Abstract
The hearts of mammalian hibernators maintain contractile function in the face of severe environmental stresses during winter heterothermy. To enable survival in torpor, hibernators regulate the expression of numerous genes involved in excitation-contraction coupling, metabolism, and stress response pathways. Understanding the basis of this transition may provide new insights into treatment of human cardiac disease. Few studies have investigated hibernator heart performance during both summer active and winter torpid states, and seasonal comparisons of whole heart function are generally lacking. We investigated the force-frequency relationship and the response to ex vivo ischemia-reperfusion in intact isolated hearts from 13-lined ground squirrels (Ictidomys tridecemlineatus) in the summer (active, July) and winter (torpid, January). In standard euthermic conditions, we found that winter hearts relaxed more rapidly than summer hearts at low to moderate pacing frequencies, even though systolic function was similar in both seasons. Proteome data support the hypothesis that enhanced Ca2+ handling in winter torpid hearts underlies the increased relaxation rate. Additionally, winter hearts developed significantly less rigor contracture during ischemia than summer hearts, while recovery during reperfusion was similar in hearts between seasons. Winter torpid hearts have an increased glycogen content, which likely reduces development of rigor contracture during the ischemic event due to anaerobic ATP production. These cardioprotective mechanisms are important for the hibernation phenotype and highlight the resistance to hypoxic stress in the hibernator.
Keywords: hibernation, isolated heart, force-frequency relationship, sarcoplasmic reticulum, Ca2+, ischemia-reperfusion injury, Langendorff, season, torpor, glycolysis, glycogenolysis
mammalian hibernators survive tremendous environmental challenges during winter by entering a transient heterothermic state, torpor (TOR). During TOR, body temperature drops to near-ambient level, yet cardiac performance is sufficiently preserved to enable life-sustaining circulation (reviewed in Ref. 2). To support this winter torpid state, hibernators orchestrate seasonal changes in the expression of genes involved in cardiac muscle metabolism, excitation-contraction coupling, stress responses, and other pathways that are thought to collectively confer resistance to cold temperature-mediated heart pump dysfunction (8, 28). These seasonal transitions in gene expression are of potential medical interest, as mechanisms underlying the remarkable stress resistance of hibernators may lead to new approaches toward remediation of heart disease in nonhibernators, including humans. Despite this potential value, relatively few studies have been conducted to elucidate hibernator cardiac function in both the summer and winter seasons.
During TOR, hibernator hearts demonstrate a physiological robust contraction-relaxation cycle, albeit at drastically fewer beats per minute under severe hypothermic conditions, that depresses contractile function and renders nonhibernator hearts highly arrhythmic (11, 23). Hibernators are also known to be highly resistant to hypoxic stress (reviewed in Ref. 20). Sarcoplasmic reticulum (SR) Ca2+ uptake capability is greater in cardiac muscle from hibernators than nonhibernators, and cardiac muscle from hibernators is capable of maintaining low diastolic Ca2+ levels at temperatures well below those tolerated by nonhibernator myocytes (35). In particular, hibernator myocardium from the winter torpid state has increased gene expression of the SR Ca2+-ATPase (SERCA2), as well as decreased expression of the SERCA2 inhibitor phospholamban (PLN) (8, 28, 53), relative to hibernator hearts during the summer months. This seasonal increase in the SERCA-to-PLN ratio is likely highly important for enabling contractility during TOR. Because SR Ca2+ uptake is impaired in human heart failure (27, 29, 47), the ability of hibernators to seasonally regulate SR function is of significant interest. Therefore, a comparison of hibernator cardiac function in the summer active and winter torpid states is likely to identify the functional significance of altered gene expression. Studies of isolated papillary muscle strips and cardiac myocytes from hibernator hearts have shown greater reliance on SR Ca2+ handling for contractility by myocardium from winter torpid than summer active ground squirrels (31, 32, 53). Seasonal changes in cardiac Ca2+ handling, however, have not been evaluated in the physiologically relevant context of the intact heart.
We investigated whether whole heart contractile performance is influenced by season in hibernators. Owing to the increased SR function in winter torpid hibernators, we hypothesized that systolic and diastolic performance would be enhanced in the hearts of winter torpid ground squirrels compared with summer active animals at 37°C. We isolated whole hearts from ground squirrels in summer (July) and winter (January) and determined the force-frequency relationship (FFR) by Langendorff-mode perfusion. By subjecting each heart to progressively increased stimulation frequencies, we stressed the heart's ability to sequester Ca2+ at each beat, revealing SR contributions to contractile function. Each heart was then subjected to ischemia-reperfusion injury to determine whether seasonal changes in Ca2+ handling or metabolism conferred additional resistance to ischemic damage in torpid hearts. To identify mechanisms underlying differences in function between summer active and winter torpid ground squirrel hearts, we compared protein expression profiles between torpid and active hibernator hearts. Although it is well established that hibernators are highly resistant to hypoxic stress, most such comparisons have been made between hibernator and nonhibernator hearts, rather than between hibernator hearts during summer and winter. Thus an additional rationale for this study was to test whether hypoxia resistance is a general property of hibernators or whether it is also regulated during TOR.
METHODS
Animal care and husbandry.
Animal husbandry and experiments were approved by the Institutional Animal Care and Use Committee of the University of Minnesota (protocols 0805A34502 and 1103A97712). Thirteen-lined ground squirrels (Ictidomys tridecemlineatus) of both sexes were trapped from the wild in the spring, dewormed, and housed individually at the University of Minnesota-Duluth. During summer months, squirrels were housed at 22°C with a 12:12-h light-dark cycle, with water and standard rodent chow available ad libitum. For induction of TOR, squirrels were transferred to a dark cold-room at 4°C and food was removed. Induction and maintenance of TOR were monitored by the sawdust method. Summer active ground squirrels were studied in July, and all winter torpid ground squirrels were studied in January. All ground squirrels studied in January were taken from the torpid state [4.1 ± 1.1 days since last interbout arousal (IBA)].
Langendorff heart preparation.
Squirrels were anesthetized with isoflurane gas (5% induction, 2% maintenance, balance pure O2). Anesthetic depth was monitored by toe pinch. Heparin (700 IU ip) was administered after anesthetic induction but prior to surgery. Upon loss of reflex, the chest was entered and the heart was surgically removed to a dish of ice-cold Krebs-Henseleit buffer (KHB; in mmol/l: 118 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 2.5 CaCl2, 0.5 NaEDTA, 15 glucose, 25 NaHCO3, 0.5 Na pyruvate). Lungs and pericardial fat were trimmed away to reveal the aortic arch. Before cannulation, each heart was briefly moved to a nearby scale and weighed. Weighing each heart prior to cannulation was necessary to prevent perfusion edema from skewing heart weight values; this crudely recorded weight included ventricles, atria, aorta, and some pulmonary vessels, but not lungs or pericardial adipose tissue.
The aorta was trimmed, and a 14-gauge notched cannula was inserted into the aortic stump and secured with a 4-0 silk suture. The cannulated heart was perfused in the Langendorff mode (Radnoti) in a two-phase protocol (see below) at a constant pressure of 75 mmHg. The KHB perfusate was bubbled with 5% CO2-95% O2, and temperature was maintained at 37°C by water-jacketed tubing for all experiments. Both atria were removed, and left ventricular (LV) pressure was measured by a pressure transducer connected to a water-filled balloon catheter inserted into the LV. The pacing frequency was artificially controlled by an electrode placed at the root of the right atrium.
Pacing challenge and ischemia-reperfusion.
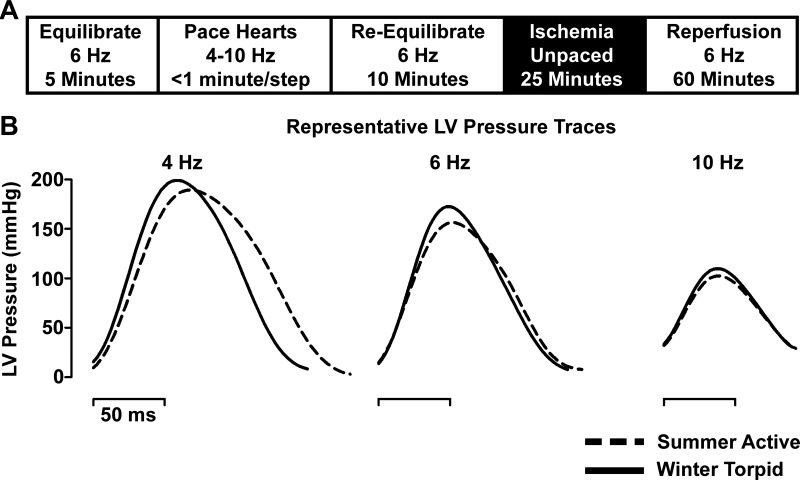
While perfused on the Langendorff apparatus, each heart was evaluated in two phases (Fig. 1A). Phase 1 consisted of 5 min of equilibration at a pacing frequency of 6 Hz. After equilibration, the pacing frequency was decreased from 6 Hz to 3 Hz in 1- and 0.5-Hz increments (6, 5, 4.5, 4, 3.5, and 3 Hz). After 3 Hz, hearts were returned to 6 Hz; then the pacing frequency was increased to 10 Hz in 1-Hz increments. At each new pacing frequency, the heart was allowed sufficient time to achieve stable performance, between 30 and 90 s, before the next frequency step. After performance at 10 Hz stabilized, the pacing frequency was returned to 6 Hz. At <4 Hz, it became difficult to reliably pace hearts at the defined frequency, so 4 Hz was the lowest pacing frequency analyzed in this study.
Fig. 1.
Protocol schematic and individual pressure traces at 37°C. A: schematic of Langendorff-isolated heart protocol. B: representative left ventricular (LV) pressure traces from summer active and winter torpid ground squirrel hearts at 4, 6, and 10 Hz. Summer and winter hearts developed similar systolic LV pressures, and relaxation was faster at low frequency in winter hearts.
Hearts were initially perfused with KHB containing 0.5 mM pyruvate to facilitate collection of pacing data. At the end of the pacing challenge phase, hearts were perfused with KHB lacking pyruvate and reequilibrated at 6 Hz for 10 min. KHB lacking pyruvate was necessary for phase 2, ischemia-reperfusion, to avoid the ischemia-protective effects of pyruvate (18, 30). After reequilibration in KHB lacking pyruvate, the pacing electrode was inactivated and perfusate flow was stopped for 25 min to cause global myocardial ischemia. After ischemia, hearts were reperfused for 60 min. At 8 min after reperfusion, the pacing electrode was reactivated at 6 Hz and remained active until the end of the experiment.
Upon completion of ex vivo perfusion, each heart was removed from the Langendorff apparatus. A portion of the LV free wall was excised, frozen in liquid nitrogen, and stored at −80°C until use. Tissue samples were pulverized with a liquid nitrogen-cooled mortar and pestle, resuspended in RIPA buffer [50 mmol/l Tris, 150 mmol/l NaCl, 1 mmol/l EDTA, and 0.5% (wt/vol) SDS] containing protease inhibitors (aprotinin, leupeptin, and pepstatin, each at 0.001 mg/ml, and 1 mmol/l phenylmethylsulfonyl fluoride), sonicated briefly, and sedimented at 15,000 g for 2 min. The protein concentration of the heart homogenate supernatant was determined by the bicinchoninic acid method (Thermo Scientific).
Animal preparation.
For proteome analysis, animals were housed as described above. Animals in TOR were collected after ≥3 days in a TOR bout and showed no visible signs of arousal. At the time the animals were euthanized, rectal temperatures were taken to verify torpid state (6–8°C body temperature). For the active time point, animals were collected in early to mid-August after 3–4 mo in Animal Services for acclimation to the laboratory. Three males and three females were euthanized at each collection point. All animals were fully anesthetized with isoflurane and then euthanized by decapitation. The pericardium was removed from around the heart, and the heart was removed from the animal and halved sagittally to include atrium and ventricle. Heart dissection was performed on ice, and dissected heart pieces were rinsed with PBS and rapidly flash-frozen in liquid nitrogen. The time from decapitation to sample freezing was <10 min. Tissue was stored at −80°C.
Protein extraction, proteolytic digestion, and labeling with isobaric tags for relative and absolute quantitation.
One-half of the heart from each animal was used for protein sample preparation. Frozen tissue was ground to a fine powder on liquid nitrogen using a mortar and pestle. Tissue powder was brought up in iTRAQ lysis buffer [7 M urea, 2 M thiourea, 0.5 M triethylammonium bicarbonate (pH 8.5), 20% acetonitrile, 4 mM tris(2-carboxyethyl)phosphine, and Roche PhosSTOP]. Probe sonication was used to lyse samples (30% amplitude, 7-s pulse). Samples were then put into PCR tubes and run in the Barocycler to efficiently isolate membrane proteins and hydrophobic proteins. Samples were transferred to a new tube, methyl methanethiosulfonate was added to a final concentration of 8 mM, and the samples were incubated at room temperature for 15 min. Bradford assay was used to determine protein concentration. To examine seasonal expression changes independent of sex effects, for each time point, three samples consisting of pooled tissue from one male and one female animal were analyzed; this limitation decreased sample size in this study. Fifty micrograms of protein from the male and 50 μg of protein from the female were combined and digested in trypsin (1:35 trypsin-total protein) overnight at 37°C. After trypsin digestion, samples were cleaned with a 4-ml Extract-Clean C18 solid-phase extraction cartridge (Grace-Davidson, Deerfield, IL). Samples were vacuum-centrifuged to dryness and resuspended in dissolution buffer (0.5 M triethylammonium bicarbonate, pH 8.5) to a final concentration of 2 μg/μl. Three micrograms of each sample were loaded onto the linear trap quadropole for a quality control run. Total ion chromatograms were produced to identify blood contamination and determine quality of each sample. For each iTRAQ 8-plex, 40 μg of each sample were labeled with iTRAQ reagent according to the manufacturer's protocol (Ab Sciex, Foster City, CA). Protein lysates were iTRAQ-labeled, with one label corresponding to each time point. The lysates from two time points were split and labeled with two separate iTRAQ labels for technical replicates. After the samples were labeled, they were multiplexed together and vacuum-dried. The multiplexed samples were cleaned with a 4-ml Extract-Clean C18 solid-phase extraction cartridge, and the eluate was dried in vacuo.
Peptide liquid chromatography fractionation and mass spectrometry.
The iTRAQ-labeled samples were resuspended in buffer A [20 mM ammonium formate (pH 10) in 98:2 water-acetonitrile] and fractionated offline by high-pH C18 reverse-phase chromatography (52). A HPLC (Prominence, Shimadzu, Columbia, MD) with a C18 XBridge column (150 mm × 2.1 mm internal diameter, 5-μm particle size; Waters, Milford, MA) was used. Buffer B contained 20 mM ammonium formate (pH 10) in 10:90 water-acetonitrile. The flow rate was 200 μl/min, with a gradient from 2 to 35% buffer B over 60 min, followed by 35–60% over 5 min. Fractions were collected every 2 min, and UV absorbances were monitored at 215 and 280 nm. Peptide-containing fractions were divided into two equal numbered groups, “early” and “late.” The first early fraction was concatenated with the first late fraction, and so on. Concatenated samples were dried in vacuo and resuspended in load solvent (98:2:0.01 water-acetonitrile-formic acid), and 1- to 1.5-μg aliquots were run on a Velos Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) as described previously (36), with the exception that the high-energy collisional dissociation activation energy was 20 ms.
Glycogen content assay.
For the glycogen assay, animals were housed as described above. Animals in TOR were collected after ≥3 days in a TOR bout and showed no visible signs of arousal. At the time of euthanization, rectal temperatures were taken to verify torpid state (6–8°C body temperature). Animals collected for the IBA collection point aroused naturally, were awake and active, and showed coordinated body movement. Rectal temperatures recorded at euthanization showed an active body temperature between 35 and 37°C. TOR and IBA animals were collected in January and February, when TOR bouts are the longest. Animals were collected for the summer active time point in August (AUG); they were awake and active and showed an active body temperature of 35–37°C. Animals were collected for the fall-active time point in October (OCT); they were awake and active and showed an active body temperature of 35–37°C. All animals were fully anesthetized with isoflurane and then euthanized by decapitation. The pericardium was removed from around the heart, and the heart was removed from the animal and halved sagittally to include atrium and ventricle. Heart dissection was performed on ice, and dissected heart pieces were rapidly flash-frozen in liquid nitrogen. The time from decapitation to sample freezing was <10 min. Tissue was stored at −80°C. Ten micrograms of tissue from each sample were ground to powder in liquid nitrogen and homogenized in 100 μl of water on ice. Homogenates were boiled for 5 min to inactivate enzymes and stored at −20°C. Glycogen assay was performed according to the manufacturer's protocol, with 12.5 μl of lysate added to each well, and included two replicates and a glucose background control (catalog no. MAK016, Sigma Aldrich, St. Louis, MO).
Data collection and statistics.
Langendorff data were collected using LabChart 6 (ADInstruments) and analyzed using Prism 5 (GraphPad). Statistical significance was evaluated by two-way analysis of variance with Bonferroni's post test or two-tailed t-test as appropriate. P < 0.05 was considered significant. The time constant of pressure decay (τ) was obtained using LabChart 7 software (ADInstruments) and the Weiss method [P(t) = Ae−t/τ + B], with a sampling range that ended at end-diastolic pressure for the beat. For determination of rigor, the initiation point of rigor was manually determined using the time at which LV pressure rose above 8 mmHg. This rule was applied prior to analysis as an estimate of the time each heart's LV pressure rose above preload levels.
For proteome analysis, Microsoft RAW files were imported into GalaxyP and searched against a customized ground squirrel database generated from the National Center for Biotechnology Information 13-lined ground squirrel genome merged with our RNA sequencing-derived protein sequences and the contaminants database. Files were processed into peak lists using ProteinPilot software (AB Sciex, Framingham, MA) for protein and quantification analysis. All peptides were identified with ≥95% confidence and <1% global false discovery rate between the three runs. Relative quantification was determined by ProteinPilot in a normalized log10-based relative iTRAQ ratio format, with the iTRAQ label corresponding to AUG as the reference denominator. Protein expression ratios were calculated in the Protein Alignment Template (AB Sciex).
RESULTS
Summer and winter ground squirrel characteristics.
Body and heart weight characteristics of the summer and winter ground squirrels used for this study are summarized in Table 1. Body weight was significantly greater in summer than winter squirrels. Heart weight and body size (tibia length) were not significantly different between seasons. Although the heart weight-to-body weight ratio was higher in winter than summer, this was due to greater body weight in summer animals, rather than any difference in heart weight. Heart weight-to-tibia length ratios were not different between summer and winter. Absolute heart weights were not significantly different between groups.
Table 1.
Animal characteristics
| Summer (n = 10) | Winter (n = 9) | P (Summer vs. Winter) | |
|---|---|---|---|
| Body wt, g | 211.54 ± 6.98 | 156.44 ± 13.58 | 0.0017 |
| Heart wt, mg | 896 ± 33.93 | 932 ± 31.13 | 0.4514 |
| Tibia length, mm | 34.30 ± 0.21 | 33.89 ± 0.42 | 0.3833 |
| Heart wt/body wt, mg/g | 4.30 ± 0.27 | 5.96 ± 0.36 | 0.0017 |
| Heart wt/tibia length, mg/mm | 26.17 ± 1.11 | 27.46 ± 1 | 0.4089 |
| Body temperature, °C | 6.96 ± 0.28 |
Values are means ± SE. Ground squirrels studied in summer (July) were significantly heavier than those studied in winter (January). Heart weights and tibia lengths were not significantly different. Heart weight-to-body weight ratios were significantly different, but this was due to differences in body weight, as heart weight-to-tibia length ratios were not significantly different between groups. P values were determined by unpaired 2-tailed t-test.
LV function.
Representative individual traces of LV pressure at 37°C are shown in Fig. 1. The magnitude of contraction (LV developed pressure) was similar at all stimulation frequencies. Pressure decay was more rapid in hearts from winter ground squirrels at low (4 Hz) and moderate (6 Hz) pacing frequencies. There was no difference in relaxation at high (10 Hz) pacing frequency (Fig. 1 and Fig. 2, B and D).
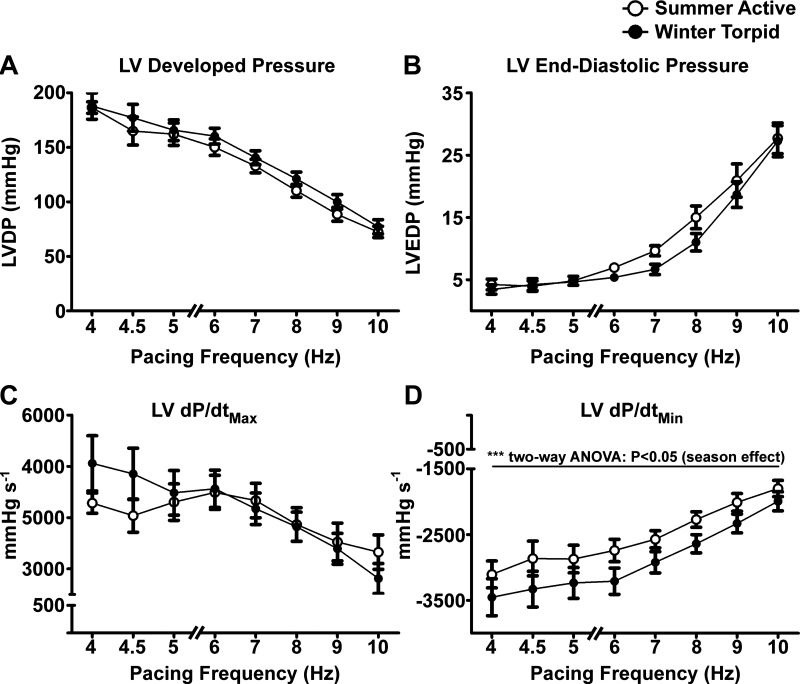
Fig. 2.
LV pressures and derivatives during force-frequency determination. A: LV developed pressure (LVDP) from 4- to 10-Hz stimulation frequency. Summer and winter hearts showed negative force-frequency relationship. LVDP was not significantly different between summer (n = 10) and winter (n = 9) hearts. B: LV end-diastolic pressure (LVEDP) from 4- to 10-Hz stimulation frequency. LVEDP was not significantly different between summer and winter hearts. C: maximal 1st derivatives of LV pressure (dP/dtmax) were not different between summer and winter hearts. D: minimal 1st derivatives of LV pressure (dP/dtmin) were greater in winter hearts, suggesting more rapid relaxation in winter than summer hearts.
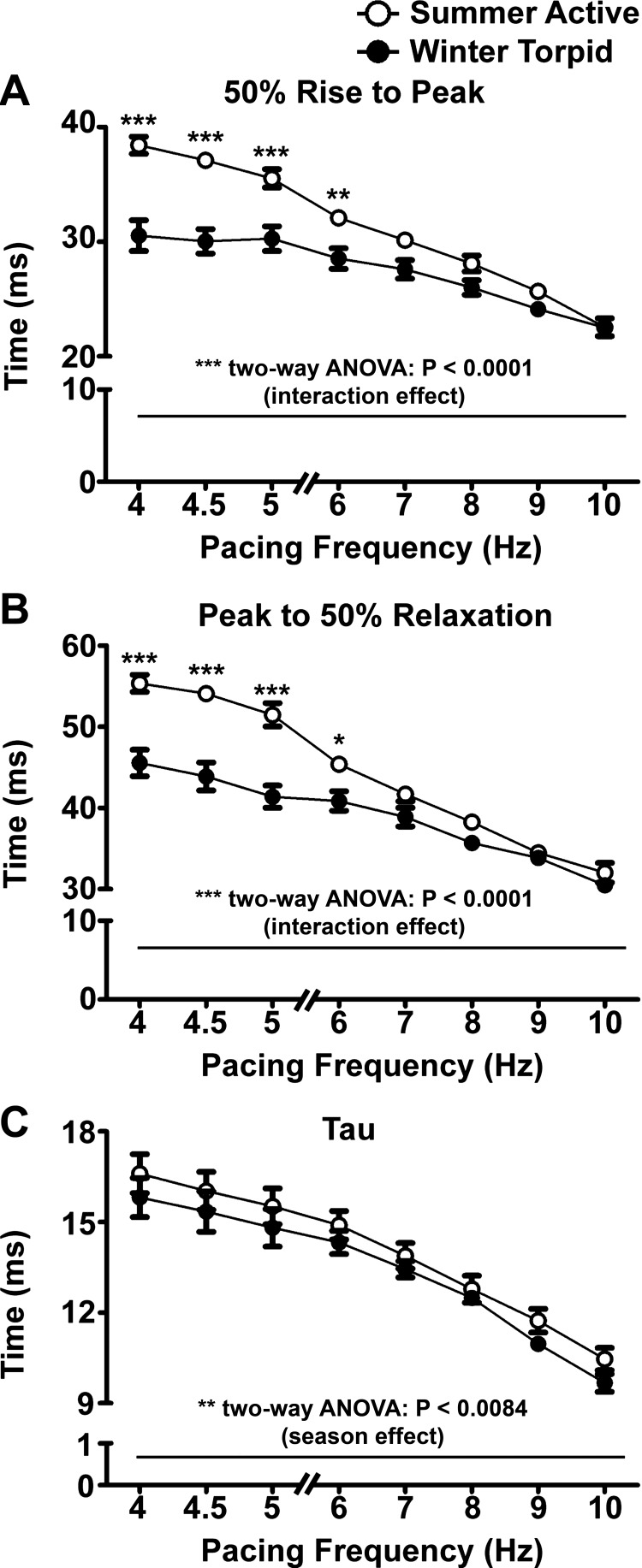
The systolic performance of perfused hearts isolated from summer and winter ground squirrels was very similar across a wide range of pacing frequencies (Fig. 2). Hearts from summer and winter squirrels underwent a negative-staircase FFR (Fig. 2A). LV developed pressures were not significantly different in hearts from winter squirrels (P = 0.0606 for season effect, by two-way ANOVA). Similarly, LV end-diastolic pressures were not significantly different between hearts from summer and winter animals from 4- to 10-Hz pacing frequency and in both groups steadily increased with each frequency step above 6 Hz (Fig. 2B). Maximal first derivatives of pressure (dP/dtmax) (Fig. 2C) were not significantly different between seasons, but minimal dP/dt (dP/dtmin) was significantly greater in winter hearts (P < 0.0001 for season main effect, by two-way ANOVA), indicating significantly faster relaxation on a beat-to-beat basis across all pacing frequencies. Winter hearts had significantly shorter times of pressure rise (Fig. 3A) and relaxation (Fig. 3B). τ was slightly, but significantly, lower in winter hearts at most pacing frequencies (Fig. 3C).
Fig. 3.
Winter hearts exhibit decreased times to peak contraction, times to 50% relaxation, and exponential time constant of pressure decay (τ). A: at low to moderate stimulation frequencies, times between 50% and peak contraction were lower in winter (n = 10) than summer (n = 9) hearts. B: at low to moderate simulation frequencies, times to 50% relaxation were lower in winter hearts. C: τ was lower in winter hearts. *P < 0.05; **P < 0.01; ***P < 0.001 (by Bonferroni's post test).
Proteomic analysis.
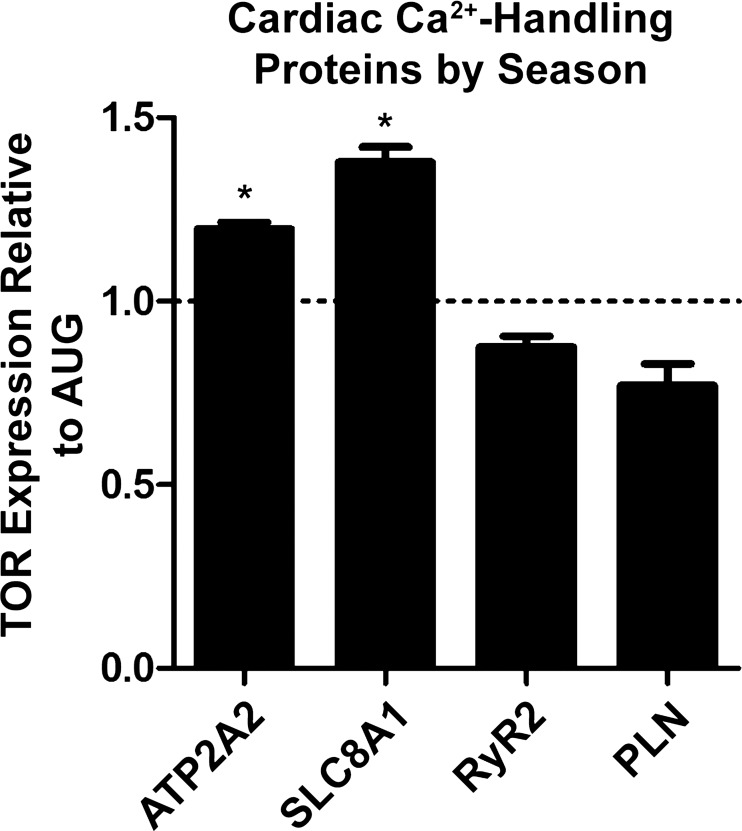
To pursue the mechanism by which winter torpid hearts are able to significantly reduce times of pressure rise and relaxation, we examined cardiac proteomic data collected from ground squirrel hearts throughout the circannual cycle. Notable results from this analysis are described in Fig. 4. We found that protein expression of several key enzymes involved in Ca2+ handling was altered in torpid hearts relative to summer active hearts. In particular, the expression of SERCA2 (or ATP2A2) and NCX1 (or SLC8A1) is significantly increased in TOR relative to AUG while expression of ryanodine receptor type 2 (RYR2) and PLN was not significantly decreased in TOR relative to AUG.
Fig. 4.
Expression of Ca2+-handling proteins in torpor (TOR) relative to the summer active time point in August (AUG). Protein expression of ATP2A2 and SLC8A1 significantly increased, while protein expression of ryanodine receptor type 2 (RYR2) and phospholamban (PLN) did not significantly decrease, in TOR. Protein expression of ATP2A2 and SLC8A1 in TOR (n = 3) was significantly different from that in AUG (n = 3) as determined by Student's t-test: *P < 0.05.
Ischemia-reperfusion.
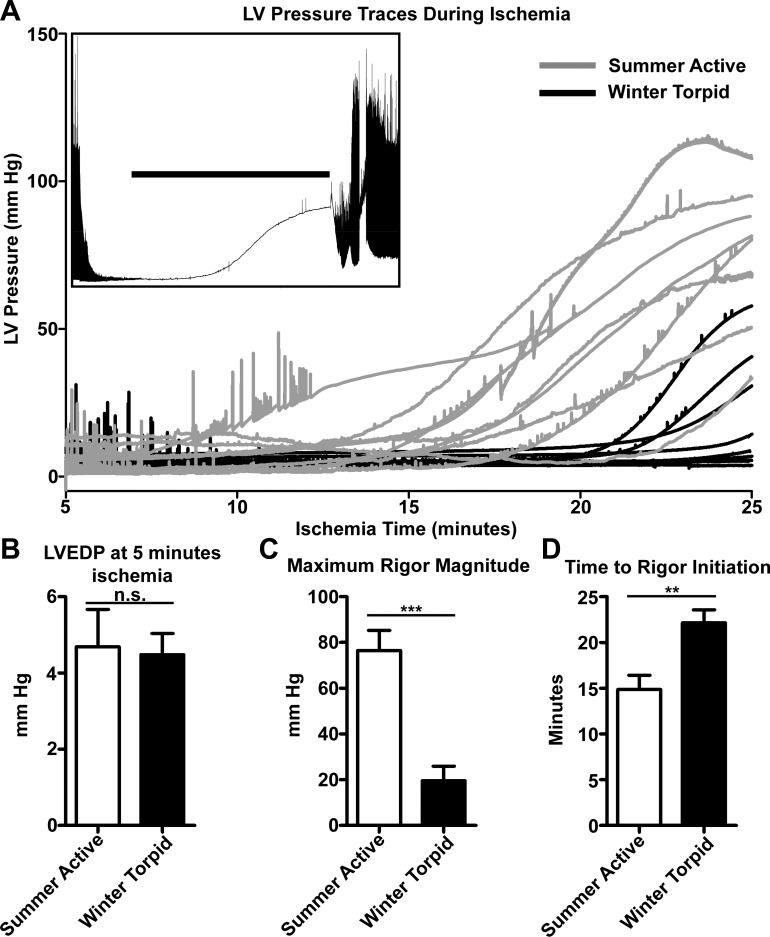
After reequilibration for 10 min with KHB lacking pyruvate, each heart was subjected to 25 min of no-flow ischemia followed by 60 min of reperfusion. Traces of LV pressure during the final 20 min of ischemia from each summer and winter heart are overlaid in Fig. 5. From these traces, it is evident that summer and winter hearts exhibit markedly different behavior during ischemia: summer hearts enter rigor more rapidly and reach higher pressures than winter hearts. Typical behavior of an isolated (summer) heart during ischemia-reperfusion is shown in the inset: upon onset of ischemia, contractile force declines rapidly, and hearts enter a relaxed phase. As ATP is gradually depleted from the myocardium, an ischemic isolated heart enters a progressively contracted rigor state (38). Upon reperfusion, hearts are arrhythmic and do not recover normal rhythm for several minutes. Winter hearts either entered a slight contracture phase before reperfusion or did not enter contracture at all. This behavior is quantified in Fig. 5, B–D: both summer and winter hearts reached similarly low pressures prior to the onset of rigor (Fig. 5B). Summer hearts attained significantly higher maximal pressures than winter hearts during ischemia (Figs. 5C and 6B) and entered contracture sooner than winter hearts (Fig. 5D). Despite these differences in heart function during ischemia, both groups recovered most of their contractile function soon after perfusion was resumed (Fig. 6A), and end-diastolic pressures were not different during reperfusion (Fig. 6B). There was no significant correlation between the maximal pressure achieved during ischemic contracture by a winter heart and the time since each winter animal's most recent IBA (Fig. 6C).
Fig. 5.
Ischemia-reperfusion contractures were different between summer and winter hearts. A: individual LV pressure traces of every summer (n = 8) and winter (n = 9) heart that underwent ischemia-reperfusion injury. Inset: representative ischemia-reperfusion behavior of 1 summer heart showing preischemic baseline and postischemia reperfusion; horizontal bar indicates time during ischemia shown for all pressure traces in A. Summer hearts entered contracture sooner in ischemia than winter hearts and developed greater pressures. Two summer hearts from experimental phase 1 were excluded from ischemia-reperfusion analysis: one due to perfusion with pyruvate during ischemia-reperfusion and the other due to a 30-min ischemia time. B: ischemic LV pressures prior to onset of contracture were not different (P > 0.05) between summer and winter hearts. C: maximal pressure during ischemia was greater in summer than winter hearts. ***P < 0.0001 (by unpaired 2-tailed t-test). D: summer hearts entered contracture sooner than winter hearts. Entry to rigor contracture was set when a heart's LV pressure passed an 8-mmHg threshold. Of 9 winter hearts, only 5 met this criterion, and only 1 winter heart entered rigor prior to 20 min of ischemia; winter hearts that did not pass 8 mmHg LV pressure were entered as “25 min.” All summer hearts entered rigor, and only 1 did so after >20 min of ischemia. **P = 0.0034, summer vs. winter (by unpaired 2-tailed t-test).
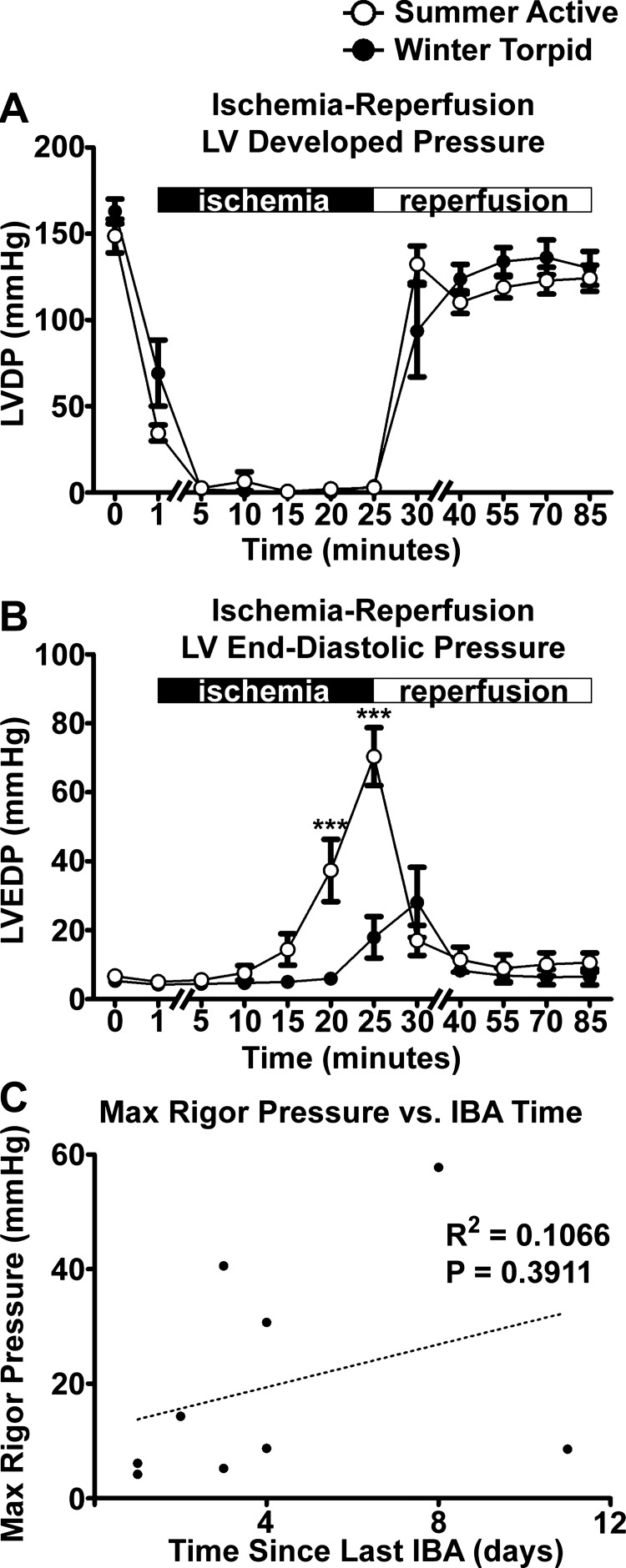
Fig. 6.
Recovery from ischemia-reperfusion injury was similar in summer and winter hearts. A: LVDPs were similar in summer (n = 8) and winter (n = 9) hearts throughout ischemia-reperfusion injury. No significant differences were found. B: LVEDPs were similar during reperfusion, suggesting no differences in severity of ischemia-reperfusion between groups. LVEDPs were higher in summer than winter hearts at 20 and 25 min of ischemia. ***P < 0.001 vs. winter torpid (by 2-way ANOVA with Bonferroni's post test). C: maximal ischemia pressures of winter hearts were compared with each heart's time since the most recent interbout arousal (IBA). No correlation between severity of ischemic contracture and IBAs was found (R2 = 0.1066, P = 0.3911).
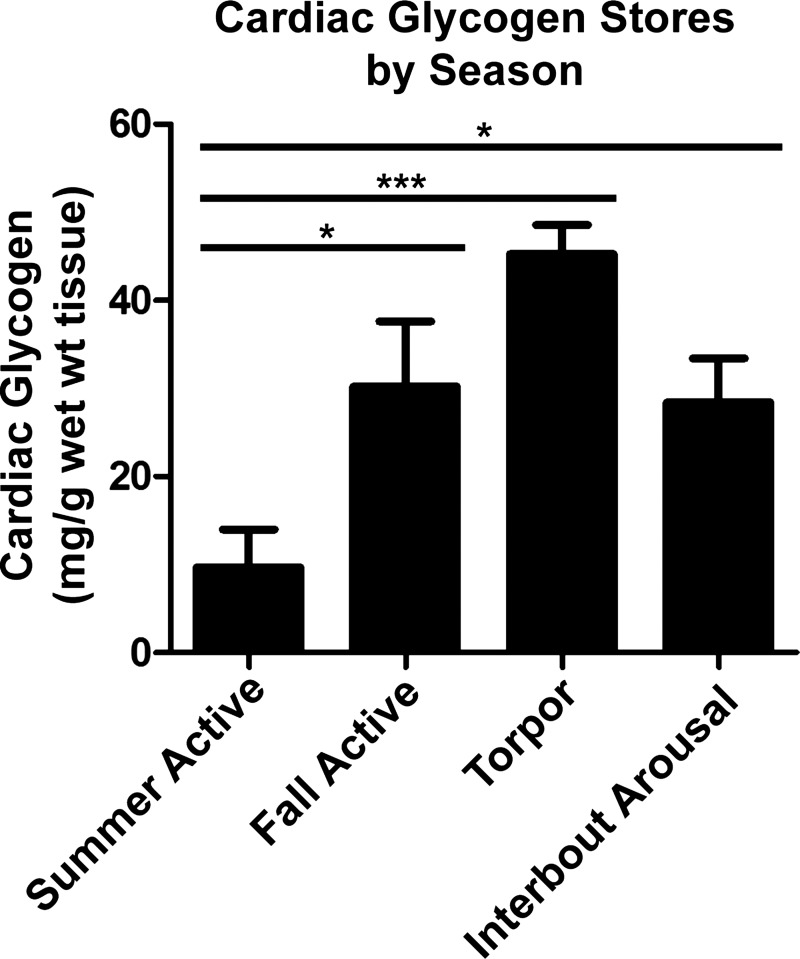
To determine if winter torpid hearts were able to resist ischemic contracture due to increased glycogen stores in the heart, we performed a glycogen assay on cardiac tissue from several time points, including AUG, OCT, TOR, and IBA. We found that cardiac glycogen content was lowest in the summer active period and rose through the fall to TOR and IBA, showing peak content in torpid animals (Fig. 7).
Fig. 7.
Cardiac glycogen content in hibernators is low during the summer but increases through the fall to TOR and IBA. Cardiac glycogen content was determined by an enzyme-coupled assay. Summer (n = 4) glycogen content was significantly different from fall (n = 5), TOR (n = 5), and IBA (n = 5) glycogen content. *P < 0.05; ***P < 0.001 (by 1-way ANOVA with Newman-Keuls post test).
DISCUSSION
Mammalian hibernators undergo remarkable adaptations in their physiology during the transition from high activity in summer to TOR in winter (2). As hibernators are highly resistant to environmental stress, the changes in gene expression that underlie this seasonal transition are of great potential medical interest (12). To understand the physiological role served by seasonal alterations in myocardial Ca2+ handling and metabolism, we evaluated the FFR and ischemia-reperfusion performance in the intact isolated hearts of active and torpid ground squirrels. Prior efforts to evaluate season-dependent function in hibernator cardiac tissue utilized isolated cardiac myocytes or papillary muscle strips (44). Although these studies have been highly informative into cellular mechanisms underlying contractility during TOR, it remained unclear whether whole-organ performance would reflect those findings. Our main new finding that ischemia-induced myocardial contracture varied significantly by season may have important implications for the treatment of ischemic injury in nonhibernators.
Whole heart relaxation performance in winter torpid hearts at 37°C is superior to that in summer active hearts, consistent with rapid diastolic Ca2+ sequestration and improved SR Ca2+-handling mechanisms in torpid hibernator myocytes (Fig. 4) (53). For relaxation of cardiac muscle to occur, intracellular Ca2+ must decrease to allow for Ca2+ release from the myofilaments. Ca2+ transport out of the cytosol occurs by four pathways involving SR Ca2+-ATPase (ATP2A2), sarcolemmal Na+/Ca2+ exchange (SLC8A1), sarcolemmal Ca2+-ATPase (ATP2B), or mitochondrial Ca2+ uniport (6). Increased protein expression of ATP2A2 and SLC8A1 in TOR relative to AUG correlates with our previous transcriptomic analysis (8). These two mechanisms account for removal of 98% of intracellular Ca2+ in rabbit ventricular myocytes (5). We found no significant differences in RYR2 expression between the active and torpid states. All these factors contribute to the increased removal of Ca2+ from the cytoplasm and would result in faster relaxation. These findings are similar to previous findings, including those reported by Yatani et al. (53), except Yatani et al. found significantly decreased protein expression of PLN. Both our study and previous work generally support increased removal of cytoplasmic Ca2+, leading to improved relaxation in hibernator hearts during the winter season.
Our finding that the FFR is negative in both summer and winter intact hearts is in apparent conflict with prior findings in papillary muscle strips, which showed divergent FFR behavior between seasons (31, 44). This difference may be due to the pacing frequency range used in the earlier studies, 0.1–2 Hz, which, although relevant to the torpid state, is well below the normal heart rate of an active ground squirrel (5–7 Hz). The present study of whole isolated hearts over a more extensive and physiologically relevant frequency range shows that the FFR of the ground squirrel, independent of season, is more similar to the FFR of the rat than of larger mammals (40, 43). The similarity in FFR between active and torpid ground squirrel hearts is intriguing, given that contractile function of the torpid heart is more dependent on SR Ca2+ flux than summer active cardiac tissue (31, 32) and that active summer hibernator heart has a far greater SR Ca2+ uptake capability than the nonhibernator heart (35, 37). Given that systolic and diastolic function were highly similar between active and torpid isolated hearts, we hypothesize that enhanced SR Ca2+ reuptake in the torpid heart is required for hypothermia tolerance and maintenance of low diastolic Ca2+, rather than alteration of systolic contractile function. The finding that intrinsic organ-level systolic function is similar between active and torpid myocardium suggests that decreased energy expenditures during TOR are a product of a much reduced heart rate, rather than intrinsic differences in contractile force.
Hypoxia resistance in hibernators has long been of interest, and numerous studies have compared hypoxia responses between hibernators and nonhibernators (9, 10, 22, 39, 41), evaluated surrogate measures of cardiac function during hypoxia in summer and winter (10, 39, 42), or compared hypoxia responses in other organs (15, 24, 33). To determine the functional importance of seasonal changes in gene and protein expression, however, a direct functional comparison of one hibernator species between seasons is necessary. Our findings show that summer active and torpid hearts diverge significantly in the development of contracture during ischemia. Hearts isolated from active ground squirrels rapidly enter ischemic contracture, and torpid hearts dramatically resist this effect. This is direct organ-level functional evidence in keeping with the known marked seasonal variations in metabolic function that underlie the ability of torpid hearts to prevent or limit ATP depletion that leads to contracture during bouts of ischemia (38). Our results are of particular interest, given recent evidence demonstrating that woodchucks similarly exhibit seasonal resistance to ischemic injury without preconditioning (51).
Myocardial ischemia, and accompanying loss of O2, makes the heart dependent on anaerobic energy production to supply energy for metabolic processes. Anaerobic energy production largely depends on the availability of adequate glycolytic substrates, with the ischemic heart greatly accelerating glycogenolysis at the onset of ischemia (4). In our comparison of the hibernator heart with the summer active heart, we found a differential response to no-flow ischemia. Summer hearts attained significantly higher maximal pressures than winter hearts during ischemia and entered contracture sooner than winter hearts. Diminished ischemic contracture in torpid hearts supports prior results that indicate that torpid hibernator hearts are primed to utilize glucose (10, 14). Additionally, we report that the glycogen content of torpid hearts is significantly increased relative to summer hearts.
This increase in cardiac glycogen content is similar to that observed during fasting in nonhibernators (3). These studies also showed that increased glycogen content results in less injury and improves recovery following no-flow ischemia in isolated hearts from nonhibernators (3, 19, 26, 48). In further comparisons of hearts from fasted and fed rats under no-flow ischemia, it was found that fasted hearts were better protected from ischemic injury and had a lower lactate-to-pyruvate ratio and increased glycogen utilization (46). These and other studies also found that ischemic contracture begins when glycogen breakdown stops and the rate of glucose uptake decreases. These data indicate that the ischemic heart is better preserved as long as glycogen is present and available for energy production (13). Our observation of higher glycogen stores in torpid hearts suggests that hibernators are able to replenish glucose and glycogen stores from glycerol liberated from fatty acids during the arousal periods. Galster and Morrison (25) found that glucose-equivalent amounts of glycerol are mobilized during the periodic arousals to restore glucose reserves and that the majority of these substrates came from fatty acids, while the rest comes from proteins from urea and ammonia in urine. They also found that glucose utilization is restricted during arousals, but not during hibernation. This is further supported by our previous findings that glucose that enters the heart during arousal remains largely intact, as evidenced by a lack of labeled metabolites derived from the tricarboxylic acid cycle (1). Although we observed a decreased glycogen content in the heart during the IBA, this could be due to the timing of collection of the IBA animals. Torpid animals are collected 3 days into a TOR bout, when replenished glucose and glycogen levels remain high, and the IBA animals are collected upon full arousal. This likely does not allow time for glycogen stores to be replenished. It would be interesting in future work to assay glycogen content over the time course of the TOR-arousal cycle to more clearly look at changing glycogen levels.
Another mechanism proposed for the differential response to ischemic events in the hearts of fasted vs. fed animals is a lower cytosolic redox state in fasted animals. The redox state can profoundly affect the rate of glycolysis by inhibiting key regulatory enzymes such as GAPDH (34, 45). A lower redox state in hearts from fasted animals would result in less inhibition of GAPDH, increased glycolysis (but not necessarily glucose oxidation), increased glycogen utilization, less accumulation of glycolytic intermediates, and greater anaerobic ATP production. An accumulation of glycogen is also observed in the ischemic heart disease hibernating myocardium, where a deregulation of glycogen metabolism, from repeated short-term ischemic events, is thought to be related to stimulation of glucose uptake for glycogen synthesis (7, 17, 21, 50). The accumulation of glycogen is also found in unloaded myocardium and in the fetal heart, suggesting that all these conditions, including hibernation, induce a reliance on glucose for energy provision (16, 49). Increased glycogen stores may provide the additional glycolytic substrates during ischemia and reperfusion. This would allow for increased ATP production and likely result in maintaining Ca2+ homeostasis, resulting in less injury and delayed entry into contracture. Resistance to ischemia and hypothermia during TOR is therefore likely due to the combined effects of metabolic and Ca2+ transport remodeling.
Perspectives and significance.
Mammalian hibernators have the unique capability to resist physiological stresses, such as hypothermia, arrhythmia, and ischemic injury, that would severely injure nonhibernators. Given the impact of these stresses on human morbidity and mortality, it is of great clinical interest to understand the mechanisms by which hibernators can resist injury and support cardiac function under adverse conditions. With this work we demonstrate that resistance to injury is seasonally regulated in hibernators, corresponding with seasonal changes in Ca2+-handling gene expression and carbohydrate storage. The regulated proteomic and metabolic changes we observed are therefore useful for informing therapeutic development and improving human clinical outcomes.
GRANTS
This work was funded by National Institute on Aging Training Grant 5T32 AG-029796-05 (to F. I. Heinis), American Heart Association Predoctoral Fellowship 14PRE18970047 (to F. I. Heinis), National Heart, Lung, and Blood Institute Grants 5R01 HL-059301-14 (to J. M. Metzger) and RC2 HL-101625 (to M. T. Andrews), and US Army Medical Research and Materiel Command Contract W81XWH-11-1-0409 (to M. T. Andrews).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
F.I.H., K.L.V., M.T.A., and J.M.M. developed the concept and designed the research; F.I.H. and K.L.V. performed the experiments; F.I.H., K.L.V., M.T.A., and J.M.M. analyzed the data; F.I.H., K.L.V., M.T.A., and J.M.M. interpreted the results of the experiments; F.I.H. and K.L.V. prepared the figures; F.I.H. and K.L.V. drafted the manuscript; F.I.H., K.L.V., M.T.A., and J.M.M. edited and revised the manuscript; F.I.H., K.L.V., M.T.A., and J.M.M. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Anne Kendall for technical assistance. We acknowledge the support of the Lillehei Heart Institute.
REFERENCES
- 1.Andrews MT, Russeth KP, Drewes LR, Henry P. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am J Physiol Regul Integr Comp Physiol 296: R383–R393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews MT. Advances in molecular biology of hibernation in mammals. Bioessays 29: 431–40, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Arnall DA, Palmer WK, Miller WC, Oscai LB. Effect of fasting on myocardial substrates in male and female rats. Am J Physiol Cell Physiol 254: C560–C563, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Bailey IA, Radda GK, Seymour AM, Williams SR. The effects of insulin on myocardial metabolism and acidosis in normoxia and ischaemia. Biochim Biophys Acta 720: 17–27, 1982. [DOI] [PubMed] [Google Scholar]
- 5.Bassani J, Bassani R, Bers D. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J Physiol 476: 279–293, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bers D. Cardiac excitation-contraction coupling. Nature 415: 198–205, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Borgers M, De Nollin S, Thoné F, Wouters L, Van Vaeck L, Flameng W. Distribution of calcium in a subset of chronic hibernating myocardium in man. Histochem J 25: 312–318, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Brauch KM, Dhruv ND, Hanse EA, Andrews MT. Digital transcriptome analysis indicates adaptive mechanisms in the heart of a hibernating mammal. Physiol Genomics 23: 227–234, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Burlington RF, Whitten BK, Sidel CM, Posivaiata MA, Salkovitz IA. Effect of hypoxia on glycolysis in perfused hearts from rats and ground squirrels (Citellus lateralis). Comp Biochem Physiol 35: 403–414, 1970. [Google Scholar]
- 10.Burlington RF, Wiebers JE. Anaerobic glycolysis in cardiac tissue from a hibernator and non-hibernator as effected by temperature and hypoxia. Comp Biochem Physiol 17: 183–189, 1966. [DOI] [PubMed] [Google Scholar]
- 11.Caprette DR, Senturia JB. Isovolumetric performance of isolated ground squirrel and rat hearts at low temperature. Am J Physiol Regul Integr Comp Physiol 247: R722–R727, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Cross HR, Opie LH, Radda GK, Clarke K. Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ Res 78: 482–491, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Dark J, Miller DR. Metabolic fuel privation in hibernating and awake ground squirrels. Physiol Behav 63: 59–65, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke 37: 1261–1265, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Depre C, Havaux X, Dion R, Vanoverschelde JL. Morphologic alterations of myocardium under conditions of left ventricular assistance. J Thorac Cardiovasc Surg 115: 478–479, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Depre C, Vanoverschelde JL, Melin JA, Borgers M, Bol A, Ausma J, Dion R, Wijns W. Structural and metabolic correlates of the reversibility of left ventricular ischemic dysfunction in humans. Am J Physiol Heart Circ Physiol 268: H1265–H1275, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Dobsak P, Courderot-Masuyer C, Zeller M, Vergely C, Laubriet A, Assem M, Eicher JC, Teyssier JR, Wolf JE, Rochette L. Antioxidative properties of pyruvate and protection of the ischemic rat heart during cardioplegia. J Cardiovasc Pharmacol 34: 651–659, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Doenst T, Guthrie PH, Chemnitius JM, Zech R, Taegtmeyer H. Fasting, lactate, and insulin improve ischemia tolerance in rat heart: a comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol 270: H1607–H1615, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Drew KL, Harris MB, LaManna JC, Smith MA, Zhu XW, Ma YL. Hypoxia tolerance in mammalian heterotherms. J Exp Biol 207: 3155–3162, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Elsasser A, Schlepper M, Klovekorn WP, Cai WJ, Zimmermann R, Muller KD, Strasser R, Kostin S, Gagel C, Munkel B, Schaper W, Schaper J. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation 96: 2920–2931, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Faleschini RJ, Whitten BK. Comparative hypoxic tolerance in the Sciuridae. Comp Biochem Physiol A Comp Physiol 52: 217–221, 1975. [DOI] [PubMed] [Google Scholar]
- 23.Fedorov VV, Li L, Glukhov A, Shishkina I, Aliev RR, Mikheeva T, Nikolski VP, Rosenshtraukh LV, Efimov IR. Hibernator Citellus undulatus maintains safe cardiac conduction and is protected against tachyarrhythmias during extreme hypothermia: possible role of Cx43 and Cx45 up-regulation. Heart Rhythm 2: 966–975, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state- and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. J Cereb Blood Flow Metab 18: 168–175, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Galster W, Morrison PR. Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol 228: 325–330, 1975. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin GW, Taegtmeyer H. Metabolic recovery of isolated working rat heart after brief global ischemia. Am J Physiol Heart Circ Physiol 267: H462–H470, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, Morgan JP. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res 61: 70–76, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Hampton M, Melvin RG, Kendall AH, Kirkpatrick BR, Peterson N, Andrews MT. Deep sequencing the transcriptome reveals seasonal adaptive mechanisms in a hibernating mammal. PLos One 6: e27021, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75: 434–442, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Kerr PM, Suleiman MS, Halestrap PA. Reversal of permeability transition during recovery of hearts from ischemia and its enhancement by pyruvate. Am J Physiol Heart Circ Physiol 276: H496–H502, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kondo N, Shibata S. Calcium source for excitation-contraction coupling in myocardium of nonhibernating and hibernating chipmunks. Science 225: 641–643, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Kondo N. Excitation-contraction coupling in myocardium of nonhibernating and hibernating chipmunks: effects of isoprenaline, a high calcium medium, and ryanodine. Circ Res 59: 221–228, 1986. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz CC, Lindell SL, Mangino MJ, Carey HV. Hibernation confers resistance to intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 291: G895–G901, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Lagunas R, McLean P, Greenbaum AL. The effect of raising the NAD+ content on the pathways of carbohydrate metabolism and lipogenesis in rat liver. Eur J Biochem 15: 179–190, 1970. [DOI] [PubMed] [Google Scholar]
- 35.Li XC, Wei L, Zhang GQ, Bai ZL, Hu YY, Zhou P, Bai SH, Chai Z, Lakatta EG, Hao XM, Wang SQ. Ca2+ cycling in heart cells from ground squirrels: adaptive strategies for intracellular Ca2+ homeostasis. PLos One 6: e24787, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin-Moshier Y, Sebastian PJ, Higgins L, Sampson ND, Hewitt JE, Marchant JS. Re-evaluation of the role of calcium homeostasis endoplasmic reticulum protein (CHERP) in cellular calcium signaling. J Biol Chem 288: 355–367, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu B, Belke DD, Wang LC. Ca2+ uptake by cardiac sarcoplasmic reticulum at low temperature in rat and ground squirrel. Am J Physiol Regul Integr Comp Physiol 272: R1121–R1127, 1997. [DOI] [PubMed] [Google Scholar]
- 38.Lowe JE, Jennings RB, Reimer KA. Cardiac rigor mortis in dogs. J Mol Cell Cardiol 11: 1017–1031, 1979. [DOI] [PubMed] [Google Scholar]
- 39.Lutton LM. The effect of hypoxia on the physical performance of the thirteen-lined ground squirrel, the eastern chipmunk and the albino Norway rat. Comp Biochem Physiol 71A: 85–91, 1982. [Google Scholar]
- 40.Maier LS, Bers DM, Pieske B. Differences in Ca2+-handling and sarcoplasmic reticulum Ca2+-content in isolated rat and rabbit myocardium. J Mol Cell Cardiol 32: 2249–2258, 2000. [DOI] [PubMed] [Google Scholar]
- 41.McKean T, Mendenhall W. Comparison of the responses to hypoxia, ischaemia and ischaemic preconditioning in wild marmot and laboratory rabbit hearts. J Exp Biol 199: 693–697, 1996. [DOI] [PubMed] [Google Scholar]
- 42.Merrill GF, White JT, Krieger LW. Coronary circulation in hearts from hibernating, normothermic, and cold-acclimated hamsters. Am J Physiol Regul Integr Comp Physiol 241: R50–R54, 1981. [DOI] [PubMed] [Google Scholar]
- 43.Morii I, Kihara Y, Konishi T, Inubushi T, Sasayama S. Mechanism of the negative force-frequency relationship in physiologically intact rat ventricular myocardium—studies by intracellular Ca2+ monitor with indo-1 and by 31P-nuclear magnetic resonance spectroscopy. Jpn Circ J 60: 593–603, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Nakipova OV, Zakharova NM, Andreeva L, Chumaeva N, Averin A, Kosarskii LS, Anufriev AI, Lewinski Von D, Kockskamper J, Pieske B. The seasonal peculiarities of force-frequency relationships in active ground squirrel Spermophilus undulatus ventricle. Cryobiology 55: 173–181, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Rovetto MJ, Lamberton WF, Neely JR. Mechanisms of glycolytic inhibition in ischemic rat hearts. Circ Res 37: 742–751, 1975. [DOI] [PubMed] [Google Scholar]
- 46.Schaefer S, Ramasamy R. Glycogen utilization and ischemic injury in the isolated rat heart. Cardiovasc Res 35: 90–98, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt U, Hajjar RJ, Helm PA, Kim CS, Doye AA, Gwathmey JK. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J Mol Cell Cardiol 30: 1929–1937, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Schneider CA, Taegtmeyer H. Fasting in vivo delays myocardial cell damage after brief periods of ischemia in the isolated working rat heart. Circ Res 68: 1045–1050, 1991. [DOI] [PubMed] [Google Scholar]
- 49.Thompson EW, Marino T, Uboh CE, Kent RL, Cooper G. Atrophy reversal and cardiocyte redifferentiation in reloaded cat myocardium. Circ Res 54: 367–377, 1984. [DOI] [PubMed] [Google Scholar]
- 50.Vanoverschelde JL, Wijns W, Borgers M, Heyndrickx G, Depre C, Flameng W, Melin JA. Chronic myocardial hibernation in humans: from bedside to bench. Circulation 95: 1961–1971, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Yan L, Kudej RK, Vatner DE, Vatner SF. Myocardial ischemic protection in natural mammalian hibernation. Basic Res Cardiol 110: 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang F, Shen Y, Camp DG, Smith RD. High-pH reversed-phase chromatography with fraction concatenation for 2D proteomic analysis. Expert Rev Proteomics 9: 129–134, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatani A, Kim SJ, Kudej RK, Wang Q, Depre C, Irie K, Kranias EG, Vatner SF, Vatner DE. Insights into cardioprotection obtained from study of cellular Ca2+ handling in myocardium of true hibernating mammals. Am J Physiol Heart Circ Physiol 286: H2219–H2228, 2004. [DOI] [PubMed] [Google Scholar]