Abstract
Megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS) is characterized by marked dilatation of the bladder and microcolon and decreased intestinal peristalsis. Recent studies indicate that heterozygous variants in ACTG2, which codes for a smooth muscle actin, cause MMIHS. However, such variants do not explain MMIHS cases that show an autosomal recessive mode of inheritance. We performed exome sequencing in a newborn with MMIHS and prune belly phenotype whose parents are consanguineous and identified a homozygous variant (c.3598A>T: p.Lys1200Ter) in MYH11, which codes for the smooth muscle myosin heavy chain. Previous studies showed that loss of Myh11 function in mice causes a bladder and intestinal phenotype that is highly reminiscent of MMIHS. All together, these observations strongly suggest that loss-of-function variants in MYH11 cause MMIHS. The documentation of variants in ACTG2 and MYH11 thus points to the involvement of the contractile apparatus of the smooth muscle in MMIHS. Interestingly, dominant-negative variants in MYH11 have previously been shown to cause thoracic aortic aneurism and dilatation. Different mechanisms of MYH11 disruption may thus lead to distinct patterns of smooth muscle dysfunction.
Introduction
Megacystis-microcolon-intestinal hypoperistalsis syndrome (MMIHS; MIM 155310), also called Berdon syndrome, is a rare and severe congenital disorder characterized by marked dilatation of the bladder, microcolon, and decreased or absent intestinal peristalsis.1, 2 Other features may also be present, including omphalocele and umbilical hernia, hydronephrosis and renal dysplasia, maldevelopment of the abdominal musculature and abdominal laxity (the so-called prune belly abnormality) as well as truncus arteriosus and other cardiovascular complications 3. MMIHS is usually sporadic but it has also been observed in the offsprings of consanguineous parents or in multiple siblings with healthy parents, suggesting an autosomal recessive pattern of inheritance in some cases.4 Recently, heterozygous variants in the ACTG2 gene were shown to cause MMIHS5 but no specific gene defect has yet been identified in recessive forms of MMIHS. Here we report the identification of a homozygous truncating variant in the MYH11 gene that is likely causal in a case with MMIHS, explaining the recessive basis of this condition.
Methods and materials
We performed exome sequencing in the proband. This study was approved by our institutional ethics committee, and informed consent was obtained from the legal guardians. Blood genomic DNA was captured and sequenced as described elsewhere.6 The variant information and phenotype have been submitted to the public database ClinVar (submission ID SCV000189224; http://www.ncbi.nlm.nih.gov/clinvar/).
Results
The proband, a male of Algerian origin, was born to healthy consanguineous parents. Antenatal ultrasound performed during the second trimester of gestation revealed the presence of megacystitis as well as severe oligohydramnios. Fetal karyotype was normal. A classic prune belly (MIM 100100) phenotype was noted at birth. The newborn was anuric. Ultrasound confirmed the presence of megacystitis with normal wall thickness. Kidneys were normal sized with slightly hyperechogenic cortices but normal corticomedullary differentiation. Because of severe lung hypoplasia, the proband was put on high-frequency oscillation shortly after birth. The patient never passed any meconium. During the third week of life, transit studies showed lack of opacification of the bowel distal to the duodenum, whereas barium enema showed filiform opacification of the distal colon with no opacification of the cecum consistent with microcolon. Heart was morphologically normal on ultrasound. Ductus arteriosus closed spontaneously between day 1 and day 3. aCGH analysis performed using a 135k-feature whole-genome microarray (SignatureChipOS, Signature Genomic Laboratories, Spokane, WA, USA by PerkinElmer, Waltham, MA USA; based on UCSC 2009 hg19 assembly) did not detect any copy number abnormality in this individual. The patient subsequently died following hemodynamic deterioration and multiple-organ system failure.
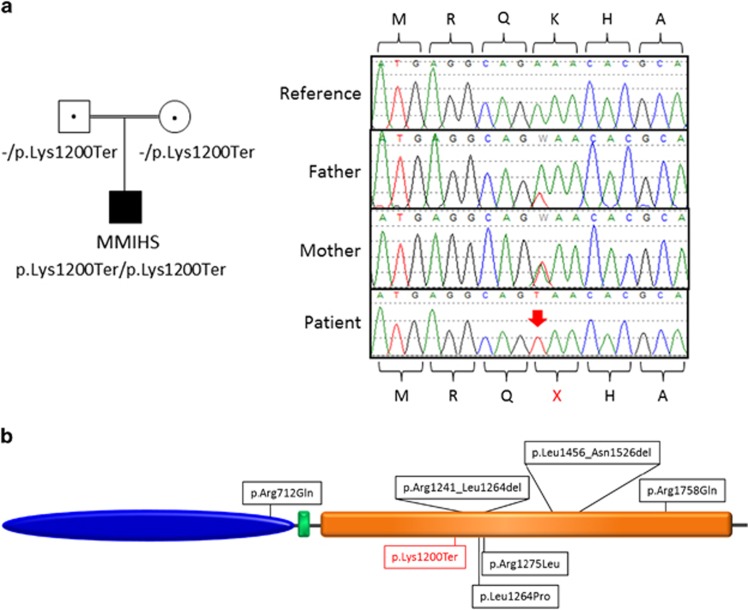
A total of 525 variants were retained as candidates according to our filtering criteria,6 including seven X-linked and 26 homozygous variants (Supplementary Table 1). Among the homozygous variants, we identified a nonsense variant, c.3598A>T (p.Lys1200Ter), in exon 27 of the MYH11 gene (NM022844; NG009299.1) (Figure 1a). This homozygous variant was confirmed by Sanger sequencing.
Figure 1.
Variant in MYH11 in a case with MMIHS. (a) Pedigree and Sanger sequencing confirmation of the c.3598A>T (p.Lys1200Ter) variant identified in our MMIHS patient using exome sequencing. Chromatograms represent the reference (top) and the mutant sequence. The homozygous variant is indicated by the red arrow. (b) Distribution of the variants identified in MYH11. The structure of the human MYH11 protein includes a motor domain (blue), a IQ motif EF-hand binding site (green) and a coiled-coil domain (orange). The variant in red was identified in the current study in a patient affected with MMIHS. Variants c.2135G>A (p.Arg712Gln), c.3791T>C (p.Arg1264Pro), c.3824G>T (p.Arg1275Leu), c.4578+1C>A (p.Leu1456_Asn1526del), c.3720_3792del (p.Arg1241_Leu1264del) and c.5273G>A (p.Arg1758Gln) were found in patients with TAAD associated with patent ductus arteriosus.
The c.3598A>T (p.Lys1200Ter) variant is located within a stretch of homozygosity extending over 5.3 Mb of sequence. This variant was not present in dbSNP, 1000 Genomes and Exome Variant Server (EVS) and in 323 ethnically matched controls. In addition, no nonsense or frameshift variants in MYH11 have been reported in the EVS data set, which includes 6258 individuals. Only two in-frame (positions 15 820 888 and 15 814 719) and several missenses variants, all in the heterozygous state, were found in EVS, further supporting the involvement of our homozygous nonsense variant in MMIHS. Segregation analysis using Sanger sequencing revealed that the father and the mother of the proband are heterozygous for the c.3598A>T (p.Lys1200Ter) variant. Examination of the MYH11 sequence of 3 additional cases with MMIHS and of 20 cases with prune belly syndrome did not reveal any splicing, truncating or novel nonsynonymous variants.
Discussion
All muscle contractions are believed to be the product of the interaction of the thin-filament actins and thick-filament myosins. Myosin and actin interactions lead to contraction through cyclic attachment and detachment of myosin to the actin filament with the concomitant hydrolysis of ATP.7 MYH11 encodes the smooth muscle myosin heavy chain, which is the major component of the thick contractile filaments of smooth muscle cells. Structurally, myosins are organized into head, neck and tail domains. The head domain binds actin and has ATPase activity, the tail domain is responsible for cargo binding and/or dimerization of heavy chains and the neck acts as a linker between the head and tail.8 The variant identified in our case is located in the region corresponding to the C-terminal coiled-coil domain and is predicted to abolish MYH11 expression by inducing nonsense-mediated mRNA decay.
Mice with a homozygous deletion of Myh11 showed several abnormalities, including a giant and thin-walled bladder and abnormal intestinal movement, the core features of MMIHS.9 These mice died within 12–24 h of birth, but could survive for up to 72 h when their bladders were emptied by applying external pressure.9 Myh11-deficient mice also show delay in closure of the ductus arteriosus.9
Variants in the MYH11 gene have previously been associated with non-syndromic thoracic aortic aneurysms and aortic dissections (TAADs) as well as with patent ductus arteriosus.10 Heterozygous variants identified in TAAD patients result in amino acid substitutions or in-frame deletions (Figure 1b). Most of the TAAD variants are located in the coiled-coil domain of the protein and are thought to act via a dominant-negative mechanism (Figure 1b). Zhu et al10 found that TAAD variants lead to an early and severe decrease in the elasticity of the aortic wall, consistent with the role of smooth muscle cells in maintaining the mechanical properties of the thoracic aorta.10 Investigation of the structural consequences for the aortic wall of an heterozygous in-frame deletion in the C-terminal region of MYH11 suggested that this variant induced a conformation change consistent with previously described heterozygous variants in the coiled-coil domain of other myosin-II heavy chains. The variant p.Lys1200Ter identified in our patient is likely to be a null, disrupting protein function either by interfering with dimerization of the protein or by inducing the degradation of the MYH11 transcript through nonsense-mediated decay. Therefore, loss-of-function variants in MYH11 appears to cause MMIHS, whereas dominant-negative variants cause TAAD. It is possible, however, that surviving MMIHS patients may be at risk of developing TAAD with age. The proband's parents and the extended family were not known to be affected with TAAD and they were not available for echocardiographic studies.
Recently, heterozygous variants in ACTG2, which codes for an enteric smooth muscle actin, have been implicated in patients affected with MMIHS.5, 11 Structural analysis and functional experiments suggested that at least some of these variants affect the polymerization of ACTG2 into thin filaments, leading to impaired contractility of the smooth muscle. In addition, a de novo mutation (p.Arg179His) in the gene ACTA2, which also codes for a smooth muscle actin, has been described in a patient with a visceral myopathy leading to aortic and cerebrovascular disease, hypotonic bladder, malrotation and hypoperistalsis of the gut.12 Examination of the exome data set of our patient showed that all the coding exons and splice sites of ACTG2 and ACTG2 were well covered and did not harbor any variants. The fact that variants in ACTG2 cause MMIHS thus further supports the involvement of the contractile apparatus of the smooth muscle in this disorder. We predict that the remaining unexplained forms of MMHIS are caused by variants in other components of this apparatus.
Acknowledgments
JLM is a National Scientist of the Fonds de Recherche du Québec-Santé (FRQS), MES is supported by the CHU Ste-Justine Research Centre and also Genome Canada and IT received a scholarship from the RMGA (Réseau de médecine génétique appliquée du FRQS). We thank the members of the RMGA bioinformatic team (Alexandre Dionne-Laporte, Dan Spiegelman, Edouard Henrion and Ousmane Diallo) for the bioinformatic analysis of the exome-sequencing data. This work was supported by March of Dimes (grant no. 12-FY10–236 to JLM).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on European Journal of Human Genetics website (http://www.nature.com/ejhg)
Supplementary Material
References
- Berdon WE, Baker DH, Blanc WA, Gay B, Santulli TV, Donovan C: Megacystis-microcolon-intestinal hypoperistalsis syndrome: a new cause of intestinal obstruction in the newborn. Report of radiologic findings in five newborn girls. AJR Am J Roentgenol 1976; 126: 957–964. [DOI] [PubMed] [Google Scholar]
- Granata C, Puri P: Megacystis-microcolon-intestinal hypoperistalsis syndrome. J Pediatr Gastroenterol Nutr 1997; 25: 12–19. [DOI] [PubMed] [Google Scholar]
- Lashley DB, Masliah E, Kaplan GW, McAleer IM: Megacystis microcolon intestinal hypoperistalsis syndrome: bladder distension and pyelectasis in the fetus without anatomic outflow obstruction. Urology 2000; 55: 774. [DOI] [PubMed] [Google Scholar]
- Mc Laughlin D, Puri P: Familial megacystis microcolon intestinal hypoperistalsis syndrome: a systematic review. Pediatr Surg Int 2013; 29: 947–951. [DOI] [PubMed] [Google Scholar]
- Wangler MF, Gonzaga-Jauregui C, Gambin T et al: Heterozygous de novo and inherited mutations in the smooth muscle actin (ACTG2) gene underlie megacystis-microcolon-intestinal hypoperistalsis syndrome. PLoS Genet 2014; 10: e1004258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srour M, Chitayat D, Caron V et al: Recessive and dominant mutations in retinoic acid receptor beta in cases with microphthalmia and diaphragmatic hernia. Am J Hum Genet 2013; 93: 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I, Rypniewski WR, Schmidt-Base K et al: Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 1993; 261: 50–58. [DOI] [PubMed] [Google Scholar]
- Krendel M, Mooseker MS: Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005; 20: 239–251. [DOI] [PubMed] [Google Scholar]
- Morano I, Chai GX, Baltas LG et al: Smooth-muscle contraction without smooth-muscle myosin. Nat Cell Biol 2000; 2: 371–375. [DOI] [PubMed] [Google Scholar]
- Zhu L, Vranckx R, Khau Van Kien P et al: Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 2006; 38: 343–349. [DOI] [PubMed] [Google Scholar]
- Thorson W, Diaz-Horta O, Foster 2nd J et al: De novo ACTG2 mutations cause congenital distended bladder, microcolon, and intestinal hypoperistalsis. Hum Genet 2014;133:737–742. [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Ostergaard JR, Ala-Kokko LM et al: De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am J Med Genet A 2010; 152A: 2437–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.