TBST, and antibody bound proteins were detected by enhanced chemiluminescence reagents (Pierce), according to the manufacturer’s protocol. To calculate fold change, density of protein bands was determined by using the ImageJ 1.38X software provided by the National Institutes of Health (Bethesda, MD). After normalization to GADPH, the control sample was assigned an arbitrary value of 1, and fold change, in response to GAA treatment, was calculated.
RNA extraction
Total RNA from GH3 and MMQ cells was isolated by binding to a silica-based membrane using a microspin column technique according to the manufacturer’s protocol (Invitrogen TRIZOL® Reagent (Cat. No. 15596-026)). Remaining traces of DNA were digested in a total volume of 100 µl with 10 units of DNaseI (FPLCpure, Pharmacia, Freiburg, Germany) in a buffer containing 10 mM Tris/HCl/10 mM MgCl2/50 mM KCl/10 mM dithiothreitol (DTT), pH 9.0 for 20 min at 37°C. With an additional clean-up procedure, DNaseI-treated RNA was recovered by adsorption on silica-based membranes as described in the total RNA extraction procedure.
cDNA synthesis was carried out in a 20 µL reverse transcription reaction containing 1 μg of total RNA, and various amounts of the corresponding internal standard RNA. RNA was reverse transcribed in a buffer containing 5 mM MgCl2, 50 mM KCl, 10 mM Tris/HCl (pH 8.3), 1 mM dNTPs, 2.5 µM random hexamers, and 20 units of RNase inhibitor and 100 units of RT. The reaction was carried out at 42°C for 20 min. Thereafter, the mixture was incubated at 99°C for 5 min to inactivate enzyme’s activity. These samples were processed for qualitative PCR (Q-PCR) analyses with primers of Bcl-2, miR-15a, miR-132, miR-16 (miR-15a RT-Primer: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGAC CACAAAC, QF: GCGGCTAGCAGCACATAATGG. U6: U6QF: CTCGCTTCGGCAGCACA, U6QR: AACGCTTCACGAATTTGCGT, miR-16 RT-Primer: GTCGTATCCAGTGCAGGGTCCGA GGTATTCGCACTGGATACGACTCACAAG, miR-16QF: GCGGCTCCCTGAGACCCT AAC, miR-132 RT Primer: GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTG GATACGAC CACAAGT, miR132 QF: GCGGCAACCCGTAGATCCGAA) under standard SYBR conditions as described previously [15]. For normalization, 18S RNA was used. MiRNA expression levels in each of the 10 microdissected pancreatic cancer tissues were compared against matched benign pancreatic tissues, and each sample was assessed in triplicate for each miRNA.
Data analysis
For cell proliferation/MTT assays, data were calculated as percent of vehicle control (dimethyl sulfoxide) and expressed as a mean ± SEM of multiple experiments, with each experiment including four to six determinations, or alternatively data are presented as mean ± SEM of OD. Statistical significance was determined using Student’s t test.
Results
Gossypol acetate inhibits the proliferation of pituitary tumor cells of GH3, MMQ
We firstly investigated the effect of GAA on GH3 cell proliferation. GH3 cells were treated with GAA in a dose-dependent manner (0-200 μM), and cell proliferation after 4 d was assessed by using the MTT assay. Results (Figure 1A) show that GAA, in a dose-dependent manner, inhibited GH3 cell proliferation with significant inhibition (60%; P < 0.005) of cell proliferation being observed with 100 μM, and maximal inhibition (90.6%; P < 0.005) being observed with 200 μM GAA. To rule out cell type-specific effects of GAA on lactotroph proliferation, we examined the effects of GAA on proliferation of another rat C cell line, MMQ cells. Our results (Figure 1B) show that like GH3 cells, MMQ cells were also susceptible to the growth-inhibitory effects of GAA with significant (80.5%; P < 0.005) inhibition of cell proliferation observed with 50 μM and maximal inhibition (95.4%; P < 0.005) being observed with 200 μM GAA. These results demonstrate that MMQ cells were more sensitive to the growth-inhibitory effect of GAA at higher concentrations.
Figure 1.
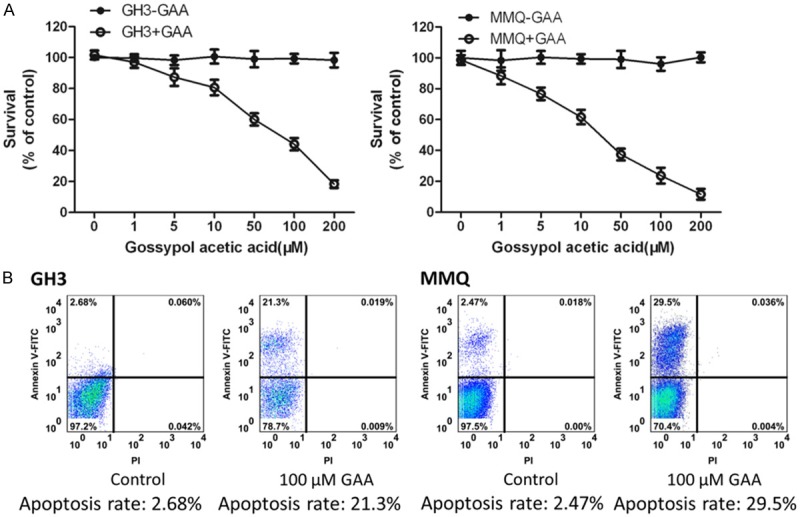
Gossypol acetate inhibits the proliferation of pituitary tumor cells of GH3, MMQ cell line). A. To see the effect of GAA concentrations on the proliferation of GH3 and MMQ. B. Apoptosis rate of GH3 and MMQ under 100 μM GAA, detecting with CK8 (the marker) by FCM.
GAA upregulate the expression of miR15a in GH3 and MMQ
We next questioned whether GAA-induced inhibition of cell proliferation was accompanied by a change in gene expression level of miR15a, since many publications have revealed that miR-15a have important roles in regulating cell proliferation [10,11,16,17]. To address this issue, GH3 and MMQ cells were treated with 100 μM of GAA for 48 h. Equal amounts of cell lysates were subjected to RNA extraction and subsequently Q-PCR was performed with the primers of miR15a, miR16, miR132. Our results (Figure 2) show that GAA caused a significant increase in the expression of miR15 in both GH3 and MMQ cells, but not miR 16 and miR132.
Figure 2.
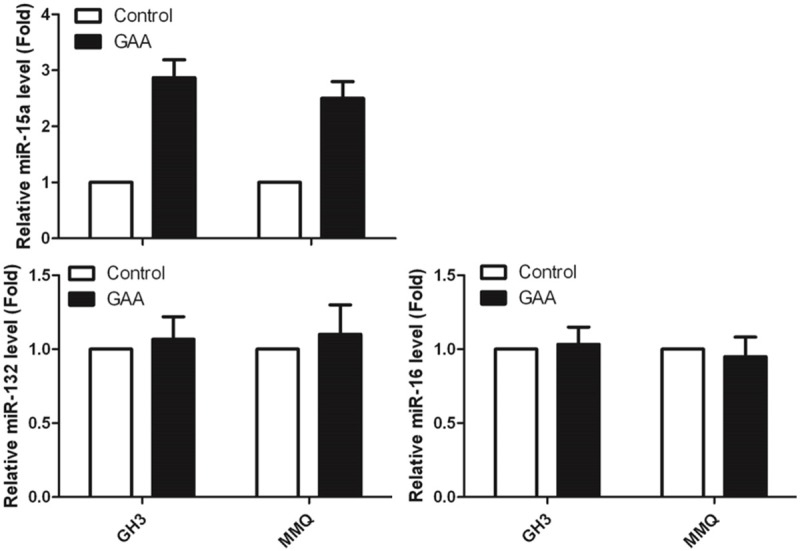
The expression level of miR15a, miR-16 and miR-132 in GH3 and MMQ.
Overexpressed miR-15a (mimics) downregulated the expression level of Bcl-2
After normalization to actin, fold change from control (arbitrary value of 1) reveals that in GH3 and MMQ cells, 100 μM GAA significantly (P < 0.05) promote miR-15a expression. We next questioned whether the GAA-induced increase in miR-15a was also accompanied by a decrease in Bcl-2, a well-known gene which has a lot of functions [8,9]. To address this issue, we generated the overexpression miR-15a cell line in both GH3 and MMQ. And, after we get the stable cell line which has overexpressed miR-15a (Figure 3A), the cell lysates from Figure 3A were subjected to RNA extraction for Q-PCR and Western blotting with anti-Bcl-2 and anti-GADPH. Our results (Figure 3B) show that GAA treatment of GH3 and MMQ cells results in decreased expression level of Bcl-2. Equal loading was confirmed by probing with total GAPDH. After normalization to total GAPDH, fold change from control (arbitrary value of 1) revealed that in GH3 and MMQ cells, overexpressed miR-15a significantly (P < 0.05) inhibited Bcl-2 expression (Figure 3C, 3D).
Figure 3.
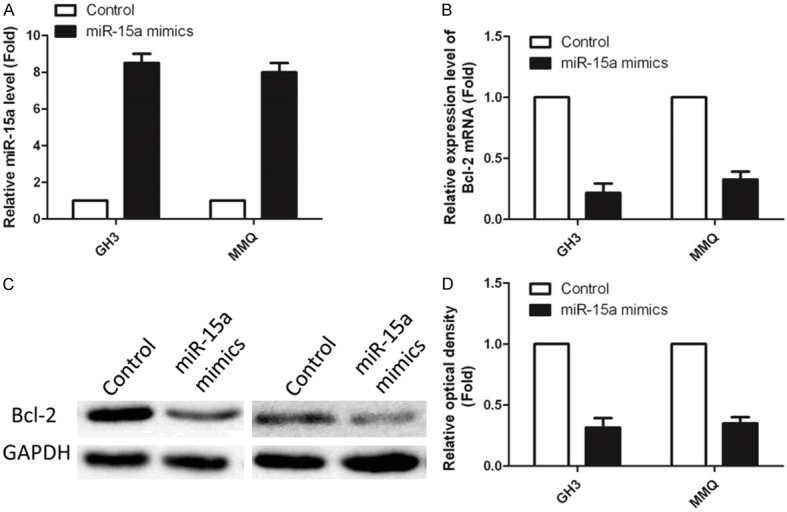
Overexpressed miR-15a (mimics) downregulated the expression level of Bcl-2. (A) Overexpression efficiency of mimics detected by Q-PCR. (B) Relative expression level of mimics in overexpression cell lines. (C) Western blotting result of Bcl-2, and relative optical density (D).
Knock down miR-15a (inhibitor) upregulate Bcl-2 and reverse the apoptosis induced by GAA
We next questioned that if the knock down miR-15a could upregulate Bcl-2 and thus reverse the apoptosis induced by GAA. Q-PCR was performed to see the efficiency of knock down in both of GH3 and MMQ. Comparing with the control, the addition of inhibitor which would inhibit miR-15a expression can significantly reduce the expression level of miR-15a (Figure 4A). Meanwhile, as the know down works, the cells with low expression level of miRNA, and the cells without the addition of inhibitor, were used for western blotting to detect the Bcl-2 protein level. As the data shows (Figure 4B), Bcl-2 is upregulated after the addition of inhibitor, also the apoptosis rate was decreased which indicates that the knock down miR-15a could upregulate Bcl-2 and thus reverse the apoptosis induced by GAA.
Figure 4.
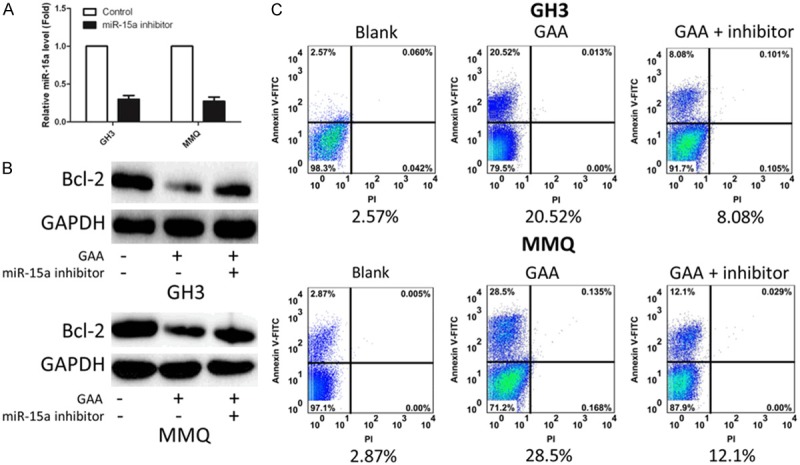
Knock down miR-15a (inhibitor) upregulate Bcl-2 and reverse the apoptosis induced by GAA. A. Q-PCR result of knock down experiment. B. Western blotting result of Bcl-2 protein level. C. Apoptosis rate detected by FCM.
Discussion
Gossypol is the active component extracted from cotton roots and seeds. It accounts for about 1.5% of the seeds in weight, mostly used as medicine in the form of Gollypol Acetate (GAA). In the late 1990s, some researchers found that GAA has obvious effect on tumors, caused a great sensation throughout the world [2-5]. After decades of clinic studies indicate that it has potent antitumor and antivirus activity and is used for treating the cancer of adrenal cortex and bladder cancer [7]. Ultrastructural study showed that GAA can cause morphological changes such as swollen mitochondria, crest fracture and so on; which will activate the endogenous pathways of apoptosis, namely the mitochondrial pathway. In our study, we found that GAA can inhibit the growth of GH3 and MMQ, two rat lactotroph cell lines (Figure 1). The reasons that we choose the two cell lines as our experimental material is that, recently, pituitary adenomas was considered to constitute a significant proportion of intracranial tumors and have several pathological effects due to excessive hormone secretion as well as visual defects due to compression of the optic chiasm. Also, prolactinomas are the most common type of pituitary adenoma, and excessive PRL secretion is a leading cause of infertility and hypogonadism. Thus, regulating lactotroph proliferation is one of therapeutic interest. Even though prolactinomas respond well to medical therapy with dopamine receptor agonists, intolerance/resistance does occur. Alternatives to medical therapy include surgery and radiation therapy. Therefore, development of alternative medical therapies is warranted. In this study we examined the role of GAA, a natural compound with proven antitumor activity and very low toxicity as determined by in vivo studies, as a potential inhibitor of pituitary tumor cell proliferation. And as we detected the inhibition of GAA on pituitary tumor cell proliferation. We next wanted to identify the mechanisms or the pathway which the GAA may take part in (Figures 3, 4). According our previous data and the information of many literatures from other groups, by using the two cell lines GH3 and MMQ, we find that the regulatory mechanism from genetic level to molecular level, that GAA really has effects on inhibit the proliferation of tumor cells by downregulating the Bcl-2 via upregulate miR15a, thus we are sure that, at least partly, GAA can induce the apoptosis of pituitary tumor cell.
In conclusion, we have shown for the first time that how GAA could inhibit proliferation, induces apoptosis, and abolishes congenic ability of pituitary tumor cells. In addition, we show that GAA-induced decrease in cell proliferation is associated with decreased expression of Bcl-2 and increased miR-15a. Based on this, we propose developing GAA as a novel therapeutic tool in the management of pituitary tumors.
Disclosure of conflict of interest
None.
References
- 1.Qian SZ, Wang ZG. Gossypol: a potential antifertility agent for males. Annu Rev Pharmacol Toxicol. 1984;24:329–360. doi: 10.1146/annurev.pa.24.040184.001553. [DOI] [PubMed] [Google Scholar]
- 2.Gadelha IC, de Macedo MF, Oloris SC, Melo MM, Soto-Blanco B. Gossypol promotes degeneration of ovarian follicles in rats. ScientificWorldJournal. 2014;2014:986184. doi: 10.1155/2014/986184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng JS, Liu CP, Lo YK, Chou KJ, Lin MC, Su W, Law YP, Chou KJ, Wang JL, Chen WC, Jan CR. Gossypol, a component in cottonseed, induced increases in cytosolic Ca2+ levels in Chang liver cells. Toxicon. 2002:851–856. doi: 10.1016/s0041-0101(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 4.Qian SZ. Gossypol: a potential antifertility agent for males. Ann Rev Pharmacol Toxicol. 1984;24:329–60. doi: 10.1146/annurev.pa.24.040184.001553. [DOI] [PubMed] [Google Scholar]
- 5.Zbidah M, Lupescu A, Shaik N, Lang F. Gossypol-induced suicidal erythrocyte death. Toxicology. 2012;302:101–105. doi: 10.1016/j.tox.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Duchen MR. Mitochondria and calcium: fromcell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin J, Wu Y, Yang D, Zhao Y. Induction of apoptosis and antitumor effects of a small molecule inhibitor of Bcl-2 and Bcl-xl, gossypol acetate, in multiple myeloma in vitro and in vivo. Oncol Rep. 2013;30:731–738. doi: 10.3892/or.2013.2489. [DOI] [PubMed] [Google Scholar]
- 8.Frenzel A, Grespi F, Chmelewskij W, Villunger A. Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis. 2009;14:584–596. doi: 10.1007/s10495-008-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Belguise K, Kersual N, Kirsch KH, Mineva ND, Galtier F, Chalbos D, Sonenshein GE. Oestrogen signalling inhibits invasive phenotype by repressing RelB and its target BCL2. Nat Cell Biol. 2007;9:470–478. doi: 10.1038/ncb1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garzon R, Calin GA, Croce CM. MicroRNAs in Cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 12.Wang V, Wu W. MicroRNA-Based Therapeutics for Cancer. BioDrugs. 2009;23:15–23. doi: 10.2165/00063030-200923010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Tashjian AH Jr, Yasumura Y, Levine L, Sato GH, Parker ML. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968;82:342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- 14.Fossum S, Gautvik KM. Stereological and biochemical analysis of prolactin and growth hormone secreting rat pituitary cells in culture. Stereology combined with non-parametrical statistics. Cell Tissue Res. 1977;184:169–178. doi: 10.1007/BF00223066. [DOI] [PubMed] [Google Scholar]
- 15.Shinden Y, Akiyoshi S, Ueo H, Nambara S, Saito T, Komatsu H, Ueda M, Hirata H, Sakimura S, Uchi R, Takano Y, Iguchi T, Eguchi H, Sugimachi K, Kijima Y, Ueo H, Natsugoe S, Mimori K. Diminished expression of MiR-15a is an independent prognostic marker for breast cancer cases. Anticancer Res. 2015;35:123–127. [PubMed] [Google Scholar]
- 16.Garzon R, Pichiorri F, Palumbo T, Visentini M, Aqeilan R, Cimmino A, Wang H, Sun H, Volinia S, Alder H, Calin GA, Liu CG, Andreeff M, Croce CM. MicroRNA gene expression during retinoic acid-induced differentiation of human acute promyelocytic leukemia. Oncogene. 2007;26:4148–4157. doi: 10.1038/sj.onc.1210186. [DOI] [PubMed] [Google Scholar]
- 17.Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356:568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]


