Abstract
The aim of this study was to examine the consistency of ultra performance liquid chromatography-tandem mass spectrometry (UPLC-TMS) in detecting the levels of para-arachidonic acids (PAAs) among differently processed plasma/serum samples. Ethylenediaminetetraacetic acid (EDTA)-K2, sodium citrate, heparin lithium, coagulant/separation gel, and coagulant-free vacuum blood-sampling tubes were used to collect the fasting blood samples from 15 volunteers. All blood samples were subjected to solid-phase extraction using an Oasis HLB μElution 96-well plate, and UPLC-TMS was used to detect 19 types of PAAs in the blood samples. Within the plasma samples, the concentrations of 5, 6-DHET; 11, 12-epoxyeicosatrienoic acid (EET); 5-hydroxyeicosatetraenoic acid (HETE); leukotriene B4 (LTB4); plasma thromboxane B2 (TXB2); and 12-HETE were significantly higher in the heparin lithium group than in the EDTA-K2 and sodium citrate groups. Within the serum samples, the concentration of LTB4 was significantly higher in the coagulant/separation gel group than in the coagulant-free group, while that of TXB2 was significantly higher in the coagulant-free group than in the coagulant/separation gel group. The levels of some types of PAAs in differently processed plasma/serum samples were inconsistent, and the concentrations of 5, 6-DHET; 5-HETE; 12-HETE; TXB2; and LTB4 were significantly higher in the two serum samples and the heparin lithium group than in the EDTA-K2 and sodium citrate groups.
Keywords: Ultra performance liquid chromatography-tandem mass spectrometry, para-arachidonic acid, arachidonic acid, plasma, serum
Introduction
Para-arachidonic acids (PAAs) are the oxidation products of arachidonic acids; the generation of PAAs from arachidonic acids is catalyzed by three enzymes, namely cyclooxygenases, lipoxygenases, and cytochrome P450 [1]. The metabolites of the cyclooxygenase pathway include prostaglandins and thromboxanes, while those of the lipoxygenase pathway include leukotrienes and hydroxyeicosatetraenoic acids, and those of the cytochrome P450 pathway include epo-eicosatrienoic acids and w-end hydroxyl hydroxyeicosatetraenoic acids. Among these metabolites, epo-eicosatrienoic acids can generate bihydroxyl hydroxyeicosatetraenoic acids under the catalysis of soluble epoxide hydrolase.
PAAs play an important role in maintaining cardiovascular system function and homeostasis within the human body, and disorders in the metabolism of PAAs are associated with many diseases [2]. Epoxyeicosatrienoic acids (EETs) are endogenous hyperpolarization factors that promote vasodilatation and vascular growth; abnormal metabolism of EETs is associated with various diseases, including high blood pressure, diabetes, and cancer [3]. The main biological functions of 20-hydroxyeicosatetraenoic acid (HETE) include contraction of blood vessels and promotion of angiogenesis, and aberrant metabolism of 20-HETE has been shown to play an important role in the occurrence and development of cardiovascular diseases, such as high blood pressure [4]. Therefore, epo-eicosatrienoic acids and 20-HETE have become therapeutic targets of vascular dysfunction-related diseases. For example, new antihypertensive drugs targeting 20-HETE are currently being developed [5]. Leukotrienes (LTs) are able to adjust the contraction and permeability of blood vessels.
In recent years, ultra performance liquid chromatography-tandem mass spectrometry (UPLC-TMS) had been used to develop suitable methods for detection of PAAs [6]. However, this method is limited by the production of matrix effects, affecting test results [7]. Some known components of plasma or serum samples, including phospholipids [8] and anticoagulants [9], can produce certain matrix effects in LP-MS. In addition, blood coagulation causes the release of blood platelets and other components, such as prostaglandins (PGs), thromboxane A2, 12-hydroxyeicosatetraenoic acid [10], and epo-eicosatrienoic acid [11,12].
Because of the important biological functions of PAAs inside the body, detection of PAA contents in the peripheral blood may help to predict and diagnose related diseases. Although no studies have demonstrated this effect, anticoagulants/coagulants and the blood coagulation responses within blood samples may affect the detection of PAAs. Therefore, it is necessary to explore variations in PAA contents among different types of serum/plasma samples. In this study, we selected 19 PAAs (EETs, HETEs, DHETs, LTs, and PGs) generated through the three metabolic pathways of arachidonic acids and used UPLC-TMS to determine the concentrations of the above 19 PAAs in five types of blood samples that were processed with ethylenediaminetetraacetic acid (EDTA)-K2, sodium citrate, heparin lithium, coagulant/separation gel, or coagulant-free medium with the objective to analyze the consistency of PAA concentrations among the five types of blood samples.
Materials and methods
Collection of blood samples
Fifteen healthy volunteers, including 5 men and 10 women (ages 21-30 years), were included in this study. Five fasting blood samples were collected from each volunteer into EDTA-K2 anticoagulant tubes, sodium citrate anticoagulant tubes, heparin lithium anticoagulant tubes, coagulant/separation gel tubes, or coagulant-free serum collection tubes (purchased from Kangkang Co., China). All blood samples were centrifuged at 3000 rpm for 10 min within 2 h of collection, the plasma and serum were separated and stored in EP tubes at -80°C. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Shanghai First People’s Hospital. Written informed consent was obtained from all participants.
Standards and internal standards
Nineteen types of PAA standards and five types of internal standards were purchased from Cayman (USA). The standards included 5, 6-DHET; 8- or 9-DHET; 11, 12-DHET; 14, 15-DHET; 5, 6-EET; 8- or 9-EET; 11, 12-EET; 14, 15-EET; 5-HETE; 8-HETE; 12-HETE; 15-HETE; 20-HETE; 6-KETO-PGF1α; TXB2; LTB4; LTC4; LTD4; and LTE4. The internal standards were D4-PGE; D4-LTB4; D11-11, 12-EET; D11-14, 15-DHET; and D8-15-HETE.
Sample preparation
For sample preparation, 400-µL blood sample was collected from each of the experimental groups, and 400 µL of 4% phosphoric acid solution and 10 μL of internal standard solution (D4-PGE; D4-LTB4; D11-11, 12-EET; or D11-14, 15-DHET at 100 ng/mL or D8-15-HETE at 2 µg/mL) were then added. Samples were mixed by vortexing, and an Oasis HLB µElution 96-well plate (Waters, USA) was used for SPE of blood samples. The elution processes were as follows: 1) activation, 200 µL methanol was added at each time to each well, a total of two times; 2) balancing, 200 µL ultrapure water was added at each time to each well, a total of three times; 3) sample loading, 700 µL of acidified samples was added to each well; 4) first rinsing, 200 µL of 5% ammonia solution was added to each well; 5) second rinsing, 200 µL methanol/water solution (70/30, v/v) was added to each well; and 6) elution, 25 µL acetonitrile/isopropanol (65/35, v/v) containing 2% formic acid was added at each time to each well, a total of two times. After the elution, 50 µL water was used to dilute the sample, and a total of 100 µL sample was then loaded into the machine for detection.
Sample detection
An ACQUITY UPLC Xevo TQ MS (Waters) with a CORTERS UPLC chromatography column (C18 1.6 μm, 2.1 × 100 mm; Waters) was used for detection of PAAs in the prepared samples. The mobile phase was 0.1% formic acid (A) (Sigma, St. Louis, MO, USA) and acetonitrile (B) (Sigma), and the elution gradient was as follows: 0-2 min, phase A from 70% to 30%; 2-5 min, phase A from 70% to 0%; 5-6 min, phase A at 0%; and 6-8 min phase A from 0% to 80%. The flow speed was 0.35 mL/min, and the sample volume was 5 μL. The mass spectrometer was a Xevo TQ-S, and the working conditions were as follows: ESI-, capillary: 2.5 kV, source temperature: 150°C, desolvation temperature: 550°C, desolvation gas flow: 800 L/h, cone gas flow: 150 L/h. The sample data were analyzed with a MassLynx4.1 workstation.
Statistical analysis
SPSS20.0 software was used for statistical analysis, and GraphPad Prism 5.0 was used for preparation of graphics. The experiment was divided into five groups; data among serum groups exhibited homogeneity of variance and were therefore analyzed using paired t-tests. Because the data between the plasma and serum groups and among plasma groups did not exhibit homogeneity of variance, statistical analysis was carried out using the randomized block method for Friedman M tests. When multiple related samples exhibited significant differences, the q test was performed for intergroup comparisons. Data analyzed using analysis of variance were expressed as the mean ± standard deviation, while data analyzed using Friedman M tests were expressed as the median (minimum and maximum). Differences with P values of less than 0.05 were considered statistically significant.
Results
Comparison of PAAs between the plasma and serum groups
The concentrations of 19 PAA metabolites in plasma samples from the EDTA-K2, sodium citrate, and heparin lithium groups and in serum samples from the coagulant/separation gel and blank tube groups were detected. Significant differences in the concentrations of 5, 6-DHET; 11, 12-EET; 14, 15-DHET; 5-HETE; 12-HETE; 15-HETE; TXB2; and LTB4 were observed between the plasma and serum groups. The q test was then performed to analyze intergroup differences. The following results were found. First, 5, 6-DHET levels were significantly higher in serum samples from the coagulant/separation gel and coagulant-free groups than in serum samples from the EDTA-K2 and sodium citrate groups. Additionally, the concentrations of 14, 15-DHET; 5-HETE; and LTB4 were significantly higher in serum samples from the coagulant/separation gel and coagulant-free groups than in the other three plasma groups. The concentration of 11, 12-EET was significantly increased in plasma samples from the heparin lithium group compared with that in serum samples from the coagulant/separation gel and coagulant-free groups, and the concentration of 11, 12-EET was significantly higher in serum samples from the coagulant-free group than in plasma samples from the EDTA-K2 group. Furthermore, the concentration of 15-HETE was significantly higher in serum samples from the coagulant-free group than in plasma samples from the EDTA-K2 and sodium citrate groups, and the concentration of 12-HETE was significantly higher in serum samples from the coagulant/separation gel and coagulant-free serum groups than in plasma samples from the EDTA-K2 and sodium citrate plasma groups. Finally, the concentration of TXB2 was significantly higher in serum samples from the coagulant-free group than in the other three plasma groups, and that of TXB2 was significantly higher in serum samples from the coagulant/separation gel group than in plasma samples from the EDTA-K2 and sodium citrate groups. These results are shown in Figure 1.
Figure 1.
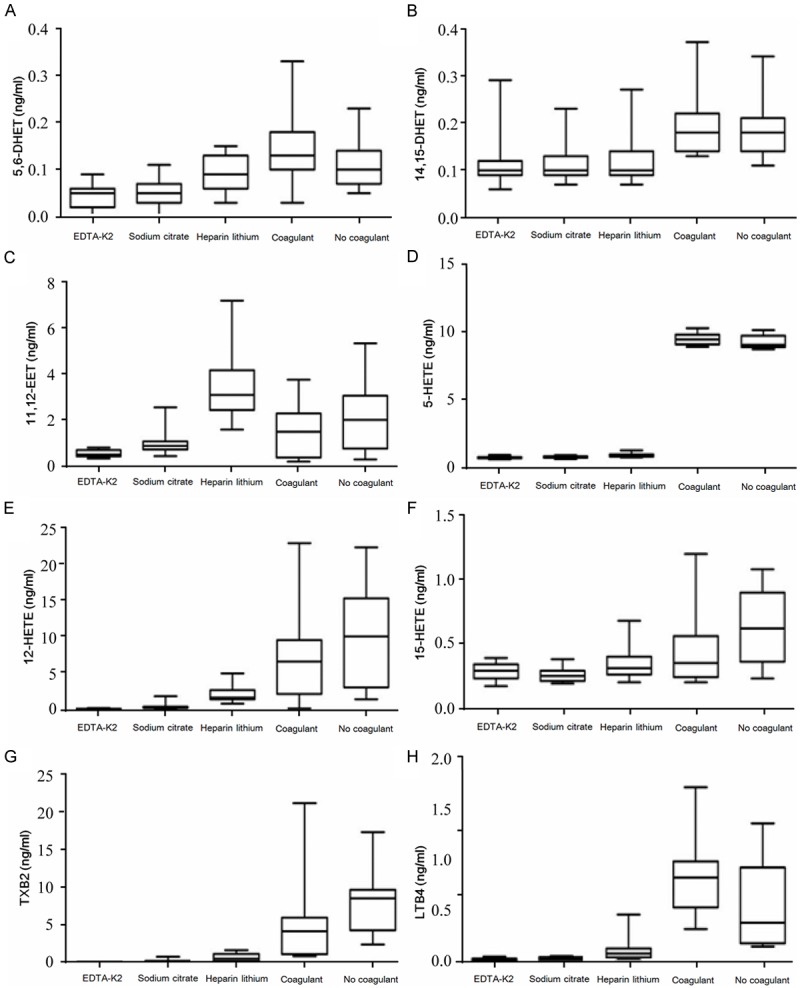
Comparison of PAAs that had the statistically significant difference between the plasma group and the serum group. A. 5, 6-DHET, B. 14, 15-DHET, C. 11, 12-EET, D. 5-HETE, E. 12-HETE, F. 15-HETE, G. TXB2, H. LTB4.
Comparison of PAAs among the plasma groups
Nineteen PAAs were detected in plasma samples from the EDTA-K2, sodium citrate, and heparin lithium groups. The results revealed that the concentrations of 5, 6-DHET; 11, 12-EET; 5-HETE; LTB4; TXB2; and 12-HETE were significantly increased in plasma samples from the heparin lithium group compared with those in plasma samples from the EDTA-K2 and sodium citrate groups. The specific results are shown in Figure 2.
Figure 2.
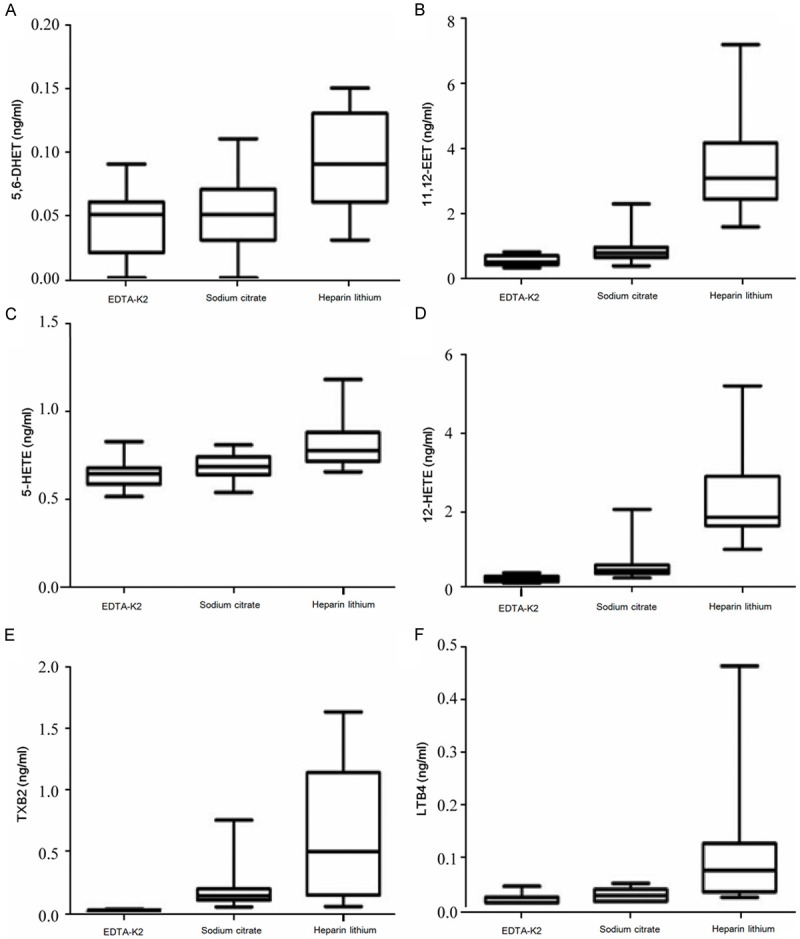
Comparison of PAAs that had the statistically significant difference among the plasma groups. A. 5, 6-DHET, B. 11, 12-EET, C. 5-HETE, D. 12-HETE, E. TXB2, F. LTB4.
Comparison of PAAs among the serum groups
Nineteen PAAs were detected in serum samples from the coagulant/separation gel and coagulant-free groups. The results revealed that the concentration of LTB4 was significantly higher in serum samples from the coagulant/separation gel serum group than in serum samples from the coagulant-free group. Additionally, the concentration of TXB2 was significantly higher in serum samples from the coagulant-free group than in serum samples from the coagulant/separation gel group. The specific results are shown in Figure 3.
Figure 3.
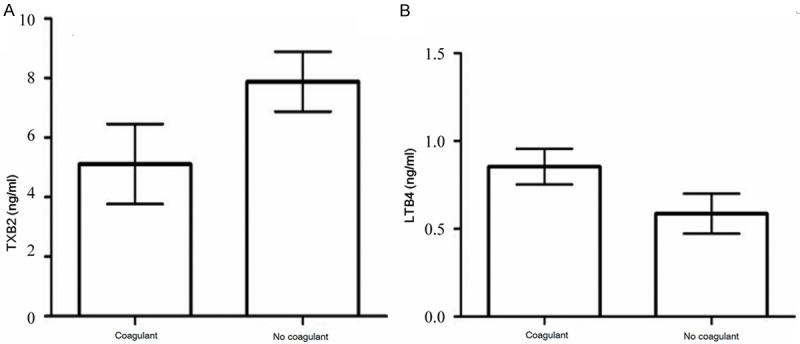
Comparison of PAAs that had the statistically significant difference among the serum groups. A. TXB2, B. LTB4.
Discussion
In recent years, the important roles of PAAs in the occurrence and development of cardiovascular diseases and cancers have received increasing attention. Detection of PAAs in blood samples may facilitate the prediction and diagnosis of related diseases. However, clinical blood types vary, and different anticoagulants, coagulants, and blood components within collection tubes can easily result in the production of matrix effects in LC-TMS. Additionally, blood coagulation is also associated with changes in the composition of PAAs; thus, these factors are likely to cause interference with the measurement of PAAs. Therefore, analysis of the consistency of PAA measurements among differently treated plasma/serum samples may provide an experimental basis for the selection of blood sample types during clinical tests and research. In this study, we used UPLC-TMS to determine the concentrations of 19 PAA metabolites, generated from the three metabolic pathways, within plasma samples collected in EDTA-K2, sodium citrate, or heparin lithium and serum samples collected in coagulant/separation gel and coagulant-free tubes.
We found that the concentrations of 5, 6-DHET; 11, 12-EET; 14, 15-DHET; 5-HETE; 12-HETE; 15-HETE; TXB2; and LTB4 were significantly different between plasma and serum samples. Indeed, the concentrations of 5, 6-DHET; 14, 15-DHET; 5-HETE; 12-HETE; TXB2; and LTB4 were significantly higher in the two serum samples than in plasma samples from the EDTA-K2 and sodium citrate groups. Among these components, 5-HETE exhibited the largest increase in concentration. Thus, our data showed that detection of PAAs in differently processed plasma/serum samples using UPLC-TMS yielded inconsistent results. While the exact reason for this inconsistency was unclear, the matrix effects and blood coagulation responses may be involved.
The generation of matrix effects by co-eluted components within samples during LC-TMS analysis is a common technical problem associated with atmospheric pressure ionization (API) [13]. In 1993, Tang and Kebarle [14] discovered matrix effects, including ion inhibition or enhancement, during LC-TMS detection [15]. While the specific mechanisms mediating these matrix effects are not fully clear, Trufelli et al [16] showed that the interfering materials causing the matrix effects could be divided into two categories. The first category was exogenous substances; these types of materials did not exist in the sample matrix, but were derived from the external environment, e.g., materials from the sample container, ion pair reagents, organic acids, and buffer, during the experimental process. The second category was endogenous substances, namely, substances derived from the matrix of the analytic targets and remaining inside the extracted species, including salt, polymer materials, surface active substances, organic molecules, and metabolites with structures similar to those of the target analyte. In addition to matrix effects, blood coagulation responses may also affect the results of PAA determination among differently processed samples. Several studies have shown that blood coagulation could cause changes in the synthesis of many types of arachidonic acids [10-12,17,18]. Based on the matrix effects and physiological mechanisms of blood coagulation, the factors affecting the results of PAA measurement among differently processed samples could be divided into three categories: 1) components of plasma and serum, such as phospholipids, proteins, and salts; 2) anticoagulants and coagulant/separation gels; and 3) blood coagulation responses.
DHETs are rapidly produced metabolites of EETs, generated by the soluble epoxide hydrolase (sEH) [19]. DHETs are stable within blood, and DHET contents are correlated with EET contents. However, no studies have reported comparisons of DHETs and EETs in plasma and serum samples. Studies have shown that EETs inhibit platelet aggregation; when blood coagulates, the Ca2+ contents inside platelets may increase significantly, thus promoting the hydrolysis of arachidonic acids from membrane phospholipids and generating EETs through a series of enzymatic reactions [11,12]. These data indicate that blood coagulation may facilitate the release of EETs and accelerate the generation of DHETs; therefore, the contents of EETs and DHETs in serum would be higher than those in plasma. This may explain the observations that the 14, 15-DHET content was significantly higher in the serum group than in the plasma group, the 5, 6-DHET content was significantly higher in the serum group than in plasma samples from the EDTA-K2 and sodium citrate groups, and the 11, 12-EET content was significantly higher in serum samples than in plasma samples from the EDTA-K2 group. During the blood coagulation process, TXB2, 12-HETE [10], and 15-HETE [18,20] are associated with platelet aggregation [18]. These three metabolites promote platelet aggregation, and activation of platelets may increase the synthesis of these three metabolites, explaining the increases in these three metabolites in serum samples compared with plasma samples. Although no comparisons of TXB2, 12-HETE, and 15-HETE between plasma and serum have been reported, we found that TXB2 content was significantly higher in serum samples from the anticoagulant-free group than in plasma samples, and that 12-HETE in serum samples, TXB2 in serum samples from the coagulant/separation gel group, and 15-HETE in serum samples from the anticoagulant-free group were significantly higher than those in plasma samples from the EDTA-K2 and sodium citrate groups. In addition, studies have shown that thrombin can activate 5-lipoxygenase on the surface of white blood cells [21], increasing the synthesis of LTB4 and 5-HETE; this may cause elevations in the levels of these two components in serum compared with those in plasma. No studies have examined differences in 5-HETE levels between plasma and serum, and comparative studies of LTB4 levels in plasma and serum have not provided consistent results. Some studies have shown that levels of LTB4 are higher in serum than in plasma, while some studies have shown that there are no obvious differences in LTB4 levels between plasma and serum [22]. In this study, 5-HETE and LTB4 levels were significantly higher in serum samples than in plasma samples, supporting that thrombin activates 5-lipoxygenase on the surface of white blood cells during blood coagulation.
In this study, we found differences in PAAs among plasma samples collected in heparin lithium, EDTA-K2, and sodium citrate; among the PAAs examined, 12-HETE, 15-HETE, and TXB2 levels did not differ significantly compared with those in serum samples. In contrast, 11, 12-EET concentrations were significantly higher in plasma samples than in serum samples. To date, no reports have described the matrix effects of anticoagulants on PAAs. However, Mei et al [9] studied the matrix effects of heparin lithium, heparin sodium, and EDTA-Na2 on eight drugs (CPMD1-8) in the plasma; the results showed that the heparin lithium enhances the matrix effects of CPMD1 in plasma samples and that this effect is increased with increased concentrations, further enhancing the matrix effects of CPMD2 at high concentrations. Chen and Li [23] reported that lithium enhances the ionization efficiency of polyethylene glycol (PEG) and further promotes the pyrolysis of PEG through low energy-collision induction. Thus, when using UPLC-TMS for detection of PAAs, heparin lithium may enhance the matrix effects of 12-HETE; 15-HETE; TXB2; and 11, 12-EET, thus resulting in an apparent increase in concentrations of these components.
In this study, we further analyzed the consistency of PAAs between plasma and serum groups. The results showed that among the three plasma groups, the contents of 5, 6-DHET; 11, 12-EET; 5-HETE; 12-HETE; LTB4; and TXB2 were significantly higher in the heparin lithium group than in the EDTA-K2 and sodium citrate groups. Additionally, in serum samples, the contents of LTB4 were significantly higher in the coagulant/separation gel group than in the coagulant-free group, while the contents of TXB2 were significantly lower in the coagulant/separation gel group than in the coagulant-free group. Because heparin lithium has been shown to enhance matrix effects, heparin lithium may affect the levels of 5, 6-DHET; 11, 12-EET; 5-HETE; 12 HETE; LTB4; and TXB2 in plasma samples, resulting in an apparent increase in samples collected in heparin lithium. In addition, the coagulant/separation gel may also result in matrix effects during measurement of LTB4 and TXB2 in serum. The coagulant/separation gel blood collection tubes used in this study are commonly used serum sample collection tubes in the clinical setting; the main components of the separating gel are silicone rubber, macromolecular hydrocarbons, and hydrophobic adhesive, while the coagulant is mainly comprised of Sio2. No reports have described the matrix effects of coagulant/separation gel components during LC-TMS detection. SiO2 is stable and insoluble in water; therefore, it is unlikely that SiO2 causes major matrix effects. However, in this experiment, we used an ordinary centrifuge, and the heat generated during the centrifugation at room temperature could lead to slight mixing of the separation gel and serum sample. Thus, some macromolecular polymers may enter the LC-TMS system and interfere with the ionization process. From these data, we can infer that the components of the separation gel may have affected the measurement of LTB4 and TXB2 in serum during LC-TMS detection.
Summary
In this study, we used UPLC-TMS technology and compared the contents of 19 PAAs among differently processed plasma/serum samples. Among plasma and serum samples, the contents of 5, 6-DHET; 14, 15-DHET; 5-HETE; 12-HETE; TXB2; and LTB4 were significantly higher in serum samples than in plasma samples from the EDTA-K2 and sodium citrate groups. Of these components, the increase in 5-HETE content was the most dramatic; this difference may have been associated with the matrix effects of the UPLC-TMS instrument and blood coagulation responses. Additionally, the contents of 5, 6-DHET; 11, 12-EET; 5-HETE; LTB4; TXB2; and 12-HETE were significantly higher in plasma samples from the heparin lithium group than in plasma samples from the EDTA-K2 and sodium citrate groups. Among these components, the increase in 11, 12-EET was the most dramatic, likely due to the matrix effects of heparin lithium during UPLC-TMS analysis. Finally, for analysis of serum samples, LTB4 contents were higher and TXB2 contents were lower in the coagulant/separation gel group than in the coagulant-free group; again, this may have been associated with the matrix effects of the UPLC-TMS instrument. Thus, our results showed that some PAA contents were not consistent between plasma and serum samples. Specifically, the levels of 5, 6-DHET; 5-HETE; 12-HETE; TXB2; and LTB4 in serum samples and in plasma samples from the heparin lithium group were significantly higher than those in the plasma samples from the EDTA-K2 and sodium citrate groups, suggesting that blood samples should be treated with the same method when using UPLC-TMS to detect PAAs and that the use of EDTA-K2 or sodium citrate may help to reduce the interference of matrix effects and blood coagulation on the test results.
Disclosure of conflict of interest
None.
References
- 1.Jadoon A, Cunningham P, Mcdermott LC. Arachidonic acid metabolism in the human placenta: identification of a putative lipoxygenase. Placenta. 2014;35:422–424. doi: 10.1016/j.placenta.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Hammond VJ, O’donnell VB. Esterified eicosanoids: generation, characterization and function. Biochim Biophys Acta. 2012;1818:2403–2412. doi: 10.1016/j.bbamem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X, Zhang XA, Wang DW. The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases. Adv Drug Deliv Rev. 2011;63:597–609. doi: 10.1016/j.addr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Ackerman R, Guo AM. 20-HETE in neovascularization. Prostaglandins Other Lipid Mediat. 2012;98:63–68. doi: 10.1016/j.prostaglandins.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinde DD, Kim KB, Oh KS, Abdalla N, Liu KH, Bae SK, Shon JH, Kim HS, Kim DH, Shin JG. LC-MS/MS for the simultaneous analysis of arachidonic acid and 32 related metabolites in human plasma: Basal plasma concentrations and aspirin-induced changes of eicosanoids. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;911:113–121. doi: 10.1016/j.jchromb.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Taylor PJ. Matrix effects: the Achilles heel of quantitative high-performance liquid chromatography-electrospray-tandem mass spectrometry. Clin Biochem. 2005;38:328–334. doi: 10.1016/j.clinbiochem.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Ismaiel OA, Zhang T, Jenkins RG, Karnes HT. Investigation of endogenous blood plasma phospholipids, cholesterol and glycerides that contribute to matrix effects in bioanalysis by liquid chromatography/m+ass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:3303–3316. doi: 10.1016/j.jchromb.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Mei H, Hsieh YS, Nardo C, Xu X, Wang S, Ng K, Korfmacher WA. Investigation of matrix effects in bioanalytical high-performance liquid chromatography/tandem mass spectrometric assays: application to drug discovery. Rapid Commun Mass Spectrom. 2003;17:97–103. doi: 10.1002/rcm.876. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto T, Takahashi Y. Arachidonate 12-hpoxygenases. Prostaglandins Other Lipid Mediators. 2002;68-69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 11.Kroetz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn HY, Campbell WB, Pohl U. Membrane potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol. 2004;24:595–600. doi: 10.1161/01.ATV.0000116219.09040.8c. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Schieber EB, Mcgiff JC, Balazy M. Identification of arachidonate P-450 metabolites in human platelet phospholipids. Hypertension. 1995;25:854–859. doi: 10.1161/01.hyp.25.4.854. [DOI] [PubMed] [Google Scholar]
- 13.Van Eeckhaut A, Lanckmans K, Sarre S, Smolders I, Michotte Y. Validation of bioanalytical LC-MS/MS assays: Evaluation of matrix effects. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2198–2207. doi: 10.1016/j.jchromb.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Tang L, Kebarle P. Dependence of ion intensity in electrospray mass-spectrometry on the concentration of the analytes in the electrosprayed solution. Analyt Chem. 1993;65:3654–3668. [Google Scholar]
- 15.Furey A, Moriarty M, Bane V, Kinsella B, Lehane M. Ion suppression; a critical review on causes, evaluation, prevention and applications. Talanta. 2013;115:104–122. doi: 10.1016/j.talanta.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 16.Trufelli H, Palma P, Famiglini G, Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrom Rev. 2011;30:491–509. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
- 17.Dolegowska B, Blogowski W, Kedzierska K, Safranow K, Jakubowska K, Olszewska M, Rać M, Chlubek D, Ciechanowski K. Platelets arachidonic acid metabolism in patients with essential hypertension. Platelets. 2009;20:242–249. doi: 10.1080/09537100902849836. [DOI] [PubMed] [Google Scholar]
- 18.Maskrey BH, Bermudez-Fajardo A, Morgan AH, Stewart-Jones E, Dioszeghy V, Taylor GW, Baker PR, Coles B, Coffey MJ, Kühn H, O’Donnell VB. Activated platelets and monocytes generate four hydroxyphosphatidylethanolamines via lipoxygenase. J Biolo Chem. 2007;282:20151–20163. doi: 10.1074/jbc.M611776200. [DOI] [PubMed] [Google Scholar]
- 19.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 20.Yeh HC, Tsai AL, Wang LH. Reaction mechanisms of 15-hydroperoxyeicosatetraenoic acid catalyzed by human prostacyclin and thromboxane synthases. Arch Biochem Biophys. 2007;461:159–168. doi: 10.1016/j.abb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 22.Seggev JS, Wiessner JH, Thornton WH Jr, Edes TE. Comparison of serum and plasma leukotriene B4 levels in normal and asthmatic subjects. Ann Allergy Asthma Immunol. 1995;75:365–368. [PubMed] [Google Scholar]
- 23.Chen R, Li L. Lithium and transition metal ions enable low energy collision-induced dissociation of polyglycols in electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2001;12:832–839. doi: 10.1016/S1044-0305(01)00261-6. [DOI] [PubMed] [Google Scholar]


