Abstract
Objective: To compare induced surgical menopause in rat models following hysterectomy with ovarian preservation, unilateral or bilateral oophorectomy, versus control. Secondary objective was evaluation of certain physiological changes in the animal following the induced menopause. Design: A prospective case control study. Setting: University Research Centre. Methodology: 80 female rats were divided into four groups (n=20). HG: hysterectomy with ovarian preservation, UOG: unilateral oophorectomy, BOG: bilateral oophorectomy and CG: control rats. Blood tests were done at day 0, one week and one month post-procedure for hormonal profile including FSH and E2, and lipid profile including cholesterol, LDL and HDL. Behavioral tests (Learning and memory tests) were also done. Results: Menopause was successfully induced by the three used surgical methods. After one week, no significant difference in FSH level between CG and HG. But its level was significantly increased in BOG and UOG. E2 level was significantly decreased in HG, UOG and BOG in comparison to CG. Its level in BOG was significantly lower than that of UOG and HG. Cholesterol level was significantly higher in HG, UOG and BOG in comparison to CG, also its level was significantly increased in UOG and BOG in comparison to HG (P<0.001). Long term memory was affected in BOG and UOG, one week and one month post-menopausal induction in comparison to the control. Conclusion: surgical menopause, induced by hysterectomy alone, unilateral, or bilateral oophorectomy has a negative impact on reproductive hormonal function, as well as cognitive & cardiovascular integrity. We suggest a possibility of early ovarian failure after hysterectomy alone or with unilateral oophorectomy.
Keywords: Menopause, memory, rat model
Introduction
The females’ reproductive system experience phases of aging, which may present at a point of time as menopause. This presents variably, with various systems’ involvement. The endocrinological changes are prominent with menopause, namely with increased serum follicle stimulating hormone (FSH) and decreased serum estradiol [1]. Typically, natural menopause sets in at a median age between 48 and 52 years, usually with a slow onset of menopausal symptoms of account of gradual decline in ovarian function after this age [2]. However, Surgically-induced menopause would follow bilateral oophorectomy, or sometimes unilateral oophorectomy. Unlike the bilateral procedure, unilateral oophorectomy is usually undertaken for a medical indication and less commonly in conjunction with hysterectomy. This indication might include prophylaxis in carriers of a high-risk gene mutation for ovarian cancer. Prophylactic oophorectomy without a clear and solid indication would have a negative impact on long term survival rates [3].
Hysterectomy procedures with ovarian preservation were also reported to induce menopause, possibly by disturbing the ovarian blood flow [4-6].
Because of human studies are being poorly feasible, experimental animal model can be used in medical testing to resemble events occurring among the human being as their genetic, biological and behavior characteristics mimic those of human [7]. Rodents pass through hormonal fluctuations occurring in middle age, similar to those in women. However, rodents enter an estropause rather than a true menopause. Rodents show irregular acyclicity at middle age (nine months to one year), after which, they enter a state of persistent estrus, with complete stoppage of reproductive cycles [8,9].
Our aim in this study was to compare induced surgical menopause in rat models following hysterectomy with ovarian preservation, unilateral or bilateral oophorectomy, versus control.Secondary objective was evaluation of certain physiological changes in the animal following the induced menopause.
Materials and methods
Prospective study was carried out on 80 adult female Sprague_Dawley rats, which were housed in an animal facility at Faculty of Medicine, Alexandria University. A minimum temperature of 10°C in winter and maximum of 35°C in the summer were maintained. A period of 12-14 hours of daylight was provided. All experimental procedures were carried out based on the ethical guidelines for care and use of laboratory animals of Alexandria University. Rats were divided into four groups (n=20). HG: rats undergone hysterectomy with ovarian preservation, UOG: rats undergone unilateral oophorectomy, BOG: rats undergone bilateral oophorectomy and CG: control sham operated female rats.
Anesthesia and surgery: On the day of the experiment, rats were anesthetized with ketamine hydrochloride (60 mg/kg IP) and xylazine hydrochloride (10 mg/kg IP). The abdominal skin was shaved, and antisepsis was performed, with a 10% povidone-iodine solution before the surgery. Using a sterile technique, a 3 cm pfenstien incision was performed and the procedures were carried out accordingly in each group. The incision was closed in two layers by 2/0 polyglactic sutures.
Blood samples: Whole-blood samples (about 2 ml) were collected from either the tail vein at day 0 and after one week or from inferior vena cava after one month (about 5 ml). Throughout our study 11, rats died. They were dissected immediately to discover the cause of death, most of which showed internal hemorrhage and pus collection on exploration.
Blood tests at day 0, one week and one month post- procedure: Serum hormonal profile including FSH and E2 using ELISA technique [10], Lipid profile including cholesterol, low density lipoprotein (LDL) and high density lipoprotein (HD) L using colorimetric method [11].
Behavioral tests (Learning and memory tests) one week and one month post-procedure: Novel object recognition test (NOR) is used to evaluate short term recognition memory. The test is based on the tendency of rodents to spend more time exploring a novel object than a familiar one spontaneously [12]. The time spent exploring each object was recorded and the results were analyzed by calculating the Recognition Index (RI), the time spent investigating the novel object (TN) relative to time spent investigating both familiar and novel object collectively (TN+TF). [RI= TN/ (TN+TF)], it is the main index of retention [12].
Passive Avoidance task (PAT) is a fear-aggravated test used to evaluate learning and long term memory. Rats learn to avoid an environment in which an aversive stimulus (a foot-shock) was previously delivered. Initial latency and retention latency (24 hours later) to pass the gate in order to avoid the stimulus is used as an indicator of learning and memory [13].
Statistical analysis
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (14) The distributions of quantitative variables were tested for normality using Kolmogorov-Smirnov test, Shapiro-Wilk test and D’Agstino test. Also Histogram and QQ plot were used for vision test. For normally distributed data, comparison between the studied groups were analyzed using F-test (ANOVA) and Post Hoc test (Scheffe), comparison between different periods using ANOVA with repeated measures and Post Hoc test was assessed using Bonferroni adjusted. For abnormally distributed data, Kruskal Wallis test was used to compare between different groups and pair wise comparison was assessed using Mann-Whitney test. To compare between the different periods Friedman test was applied and Wilcoxon signed ranks test. Significance test results are quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level.
Results
FSH and E2
Menopause was successfully induced by the three used surgical methods as shown by FSH and E2 hormonal levels measured at day zero, one week and one month following the surgical procedure in all experimental groups.
Results of BOG, UOG and HG revealed an increase in the FSH and a decrease in the E2 levels with progression of time, in a way that the levels varied significantly after one week in comparison to day zero and after one month in comparison to after one week in the above mentioned pattern. On contrary, the levels of such hormones showed no significant difference across time in the sham operated control group (Figure 1).
Figure 1.
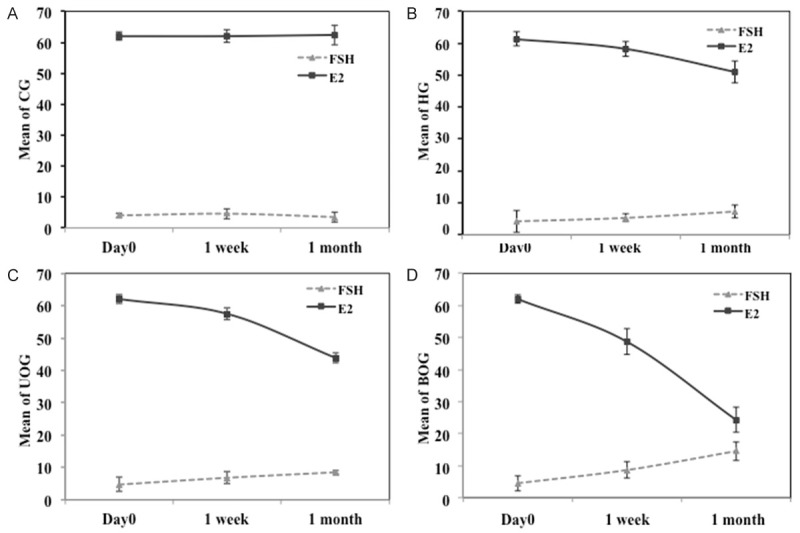
FSH and E2 levels across time shown as Mean ± SD; Day 0, same day of surgical procedure, 1 week and 1 month past-operative. A. CG control sham operated group; Day 0, FSH (4.0±0.8) E2 (62.0±1.3) after 1 week, FSH (4.6±1.7) E2 (62.0±2) after 1 month, FSH (3.5±1.6) E2 (62.4±3.2). B. HG hysterectomy group; Day 0, FSH (4.2±3.4) E2 (61.4±2.3) after 1 week, FSH (5.3±1.2) E2 (58.2±2.3) after 1 month, FSH (7.3±2.0) E2 (51.0±3.4). C. UOG unilateral oophorectomy group; Day 0, FSH (4.7±2.2) E2 (62.0±1.3) after 1 week, FSH (6.8± 1.8) E2 (57.5±1.8) after 1 month, FSH (8.5±0.6) E2 (43.9±1.6). D. BOG bilateral oophorectomy group; Day 0, FSH (4.5±2.4) E2 (62.0±1.3) after 1 week, FSH (8.6±2.6) E2 (48.7±4) after 1 month, FSH (14.5±2.9) E2 (24.3±3.9).
Comparison between different groups was done to evaluate the effectiveness of the different surgical methods of premature menopausal induction, and the results revealed that; after one week, no significant difference in FSH level between CG and HG. But its level was significantly increased in BOG and UOG in comparison to the two former groups, in a way that its level in BOG was significantly higher than that of UOG. Regarding E2 level, it was significantly decreased in HG, UOG and BOG in comparison to CG. Its level in BOG was significantly lower than that of UOG and HG (P<0.001) (Table 1).
Table 1.
Comparison between the studied groups according to FSH and E2 levels
| FSH | CG | HG | UOG | BOG | P |
|
| |||||
| Day 0 | N=20 | N=20 | N=20 | N=20 | 0.310 |
| Mean ± SD | 4.0±0.8 | 4.2±3.4 | 4.7±2.2 | 4.5±2.4 | |
| Median (min, max) | 3.9 (2.9, 5.5) | 2.5 (0.7, 14.2) | 4.69 (1.1, 10.0) | 3.3 (2.5, 11.4) | |
| After 1 week | N=20 | N=19 | N=19 | N=18 | <0.001* |
| Mean ± SD | 4.6±1.7 | 5.3±1.2 | 6.8±1.8 | 8.6±2.6 | |
| Median (min, max) | 5.0 | 5.2 | 6.7 | 7.9 | |
| Sig. bet. Groups | CG-UOG**, CG-BOG***, HG-UOG**, HG-BOG***, UOG-BOG** | ||||
| After 1 month | N=20 | N=18 | N=18 | N=17 | <0.001* |
| Mean ± SD | 3.5±1.6 | 7.3±2.0 | 8.5±0.6 | 14.5±2.9 | |
| Median (min, max) | 3.5 | 8.2 | 8.5 | 14.6 | |
| Sig. bet. Groups | CG-HG***, CG-UOG***, CG-BOG***, HG-UOG**, HG-BOG***, UOG-BOG*** | ||||
|
| |||||
| E2 | CG | HG | UOG | BOG | P |
|
| |||||
| Day 0 | N=20 | N=20 | N=20 | N=20 | 0.580 |
| Mean ± SD | 62.0±1.3 | 61.4±2.3 | 62.0±1.3 | 62.0±1.3 | |
| Median (min, max) | 62.4 (59, 63.3) | 61.5 | 62.4 | 62.4 | |
| After 1 week | N=20 | N=19 | N=19 | N=18 | <0.001* |
| Mean ± SD | 62.0±2.0 | 58.2±2.3 | 57.5±1.8 | 48.7±4.0 | |
| Median (min, max) | 62.0 (58, 65) | 58.2 (53, 62) | 58.0 (54, 60) | 49.7 (39, 55) | |
| Sig. bet. Groups | CG-HG***, CG-UOG***, CG-BOG***, HG-BOG***, UOG-BOG*** | ||||
| After 1 month | N=20 | N=18 | N=18 | N=17 | <0.001* |
| Mean ± SD | 62.4±3.2 | 51.0±3.4 | 43.9±1.6 | 24.3±3.9 | |
| Median (min, max) | 62.3 (57.9, 67.7) | 50.0 (45, 58) | 43.7 (41.5, 46.4) | 24.5 (17.6, 30) | |
| Sig. bet. Groups | CG-HG***, CG-UOG***, CG-BOG***, HG-UOG***, HG-BOG***, UOG-BOG*** | ||||
F test (ANOVA) was used, Sig. bet. grps was done using Post Hoc Test (Scheffe).
Statistically significant at P≤0.05;
Statistically significant at P≤0.01;
Statistically significant at P≤0.001.
After one month, FSH level was significantly increased while the E2 level was significantly decreased in the three groups in comparison to CG. In comparing different experimental groups, FSH level was significantly higher and E2 level was significantly lower in BOG and UOG in comparison to HG, similar to results after one week. The increase in FSH and decrease in E2 levels were significantly more in BOG than in UOG (P<0.001) (Table 1).
Cholesterol, HDL and LDL
After one week, there was significant increase in the cholesterol level in HG, UOG and BOG in comparison to CG, also its level was significantly increased in UOG and BOG in comparison to HG (P<0.001). While, the HDL level showed a significant decrease only in BOG in comparison to CG but not among other groups (P<0.05). In accordance the LDL level showed no significant difference among the studied groups (Table 2).
Table 2.
Comparison between the studied periods according to cholesterol, LDL and HDL
| CG | HG | UOG | BOG | P | ||
|---|---|---|---|---|---|---|
| Cholesterol | Day 0 | N=20 | N=20 | N=20 | N=20 | 0.941 |
| Mean ± SD | 106.5±13.9 | 104.6±8.5 | 105.4±7.9 | 105.7±8.6 | ||
| After 1 week | N=20 | N=19 | N=19 | N=18 | <0.001* | |
| Mean ± SD | 106.5±6.3 | 112.2±6.5 | 119.1±3.0 | 121.8±5.0 | ||
| Sig. bet. Groups | CG-HG*, CG-UOG***, CG-BOG***, HG-UOG**, HG-BOG*** | |||||
| After 1 month | N=20 | N=18 | N=18 | N=17 | <0.001* | |
| Mean ± SD | 106.3± 5.9 | 120.8± 4.1 | 129.4± 4.1 | 140.3± 6.7 | ||
| Sig. bet. Groups | CG-HG***, CG-UOG***, CG-BOG***, HG-UOG***, HG-BOG***, UOG-BOG*** | |||||
| LDL | Day 0 | N=20 | N=20 | N=20 | N=20 | 0.997 |
| Mean ± SD | 51.1±13.3 | 51.8±4.2 | 51.8±2.8 | 51.4±18.5 | ||
| After 1 week | N=20 | N=19 | N=19 | N=18 | 0.055 | |
| Mean ± SD | 51.6± 13.1 | 56.6±6.5 | 57.2±5.7 | 60.8±12.6 | ||
| After 1 month | N=20 | N=18 | N=18 | N=17 | 0.020* | |
| Mean ± SD | 51.5±12.8 | 61.3±6.1 | 67.62±28.2 | 71.7±28.7 | ||
| Sig. bet. Groups | CG-BOG* | |||||
| Day 0 | N=20 | N=20 | N=20 | N=20 | 0.996 | |
| HDL | Mean | 64.4±5.7 | 64.5±6.5 | 64.8±1.2 | 64.3±10.1 | |
| After 1 week | N=20 | N=19 | N=19 | N=18 | 0.022* | |
| Mean | 64.4±5.9 | 62.6±6.3 | 62.5±2.2 | 59.1±5.4 | ||
| Sig. bet. Groups | CG-BOG* | |||||
| After 1 month | N=20 | N=18 | N=18 | N=17 | <0.001* | |
| Mean | 64.4±5.4 | 59.5±4.5 | 57.3±5.2 | 53.6±9.5 | ||
| Sig. bet. Groups | CG-UOG*, CG-BOG*** | |||||
F test (ANOVA) was used, Sig. bet. grps was done using Post Hoc Test (Scheffe).
Statistically significant at P≤0.05;
Statistically significant at P≤0.01;
Statistically significant at P≤0.001.
After one month, results were changed regarding LDL and HDL levels. As LDL level was significantly increased in BOG in comparison to CG (P<0.05). While, HDL level in BOG and UOG was significantly decreased in comparison to CG; as such its level in BOG was significantly lower than UOG (P<0.001). On the other hand, cholesterol level remained significantly higher in the three groups in comparison to CG, as well as, in UOG and BOG in comparison to HG (P<0.001). In addition, its level in BOG was significantly higher than UOG (Table 2).
Cognitive functions
Passive avoidance test (PAT)
Long term memory was affected in BOG and UOG, one week and one month post-menopausal induction in comparison to the control group. Such affection was more announced in BOG after one month so that its retention latency was significantly decreased in comparison to UOG and HG as well (P<0.001) (Table 3). Retention latency in BOG after onemonth showed a positive correlation to the E2 level, denoting that the decrease in estradiol level coincided with long term memory affection (Figure 2). No significant difference was shown in HG in comparison to CG neither after one week nor after one month of menopausal induction.
Table 3.
Comparison between the studied groups according to retention laten
| Retention latency | CG | HG | UOG | BOG | P |
|---|---|---|---|---|---|
| After 1 week | N=10 | N=9 | N=9 | N=9 | 0.019* |
| Mean ± SD | 300.0±0.0 | 232.11±105.71 | 183.44±138.61 | 144.67±95.21 | |
| Median (min, max) | 300 (300, 300) | 300 (43, 300) | 300 (19, 300) | 100 (67, 300) | |
| Sig. bet. Groups | CG-UOG*, CG-BOG** | ||||
| After 1 month | N=10 | N=9 | N=9 | N=8 | <0.001* |
| Mean ± SD | 300.0±0.0 | 206.89±140.06 | 165.33±133.49 | 15.63±8.47 | |
| Median (min, max) | 300 (300, 300) | 300 (8, 300) | 148 (10, 300) | 14.00 (4, 30) | |
| Sig. bet. Groups | CG-UOG**, CG-BOG***, HG-BOG*, UOG-BOG** | ||||
| p1 | 1.000 | 0.713 | 0.741 | 0.001* | |
χ2: Chi square for Kruskal Wallis test. Sig. between groups was done using Mann Whitney test. p1: p value for Mann Whitney test for comparing between 1 week and 1 month in each group.
Statistically significant at P≤0.0;
Statistically significant at P≤0.01;
Statistically significant at P≤0.001.
Figure 2.
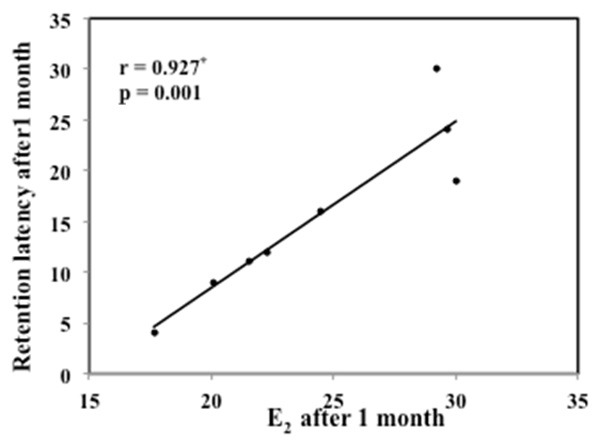
Correlation between E2 after 1 month with retention latency after 1 month in BOG. r: Pearson coefficient, *: Statistically significant at P≤0.05.
Memory affection was not progressive with time in UOG as there was no significant difference between the RL after one week and after one month. On contrary, in BOG, the latency was significantly decreased after one month in comparison to one week post-menopausal induction (P<0.001) (Table 3).
Novel object recognition test (NOR)
Same results were shown one week and one month post-menopausal induction. RI was significantly decreased in HG, UOG and BOG in comparison to CG (P<0.001). It was significantly lower in BOG in comparison to HG and UOG. Similarly, in UOG in comparison to HG (P<0.001) (Table 4).
Table 4.
Comparison between the studied groups according to recognition index %
| Recognition index % | CG | HG | UOG | BOG | P |
|---|---|---|---|---|---|
| After 1 week | <0.001* | ||||
| Mean ± SD | 85.6±5.9 | 65.7±7.4 | 53.6±5.4 | 34.0±5.1 | |
| Sig. bet. Groups | HG-UOG**, HG-BOG***, HG-CG***, UOG-BOG***, UOG-CG***, BOG-CG*** | ||||
| After 1 month | <0.001* | ||||
| Mean ± SD | 85.8±5.7 | 65.6±6.5 | 47.7±4.1 | 16.3±4.5 | |
| Sig. bet. Groups | HG-UOG***, HG-BOG***, CG-HG***, UOG-BOG***, CG-UOG***, CG-BOG*** | ||||
| p1 | 0.929 | 0.960 | 0.042* | <0.001* | |
F test (ANOVA) was used, Sig. bet. grps was done using Post Hoc Test (Scheffe).
Statistically significant at P≤0.05;
Statistically significant at P≤0.01;
Statistically significant at P≤0.001.
Short term memory affection wasn’t progressive after hysterectomy (HG) induced menopause, as results showed no significant change between the RI, one week and one month post-surgical induction. On contrary, the short term memory affection increased by time post bilateral and unilateral oophorectomy induced menopause. As, RI was significantly decreased one month in comparison to one week after either surgical induction (P<0.001) (Table 4).
Discussion
In terms of studies on human physiological functions, it is mandatory to find a model with similar physiological functions and/or patterns to conduct the research on, thus overcoming the lack of feasibility sometimes met within human subjects. This is essentially needed for research on the ovarian function. Having an ideal model is difficult; however, a rat model has been used to add knowledge on hormonal function, ovarian aging and changes related to the menopausal transition in women. It has been demonstrated that rats have multiple features and endocrine changes common with human, such as decline in follicles, irregular cycling, steroid hormone fluctuations and irregular fertility [15].
We compared surgical menopause induction in rat models following hysterectomy with or without ovarian preservation, versus control. We also followed up the animal for physiological and behavioral changes after menopause.
We confirmed FSH levels to be significantly higher after one week & one month in the bilateral oophorectomy group (BOG) followed by the unilateral oophorectomy group (UOG), then the hysterectomy group (HG), meanwhile E2 levelswere significantly lower following the same order.
The effect of hysterectomy & oophorectomy upon the hormonal profile was also reported by, Moorman PG. et al. who, in agreement with our results, concluded that women undergoing hysterectomy were at significantly increased risk for ovarian failure notably those having a unilateral oophorectomy along with hysterectomy [16]. Farquhar et al. reported similar conclusions to those of this study [17]. A recent study by Hasan Toyganözü et al. reported changes in the ovarian environment after excision of the rudimentary horn in female Wistar albino rats. They suggested that the excision of rudimentary horn could have negative effects on ipsilateral ovarian functions [18]. Derksen et al. reported that hysterectomy and endometrial ablation were associated with elevated FSH levels one year after surgery [19].
Compromised blood supply to the ovaries during hysterectomy procedures, an effect that might occur after uterine artery embolization procedures, could explain the change in ovarian function following the surgery. It could also be explained by the pathology that indicated the hysterectomy, rather that the surgery itself, as suggested by Moorman PG [16].
In our study, both cholesterol & LDL serum levels were significantly higher in BOG, UOG and to a lesser extent in HG after one week and one month. Meanwhile, HDL serum levels were significantly lower in BOG, and to a lesser extent UOG and HG respectively.
Estrogen-related impact on lipids and lipoproteins’ metabolism would include a decrease in total cholesterol and LDL cholesterol levels in serum by about 5-15%, due to an increase in the liver LDL-receptors, and enhancement of LDL-catabolism and clearance [20]. A potential cardio-protective role of estrogen was suggested by William H. et al. in a large scale prospective study onwomen undergoing hysterectomy for benign disease. They concluded that bilateral oophorectomy is associated with increased mortality in women younger than 50 years who never used estrogen therapy, and that oophorectomy did not lead to a better outcome at any age group [21]. It was also suggested by Swarnalatha PK et al that most cardiac related disorders associated with menopause might be affected by the hormonal changes on the serum lipid levels, which lead them to the conclusion that estrogen may be aprotective factor [22].
Ovarian hormones affect cognition especially learning and memory functions, in both rodents and humans. The decrease of estrogen following ovariectomy or menopause could have a role in the development of cognitive impairment. Actually, estrogen enhances the encoding of information into short-term memory. Estrogen receptors were detected in the cortex of rodents [23,24]. In our study, we tested for cognition changes in rats. Learning and long term memory were tested by the passive avoidance test, results showed a significant impact in terms of retention latency after one week in BOG and UOG respectively compared to controls. HG group showed no change after one week, but significantly changed after one month, and came third in order of impact after BOG and UOG respectively. Novel object recognition showed also a short memory affection in BOG and UOG both after one week and one month. Walter A. Rocca et al. suggested an increased risk of cognitive decline and dementia in women who underwent bilateral oophorectomy before menopause. They concluded that estrogenmay have a neuroprotective effect [25]. Riley Bove et al concluded - from 2 longitudinal studies of cognitive decline- that early age at surgical menopause was associated with cognitive decline and Alzheimer disease neuropathology [26]. The effect of estrogen deprivation on cognition was probed by Kiss A et al., who examined the effects of estrogen treatment over cognition and depressive-like behaviors in female Wistar rats. They confirmed estrogen’s beneficial effect [27]. Moreover, Sarkaki A et al. reported that ovariectomy impaired spatial reference memory in Wistar rats [28].
Interestingly, a recent survey on elective bilateral oophorectomy at the time of hysterectomy in the United States, revealed that approximately one third of surgeons prefer elective oophorectomy before menopause despite the American college for Obstetricians and Gynecologists (ACOG)’s statement against it. However, most gynecologists comply with ACOG’s position recommending ovarian conservation in postmenopausal women [29].
In our study, comparison between the effects of the three procedures; namely hysterectomy, unilateral, and bilateral oophorectomy, would represent-up to our knowledge- an unprecedented report, describing different physiological effects of surgical menopause following the three procedures compared to control. The studied sample size was calculated so that, more credibility of our results would be achieved.
Conclusion
This study is among a few reports comparing the effects of surgical menopause, induced by hysterectomy alone, unilateral, or bilateral oophorectomy in female rats, on reproductive hormonal function, as well as cognitive & cardiovascular integrity. We reported the negative impact of the three procedures and warned from the possibility of early ovarian failure after hysterectomy alone or with unilateral oophorectomy.
Acknowledgements
The authors wish to thank the University of Alexandria for the unlimited support. The study was formally approved by the Ethical Committee of the Faculty of Medicine, Alexandria University.
Disclosure of conflict of interest
None.
Abbreviations
- ACOG
American college for Obstetricians and Gynecologists
- BOG
Bilateral oophorectomy group
- CG
Control group
- FSH
Follicle stimulating hormone
- HDL
High density lipoprotein
- HG
Hysterectomy group
- LDL
Low density lipoprotein
- NOR
Novel object recognition test
- PAT
Passive Avoidance task
- RI
Recognition Index
- UOG
Unilateral oophorectomy group
References
- 1.Butler L, Santoro N. The reproductive endocrinology of the menopausal transition. Steroids. 2011;76(Suppl 7):627–35. doi: 10.1016/j.steroids.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora P, Polson DW. Diagnosis and management of premature ovarian failure. The Obstet Gynaecol. 2011;13:67–72. [Google Scholar]
- 3.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. “Prophylactic oophorectomy in premenopausal women and long-term health”. Menopause Int. 2008;14(Suppl 3):111–6. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiangying H, Lili H, Yifu S. The effect of hysterectomy on ovarian blood supply and endocrine function. Climacteric. 2006;9:283–9. doi: 10.1080/13697130600865774. [DOI] [PubMed] [Google Scholar]
- 5.Lee DY, Park HJ, Kim BG, Bae DS, Yoon BK, Choi D. Change in the ovarian environment after hysterectomy as assessed by ovarian arterial blood flow indices and serum anti-Mullerian hormone levels. Eur J Obstet Gynecol Reprod Biol. 2010;151:82–5. doi: 10.1016/j.ejogrb.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 6.Chan CC, Ng EH, Ho PC. Ovarian changes after abdominal hysterectomy for benign conditions. J Soc Gynecol Investig. 2005;12:54–7. doi: 10.1016/j.jsgi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Chakraborty C, Hsu CH, Wen ZH, Lin CS, Agoramoorthy G. Zebrafish: a complete animal model for in vivo drug discovery and development. Curr Drug Metab. 2009;10:116–24. doi: 10.2174/138920009787522197. [DOI] [PubMed] [Google Scholar]
- 8.Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn MP, editor. In Handbook of models for human aging. San Diego: Academic Press and Elsevier; 2006. pp. 533–52. [Google Scholar]
- 9.Frick KM. Estrogens and age-related memory decline in rodents: What have we learned and where do we go from here? Horm Behav. 2008;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lequin R. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA) Clin Chem. 2005;51:2415–8. doi: 10.1373/clinchem.2005.051532. [DOI] [PubMed] [Google Scholar]
- 11.Housecroft CE, Constable EC. In Chemistry. 3rd edition. Switzerland: Prentice Hall; 2006. An introduction to organic, inorganic, and physical chemistry; pp. 349–53. [Google Scholar]
- 12.Gaskin S, Tardif M, Cole E, Piterkin P. Object familiarization and novel-object preference in rats. Behav Processes. 2010;83:61–71. doi: 10.1016/j.beproc.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Ishiyama T, Tokuda K, Ishibashi T, Ohno Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur J Pharmacol. 2007;572:160–70. doi: 10.1016/j.ejphar.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick LA, Feeney BC. A simple guide to IBM SPSS statistics for version 20.0. 12th edition. Belmont Calif: Wadsworth, Cengage Learning; 2013. p. 115. [Google Scholar]
- 15.Brinton RD. Translational Animal Models of Human Menopause: Challenges and Emerging Opportunities. Endocrinology. 2012;153:3571–8. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moorman PG, Myers ER, Schildkraut JM. Effect of hysterectomy with ovarian preservation on ovarian function. Obstet Gynecol. 2011;118:1271–9. doi: 10.1097/AOG.0b013e318236fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956–62. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 18.Toyganözü H, Nazik H, Narin R. Examination of the Ovarian Reserve after Generation of Unilateral Rudimentary Uterine Horns in Rats. ScientificWorldJournal. 2014;2014:918496. doi: 10.1155/2014/918496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derksen JGM, Brölmann HAM, Wiegerinck MAHM, Vader HL. The effect of hysterectomy and endometrial ablation on follicle stimulating hormone (FSH) levels up to 1 year after surgery. Maturitas. 1998;29:133–8. doi: 10.1016/s0378-5122(98)00018-8. [DOI] [PubMed] [Google Scholar]
- 20.Lemieux C, Phaneuf D, Labrie F, Giguère V. Estrogen receptor α-mediated adiposity-lowering and hypocholesterolemic actions of the selective estrogen receptor modulator acolbifene. Int J Obes (Lond) 2005;29:1236–44. doi: 10.1038/sj.ijo.0803014. [DOI] [PubMed] [Google Scholar]
- 21.William H, Michael S, Cynthia M, Jonathan S. Long-Term Mortality Associated With Oophorectomy Compared With Ovarian Conservation in the Nurses’ Health Study. Obstet Gynecol. 2013;121:709–16. doi: 10.1097/AOG.0b013e3182864350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swarnalatha PK, Ebrahim NK. A correlative study of estrogen and lipid profile in premenopausal and post-menopausal women. IJBAR. 2012;3:18–22. [Google Scholar]
- 23.Gasbarri A, Tavares MC, Rodrigues RC, Tomaz C. Estrogen, cognitive functions and emotion: an overview on humans, non-human primates and rodents in reproductive years. Rev Neurosci. 2012;23:587–606. doi: 10.1515/revneuro-2012-0051. [DOI] [PubMed] [Google Scholar]
- 24.Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–72. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 25.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, estrogen, and dementia: A 2014 update. Mol Cell Endocrinol. 2014;124:840–1. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bove R, Secor E, Chibnik LB, Barnes LL, Schneider JA, Bennett DA, De Jager PL. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology. 2014;82:222–9. doi: 10.1212/WNL.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiss A, Delattre AM, Pereira SI. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav Brain Res. 2012;227:100–8. doi: 10.1016/j.bbr.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 28.Sarkaki A, Amani R, Badavi M, Safahani M. Effect of ovariectomy on reference memory version of Morris water maze in young adult rats. Iran Biomed J. 2008;12:123–8. [PubMed] [Google Scholar]
- 29.Harmanli O, Shinnick J, Jones K, St Marie P. Obstetrician-Gynecologists’ Opinions on Elective Bilateral Oophorectomy at the Time of Hysterectomy in the United States. Menopause. 2014;21:355–60. doi: 10.1097/GME.0b013e31829fc376. [DOI] [PubMed] [Google Scholar]


