Abstract
Currently, the physiological factors responsible for exercise intolerance and bioenergetic alterations with age are poorly understood due, at least in art, to the confounding effect of reduced physical activity in the elderly. Thus, in 40 healthy young (22 ± 2 yr) and old (74 ± 8 yr) activity-matched subjects, we assessed the impact of age on: 1) the relative contribution of the three major pathways of ATP synthesis (oxidative ATP synthesis, glycolysis, and the creatine kinase reaction) and 2) the ATP cost of contraction during high-intensity exercise. Specifically, during supramaximal plantar flexion (120% of maximal aerobic power), to stress the functional limits of the skeletal muscle energy systems, we used 31P-labeled magnetic resonance spectroscopy to assess metabolism. Although glycolytic activation was delayed in the old, ATP synthesis from the main energy pathways was not significantly different between groups. Similarly, the inferred peak rate of mitochondrial ATP synthesis was not significantly different between the young (25 ± 8 mM/min) and old (24 ± 6 mM/min). In contrast, the ATP cost of contraction was significantly elevated in the old compared with the young (5.1 ± 2.0 and 3.7 ± 1.7 mM·min−1·W−1, respectively; P < 0.05). Overall, these findings suggest that, when young and old subjects are activity matched, there is no evidence of age-related mitochondrial and glycolytic dysfunction. However, this study does confirm an abnormal elevation in exercise-induced skeletal muscle metabolic demand in the old that may contribute to the decline in exercise capacity with advancing age.
Keywords: adenosine triphosphate synthesis pathways, glycolysis, phosphorus-31 magnetic resonance spectroscopy
during the transition from rest to exercise, ATP demand from cross-bridge cycling and ionic pumping may increase more than 100-fold. This requires a tight coupling between the rate of ATP hydrolysis and ATP supply, since the ATP content of skeletal muscle is low and remains relatively constant during exercise until extreme levels of fatigue (79). Interestingly, with age, at the onset of whole body exercise, the kinetics of aerobic ATP generation are slowed, and this is accompanied by an increased contribution from anaerobic sources (25). However, given the age-related decline in central and peripheral hemodynamics (53, 71), the recognized slowing of the pulmonary V̇o2 on-transient during whole body exercise may be a consequence of limited O2 delivery (9), precluding inferences regarding the effect of age on the intrinsic ability of the skeletal muscle energy system to cope with a given ATP demand.
To dissociate systemic age-related changes from skeletal muscle metabolism, small muscle mass modalities such as plantar- or dorsiflexion exercise have been used (13, 49, 64, 88). However, even with this approach, conflicting results have still been reported in terms of aging and the interplay between the energy pathways during exercise. For instance, several studies have reported a higher Pi-to-PCr ratio for a given dynamic plantar flexion exercise work rate in the old, suggesting higher metabolic demand and/or a greater reliance on oxidative ATP synthesis, compared with their younger counterparts (13, 64, 88) while, contrasting with these findings, Chilibeck et al. (8) have consistently reported similar PCr and pulmonary V̇o2 kinetics in young and old subjects at the onset of submaximal plantar flexion exercise (7–9). These differences in findings are likely mutifactorial (discrepancies in exercise paradigm, use of differing absolute or relative exercise intensities between age groups, assessment techniques, etc.); however, it is highly likely that variations in physical activity, both within and between studies, a factor accepted to affect the relative contribution of aerobic and anaerobic processes, may have also played an important role.
Interestingly, a reduced contribution from anaerobic glycolysis to ATP synthesis (49) has been proposed as the explanation for the smaller reduction in pH (44, 49) in the tibialis anterior muscle of the old during isometric exercise. Indeed, because the rates of ATP synthesis from anaerobic glycolysis were comparable between old and young subjects under ischemic conditions (50), it has been suggested that the lower glycolytic flux during isometric exercise is related to a preferential reliance upon oxidative phosphorylation rather than impaired glycolytic function. However, an alternative explanation for these and other alterations in muscle energetics documented in older individuals during dynamic exercise (13, 64, 88) may be an increased cost of contraction rather than a change in the ATP synthesis pathways. However, this possibility has rarely been investigated in human locomotor muscles (15, 58). In this context, the supramaximal model (i.e., above maximal aerobic power) during a small muscle mass exercise, in which all the skeletal muscle energy systems are stressed to their functional limit, represents an appealing approach to quantitatively evaluate the mechanisms that couple ATP synthesis to metabolic demand with age.
In a quest to unveil the physiological factors responsible for exercise intolerance and bioenergetic alterations with age, our group has recently demonstrated that, unlike muscle oxidative phosphorylation capacity, which appears to be preserved during the process of aging when physical activity is controlled (30), the ATP cost of contraction during submaximal exercise is significantly higher in older individuals (58). Mechanistically, we suggested that this is likely a result of greater metabolic demand from noncontractile processes (57). Building upon these prior investigations, the purpose of the present study was, therefore, in activity-matched young and old subjects, to quantitatively assess the impact of age on the contribution of the major energy pathways to muscular work and the ATP cost of these contractions utilizing 31P-labeled-magnetic resonance spectroscopy (MRS) during high-intensity exercise. Specifically, we hypothesized that, during supramaximal plantar flexion exercise: 1) the old would exhibit preserved oxidative and glycolytic function such that the relative contribution from the main pathways of ATP synthesis would be similar between groups, however, 2) the ATP cost of contraction would be higher in the old compared with their younger counterparts.
METHODS
Subjects.
Following informed consent procedures, 40 subjects (20 older and 20 young) with activity levels similar to the estimates of U.S. adults' habitual physical activity levels (84) participated in this study (Table 1). Because of poor 31P-MRS signal-to-noise ratio or an inability to maintain the required power output during the exercise protocol, two young and two older subjects were excluded from the study, and their data were therefore not analyzed further nor included in the current results. Healthy subjects were recruited based on age (18–25 yr for the young and >65 yr for the old) and no evidence of regular physical activity, above that required for activities of daily living, which was assessed by both questionnaire and accelerometry. All subjects were nonsmokers, free of diabetes, and diagnosed cardiovascular, peripheral vascular, neuromuscular, or pulmonary disease. Premenopausal women were studied during days 1-7 of their menstrual cycle to standardize the influence of female hormones. Women taking hormone replacement therapy were excluded from the study. Other experimental data from this cohort of subjects have already been published elsewhere (30, 58). The study was approved by the Human Research Protection Program of the University of Utah and the Salt Lake City Veterans Affairs Medical Center.
Table 1.
Subject characteristics
| Young | Old | |
|---|---|---|
| n (female/male) | 18 (9/9) | 18 (9/9) |
| Age, yr | 22 ± 1.6 | 74 ± 8.1* |
| Height, cm | 172 ± 10.1 | 170 ± 9.5 |
| Weight, kg | 69 ± 12.2 | 74 ± 15.1 |
| BMI, kg/m2 | 23 ± 3.2 | 25 ± 4.1 |
| Muscle volume, liters | 2.0 ± 0.6 | 2.1 ± 0.6 |
| Peak work rate, watts | 13 ± 5 | 9 ± 4* |
| Step, count/day | 5,927 ± 2,252 | 6,883 ± 2,766 |
| Physical activity, count/min | 157 ± 46 | 165 ± 89 |
| Glucose, mg/dl | 71.4 ± 6.4 | 80.1 ± 13.8* |
| Cholesterol, mg/dl | 177.2 ± 43.3 | 198.7 ± 28.3 |
| Triglycerides, mg/dl | 107.4 ± 78.0 | 122.1 ± 63.0 |
| HDL, mg/dl | 53.9 ± 11.0 | 54.6 ± 13.8 |
| LDL, mg/dl | 109.3 ± 35.4 | 125.7 ± 25.3 |
| WBC, K/μl | 5.8 ± 0.9 | 5.7 ± 1.1 |
| RBC, M/μl | 5.1 ± 0.4 | 4.8 ± 0.3* |
| Hemoglobin, g/dl | 15.3 ± 1.5 | 14.8 ± 1.0 |
| Hematocrit, % | 45.0 ± 3.6 | 44.3 ± 2.4 |
| Neutrophil, K/μl | 3.2 ± 0.8 | 3.4 ± 1.0 |
| Lymphocyte, K/μl | 2.0 ± 0.5 | 1.6 ± 0.5* |
| Monocyte, K/μl | 0.5 ± 0.1 | 0.5 ± 0.1 |
Values expressed as means ± SD;
n, no. of subjects; BMI, body mass index; HDL, high-density cholesterol; LDL, low-density cholesterol; WBC, white blood cells; RBC, red blood cells
P < 0.05, significantly different from young.
Exercise protocol.
After familiarization, individual maximum dynamic plantar flexion work rate (WRmax) was determined by performing incremental exercise to exhaustion (0.5- to 2-watt increments/min) to determine the maximal power corresponding to the peak aerobic capacity of the calf muscle. On a separate day, subjects performed constant-load supramaximal plantar flexion at 120% of WRmax (frequency of 1 Hz) in the whole body MR system (TimTrio 2.9T; Siemens Medical Systems, Erlangen, Germany), with the thigh and hip secured to the patient bed to isolate power production from the plantar flexors and minimize movement. Specifically, after 1 min of resting baseline assessment, subjects exercised for 1 min, followed by 5 min of recovery. The intensity and the duration of the exercise protocol were chosen based upon preliminary work to ensure that the majority of the participants would be very close to exhaustion at the end of the exercise, but would still be able to maintain the required power output throughout the protocol. An inability to maintain the same range of motion, the contraction frequency, or evidence of PCr resynthesis during the exercise were each used as criteria to exclude a subject's data from the analysis. On a different day, blood samples were collected to perform a complete blood cell count. All experimental trials were performed with participants in an overnight fasted state, and having refrained from any physical activity for 24 h.
31P-MRS.
MRS was performed using a clinical 2.9T MRI system (TimTrio; Siemens Medical Solutions) operating at 49.9 MHz for 31P resonance. 31P-MRS data were acquired with a 31P-1H dual-surface coil with linear polarization (Rapid Biomedical, Rimpar, Germany) positioned around the calf at its maximum diameter. The 31P single-loop coil diameter was 125 mm surrounding a 110-mm 1H coil loop. After a three-plane scout image was acquired, advanced localized volume shimming (9 cm × 9 cm × 9 cm) was performed. Before each experiment, two fully relaxed spectra were acquired at rest with three averages per spectrum and a repetition time (TR) of 30 s. Next, MRS data acquisition was performed throughout the rest-exercise-recovery protocol using a free induction decay pulse sequence with a 2.56-ms adiabatic-half-passage excitation radio frequency pulse and the following parameters: TR = 2 s, receiver bandwidth = 5 kHz, 1,024 data points, and 3 averages/spectrum. Saturation factors were quantified by the comparison between fully relaxed (TR = 30 s) and partially relaxed (TR = 2 s) spectra.
As previously described (55), relative concentrations of phosphocreatine ([PCr]), inorganic phosphate ([Pi]), phosphomonoester ([PME]), and ATP ([ATP]) were obtained by a time-domain fitting routine using the AMARES algorithm (85) incorporated into the CSIAPO software (60). Intracellular pH was calculated from the chemical shift difference between the Pi and PCr signals. The free cytosolic ADP concentration ([ADP]) was calculated from [PCr] and pH using the creatine kinase equilibrium constant (KCK = 1.66 × 109 M−1), with the assumption that phosphocreatine represents 85% of the total creatine content (37). The resting concentrations were calculated from the average peak areas of the two relaxed spectra (TR = 30 s; n = 3) recorded at rest and assuming an 8.2 mM ATP concentration at rest (29). When Pi splitting was evident, the pH corresponding to each Pi pool was calculated separately as pH1 and pH2 on the basis of the chemical shift of each peak relative to PCr. The overall muscle pH was then calculated as pH = pH1 (area Pi1/total Pi area) + pH2 (area Pi2/total Pi area). Most PME generated during exercise are hexose phosphate, i.e., glycolytic intermediates such as glucose 6-phosphate (∼80%) and fructose 6-phosphate (∼15%) (4, 5). Therefore, as previously suggested (19, 20), changes in [PME] were considered equivalent to changes in hexose phosphate such that accumulation of [PME] reflects additional glycogenolytic flux that has not passed through the glycolytic pathway (19, 20). Changes in pH and in the concentration of phosphorus metabolites during contraction and recovery phases were used to calculate oxidative capacity and the rates of ATP synthesis through the creatine kinase reaction, oxidative phosphorylation, and anaerobic glycolysis as previously described (40) and are detailed in the appendix. The data during the exercise were averaged over 12 s for the flux analysis.
Lower leg volume.
Lower leg volume was calculated based on lower leg circumference (three sites: distal, middle, and proximal), lower leg length, and skinfold measurements (39). This method has recently been confirmed to provide a valid estimate for muscle volume across a spectrum of individuals with normal muscle mass and severe muscle atrophy (59).
Physical activity level.
Physical activity level (PAL) was assessed using both a subjective PAL recall questionnaire and objective accelerometer data. The PAL questionnaire included items regarding the average type, frequency, intensity, and duration of physical activity in any given week. After receiving standardized operating instructions, subjects wore an accelerometer (GT1M; Actigraph, Pensacola, FL) for seven continuous days, with adherence automatically assessed. Average daily physical activity was expressed as both steps per day and total accelerometer counts per minute.
Statistical analysis.
Because there was no evidence of a significant sex effect on the main variables of the study, assessed in an initial preliminary analysis, only a main effect of age on the variables, irrespective of sex, was assessed in this study. For variables evolving with respect to time during exercise, the effect of age on the overall time course was determined using a two-way ANOVA with repeated measurements (group × time) (Statistica software; Statsoft). For each time point, post hoc comparisons (Bonferroni test) were used to compare values between the young and the old. Specifically, the first data point significantly greater than zero (i.e., baseline) was considered to reflect the onset of glycolytic flux (19). The assessment of differences between the young and the old for the other variables was performed with either independent t-tests or nonparametric Mann-Whitney tests, where appropriate (Statsoft, version 5.5; Statistica, Tulsa, Oklahoma). Statistical significance was accepted at P < 0.05. Results are presented as means ± SD in Table 1 and means ± SE in Figs. 1–5 for clarity.
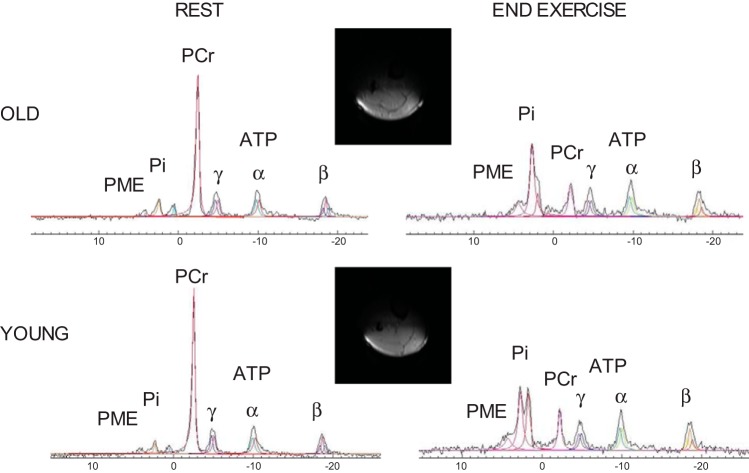
Fig. 1.
Representative examples of rapid 1H imaging transverse slices, illustrating the spatial sensitivity of the coil, and 31P spectra acquired from the plantar flexor muscles of a young and old subject at rest and at the end of the 1-min exercise bout. The signal-to-noise ratio from phosphocreatine (PCr) was 61 in the old and 95 in the young with a time resolution of 6 s. A double inorganic phosphate (Pi) peak was detected in both subjects. PME, phosphomonoester; ATP, adenosine triphosphate (α, β, γ).
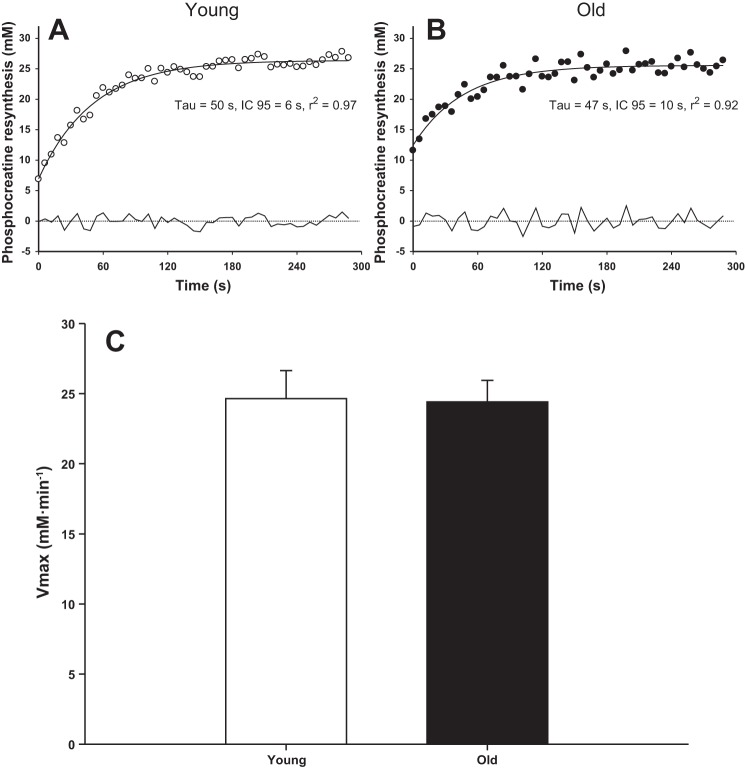
Fig. 5.
Phosphocreatine dynamics fitted according to a monoexponential function during the recovery period in a representative young (○, A) and old (●, B) subject and the mean peak rate of mitochondrial ATP synthesis (Vmax, C). The residuals to the fitted responses are documented below the curves. Vmax was not significantly different between the young and old (P > 0.05).
RESULTS
Subject characteristics and physical activity assessment.
Apart from age, the young and old subjects only differed significantly in terms of peak plantar flexion work rate, resting blood glucose levels, red blood cells, and lymphocytes (Table 1). By experimental design, the young and old subjects did not differ from each other in terms of the PAL as assessed by steps per day and total accelerometer counts per minute (Table 1).
Muscle volume and plantar flexion exercise.
Although muscle volume was not different between young and old (P > 0.05), plantar flexion WRmax was significantly lower in the old compared with the young group (Table 1, P < 0.05). Thus, the corresponding power output used for supramaximal exercise was 16 ± 6 and 11 ± 5 W in the young and old subjects, respectively (P < 0.05). Also, skinfold thickness was not significantly different between groups (young: 7.4 ± 2.4 mm; old: 7.7 ± 2.9 mm, P > 0.05).
High-energy phosphate compounds and intracellular pH.
Example MR spectra acquired from the plantar flexor muscles during the protocol for both young and old subjects are illustrated in Fig. 1. The effects of supramaximal dynamic plantar flexion exercise on phosphorylated compounds and pH in the young and old are illustrated in Fig. 2. [ATP] did not change significantly during the exercise in either group (P > 0.05).
Fig. 2.
Changes in PCr (A), adenosine diphosphate (ADP, B), pH (C), and Pi (D) with respect to time during supramaximal plantar flexion exercise. Values are presented as means ± SE. None of the variables were significantly different between the young and old (P > 0.05), and none of the subjects were eliminated from this analysis because of task failure.
ATP synthesis rates and energy cost.
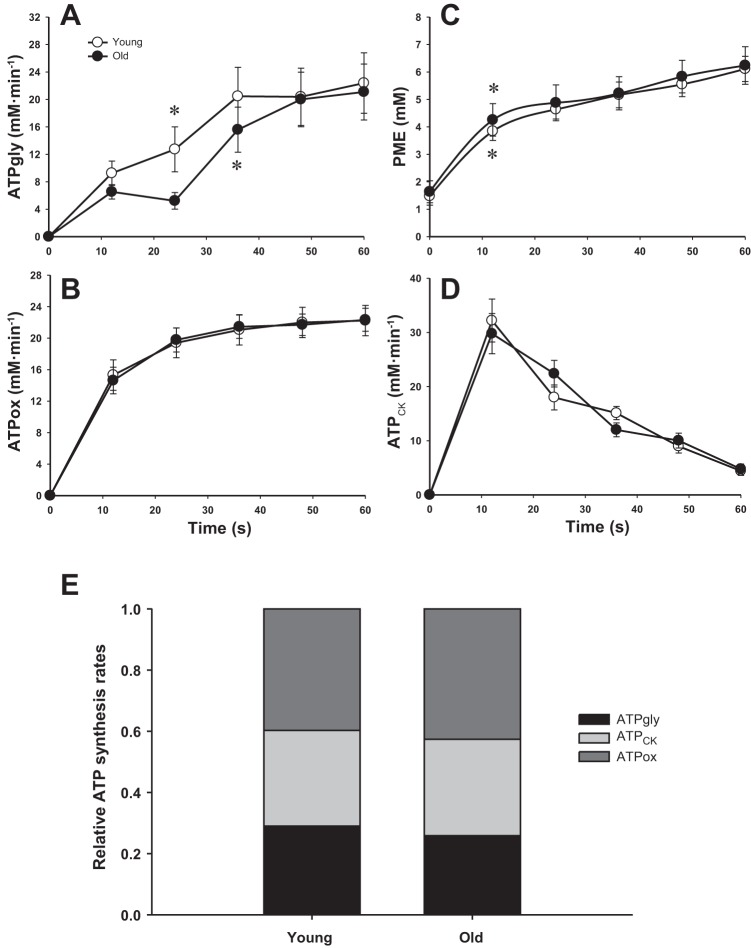
Neither absolute nor relative rates of ATP synthesis (i.e., oxidative ATP synthesis, glycolysis, and the creatine kinase reaction) were significantly different between the young and old (P > 0.05, Fig. 3). Thus, the corresponding total ATP synthesis rate during supramaximal exercise was also not significantly different between groups (P > 0.05, Fig. 4A). Because these were nonsignificant findings, as hypothesized, it should be noted that post hoc power calculations confirmed a very small effect size (d = 0.2). Indeed, these calculations revealed the need for a very large sample size (∼1,300 subjects) to affirm this as a significant difference between groups, supporting the conclusion that there was actually no effect of age. PME accumulation, which is related to glycogenolytic activation, occurred after ∼12 s in both groups (Fig. 3C), whereas glycolytic activation occurred after ∼24 s in the young and ∼36 s in the old (Fig. 3A). Despite similar total ATP synthesis rates, but as a result of the lower power output developed during the exercise, the energy cost of contraction was significantly elevated in the old subjects compared with the young (P < 0.05, Fig. 4B). In this case, post hoc power analysis revealed 1 − β = 0.6.
Fig. 3.
The rate of ATP synthesis through anaerobic glycolysis (ATPgly, A), oxidative phosphorylation (ATPox, B), and the creatine kinase reaction (ATPCK, D) with respect to time during supramaximal plantar flexion exercise. The time course of PME (C) illustrates the glycogenolytic flux that has not passed through the glycolytic pathway. Relative contribution of the anaerobic glycolysis, oxidative phosphorylation, and the creatine kinase reaction to total ATP synthesis during exercise (E). Values are presented as means ± SE. None of the ATP synthesis rates were significantly different between the young and old (P > 0.05). *First data point that is significantly greater than 0 (glycolysis) or baseline (PME).
Fig. 4.
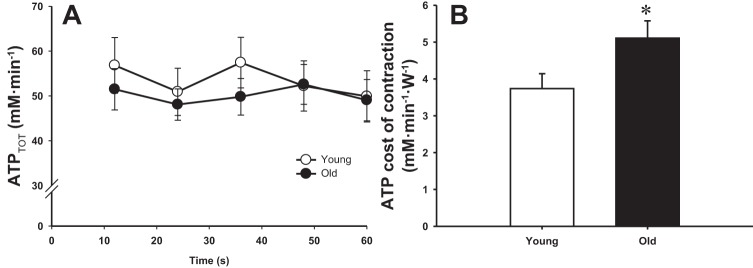
Total ATP synthesis rates (ATPTOT; A) with respect to time during supramaximal plantar flexion exercise and averaged ATP cost of contraction over the entire exercise period (B). Values are presented as means ± SE. *P < 0.05, significantly different from the young.
PCr kinetics at the offset of exercise and muscle oxidative capacity.
Representative examples of the PCr dynamics during the postexercise recovery period in the young and old are displayed in Fig. 5. The PCr recovery time constant was 45 ± 18 s in the young and 41 ± 12 s in the old (P > 0.05). The initial PCr resynthesis rate in the old (22 ± 6 mM/min) was akin to that of the young subjects (22 ± 8 mM/min, P > 0.05). Similarly, the inferred peak rate of mitochondrial ATP synthesis (Vmax) was not significantly different between the young and old (P > 0.05, Fig. 5C).
DISCUSSION
With the use of 31P-MRS, this study sought to determine the contribution of the three major energy pathways during dynamic supramaximal plantar flexor exercise in young and old physical activity-matched subjects. The principal novel findings of this study were that 1) both oxidative and glycolytic function were preserved with age such that the relative contribution from the main ATP synthesis pathways in the old were not different from the young, 2) glycolytic activation was delayed in the old, and 3) the ATP cost of contraction was substantially higher in the old compared with their younger counterparts. Overall, these findings imply that an abnormally elevated metabolic demand from skeletal muscle, rather than the capacity of the three main energy pathways to generate ATP, may play a role in the decline in skeletal muscle exercise capacity with advancing age.
Oxidative ATP synthesis.
A novel and important finding of the present study was the comparable oxidative ATP synthesis, expressed either as absolute or relative rates, during dynamic supramaximal exercise in old and young subjects (Fig. 3). Therefore, aging does not compromise the intrinsic aerobic capacity of skeletal muscle to adequately support a sudden increase in ATP demand caused by muscle contraction. Consistent with the results obtained during exercise, the peak rate of mitochondrial ATP synthesis (Vmax) inferred from the postexercise recovery period was also not significantly different between the young and old subjects (Fig. 5C), which further supports the suggestion that age per se does not impair mitochondrial function in vivo (30) and ex vivo (72).
Of note, the peak rates measured during the supramaximal exercise were close to the Vmax calculated during the recovery period in both young and old (∼24 mM/min), supporting both the maximal nature of our protocol and the low-activity status of our subjects. Indeed, the peak rates of oxidative ATP synthesis during dynamic plantar flexion (∼22 mM/min) were consistent with those of previous studies conducted in untrained individuals (2, 34, 69). For instance, Hunter et al. (34) and Newcomer and Bosca (69) reported a rate of ∼18–19 mM/min during a 90-s maximal isometric contraction of the calf muscle in untrained adults. With moderately active or trained sprinters as subjects, which can dramatically increase the peak rate of mitochondrial respiration (54), Walter et al. (86) reported much higher values (∼40 mM/min) during similar supramaximal dynamic plantar flexion exercise.
Consistent with the current study, Chilibeck et al. (7–9) have previously reported similar PCr and pulmonary V̇o2 kinetics in young and old subjects during the transition from rest to submaximal plantar flexion exercise. However, unlike these former studies, an advantage of the 31P-MRS approach employed here is that skeletal muscle energy systems were stressed to their functional limit during a supramaximal exercise, and all three major ATP synthesis pathways (oxidative phosphorylation, glycolysis, and creatine kinase reaction) were examined quantitatively. Our findings do, however, somewhat contrast with a previous study documenting a greater reliance upon oxidative ATP synthesis during a 60-s maximal isometric voluntary contraction in the tibialis anterior of older individuals (49). However, the discrepancy between this study and the current results may be explained by the differences in force generation between the young and old subjects. Indeed, the young men in the study by Lanza et al. (49) generated an ∼50% greater force during the isometric contraction, which likely generated higher intramuscular pressure and potentially compromised blood flow (87). Accordingly, the lower oxidative ATP synthesis reported in the young subjects might, in fact, be attributable to a compromised O2 availability rather than a greater reliance on oxidative phosphorylation in the elderly (49). This confounding factor was likely less of an issue in the current study, since subjects performed dynamic contractions, likely preserving peripheral hemodynamics, even at the higher work rates.
A similar finding to the study by Lanza et al. (49) (i.e., a greater reliance on oxidative phosphorylation) was recently reported by the same group in old compared with young subjects matched for maximum voluntary contraction (10). However, it should be underscored that the muscle investigated by both Lanza et al. (49) and Christie et al. (10), the tibialis anterior, exhibits some unique features, most notably a preserved mitochondrial efficiency with age (1) and a maintained or even increased oxidative capacity in the elderly (10, 51, 80), perhaps as a result of a greater activation of this muscle for postural control in this population (52). These potentially unique age- and muscle-specific adaptations render the comparison with these studies more difficult. Other studies have used dynamic plantar flexion to investigate the effects of age on muscle energetics (13, 64, 88). Although not quantitative, these studies suggested a greater reliance on oxidative phosphorylation in older individuals as inferred from a higher Pi-to-PCr ratio for a given work rate. However, in these cases, either physical activity was not controlled (13, 64) or relative exercise intensity between the age groups was different (88), which may explain the discrepancy with the current results.
ATP synthesis from glycolysis and the creatine kinase reaction.
There is accumulating evidence that glycolysis is activated according to a “dual control” model (14, 16, 19, 20). Accordingly, both a feedforward signal related to muscle contraction (e.g., intracellular Ca2+) and a feedback signal related to metabolic demand/metabolite accumulation [ADP, AMP, and Pi affecting phosphofructokinase activity (17) or glycogen phosphorylase activity via Pi and H+ (73)] regulates glycolytic rate. In light of this, a major goal of the present study was to comprehensively compare and contrast the anaerobic ATP synthesis flux within the skeletal muscle of young and old subjects in response to supramaximal dynamic plantar flexion exercise. Interestingly, although the relative ATP synthesis rates from glycolysis and the creatine kinase reaction averaged over the entire exercise bout were similar in the young and old, glycolytic activation was delayed in the old subjects (∼36 s) compared with the young (∼24 s, Fig. 3A).
In terms of metabolic control, it has been suggested that a threshold of metabolite accumulation must be achieved before contraction-related signals can increase glycolytic flux (19). However, it is unlikely that the longer delay in glycolysis activation in the old subjects can be explained by different time to reach this threshold, since the levels of [Pi] (old: ∼15 mM; young: ∼13 mM) and [ADP] (old: ∼93 μM; young: ∼61 μM) were actually higher, not lower, in the old at the activation of glycolysis. In addition, the levels of these metabolites are well above the Km for phosphofructokinase activation in vitro (1 mM and 30 μM for Pi and ADP, respectively) (17). A shortage in substrate such as glucose 6-phosphate and fructose 6-phosphate can also be ruled out since [PME] levels significantly increased after only ∼12 s in both groups, implying that hexokinase was activated and glycogenolysis generation of subtraste was actually in excess, compared with glycolytic flux, in both the young and old (Fig. 3C).
A mechanism that may explain the longer delay for the onset of glycolysis rate in the elderly is a different intracellular Ca2+ level between groups. Indeed, based on in vitro experiments (62, 63) and computer simulation (47, 77), it has been suggested that Ca2+-calmodulin activation of the phosphofructokinase enzyme has a dominant role in controlling the glycolytic flux during skeletal muscle contraction, although metabolite feedback and H+ concentration accumulation are also required (19, 20, 41, 47). In the present study, although the total rate of ATP turnover was similar between the old and young (Fig. 4A), elderly subjects exercised at a lower absolute power output. This implies that the Ca2+ release from the sarcoplasmic reticulum and the intracellular Ca2+ may have been lower in the old compared with the young. This would lead to a lower feedforward signal requiring greater level of metabolites (Pi, ADP, and AMP) and lower pH to activate an increase in glycolysis rate. In support of this theory, as already indicated, compared with the young subjects, the old tended to exhibit higher levels of [Pi] and [ADP] at the activation of glycolysis.
Another interesting observation from the present study was the similar rates of ATP synthesis from glycolysis and the creatine kinase reaction over the entire exercise in the young and old subjects. This was the case whether expressed as absolute or relative total ATP synthesis (Fig. 3E) and is in agreement with a previous study demonstrating an unchanged creatine kinase reaction flux using the magnetization transfer technique (33).
ATP cost of contraction.
In agreement with a previous study investigating the same muscle group but at a lower exercise intensity (58), the ATP cost of contraction during dynamic supramaximal plantar flexion exercise was substantially elevated in the old (∼37%) compared with the young (Fig. 4B). Interestingly, previous studies have reported that the ATP cost of contraction was improved (80), unchanged (10, 15, 56), or impaired (56) during exercise localized to the tibialis anterior and the quadriceps muscles. Taken together, these results provide support for the hypothesis that age-related alterations in muscle efficiency may vary among muscles depending on the chronic activity of the muscle itself. Additionally, the rate of contraction employed during the task can substantially affect conclusions regarding muscle metabolism, which could also explain some of the discrepancies in this field of study, since older individuals have been documented to exhibit an impaired ATP cost of contraction during intermittent contractions, but not during a continuous isometric contraction (56).
Although very interesting and innovative, some methodological concerns have been raised (57) regarding a recent study reporting a preserved cost of contraction in the quadriceps with age by Conley and colleagues (15). Specifically, the combined analysis of data from different exercise modalities, and assumptions about muscle recruitment during exercise in both young and old, had some bearing on the interpretation of the findings. Despite these limitations, the suggestion in this study (15) that mitochondrial dysfunction can contribute to a reduction in muscle efficiency should not be ruled out, since these two mechanisms (contractile and/or mitochondrial inefficiency) are not mutually exclusive and, in fact, may both explain the increase in the cost of locomotion and greater fatigability associated with aging (12, 76).
The mechanisms underlying this age-related increase in ATP cost of muscle contraction are likely numerous, but an increase in the noncontractile processes of ion transport (Ca2+-ATPase and Na+-K+-ATPase) may account for much of the present findings. Indeed, an excessive energy demand from ion pumping in the skeletal muscle of older individuals has recently been implied, by our group (57), to contribute to the decline in muscle efficiency with age. In line with this concept, slower rates of relaxation after contractions evoked by electrical stimulation have been consistently documented in older subjects (3, 28, 36, 74) and associated with lower Ca2+ uptake and Ca2+-ATPase activity measured in vitro (36, 45). This impairment in Ca2+ sequestration by the sarcoplasmic reticulum would further contribute to the slowing of muscle fiber cross-bridge dissociation, prolonging relaxation and total contraction time, further elevating the metabolic cost of dynamic exercise.
Additionally, an age-related slowing of the contractile properties specific to the plantar flexor muscle has previously been documented (22, 24, 81), likely affecting skeletal muscle metabolic demand in older individuals during dynamic exercise. This is potentially the consequence of a slower rate of myosin attachment and detachment to actin (66), which may eventually decrease fiber power output, although this has yet to be confirmed. In addition, there is a growing appreciation that older individuals rely more on torque-generating capacity for power production due to an impaired velocity of contractile function (21) and impaired membrane excitability (24), thus impeding rapid muscular contraction and relaxation. Therefore, it is likely that, in combination, the slower contractile properties and excessive energy demand from the noncontractile processes of ion transport may all contribute to exacerbate the energy cost of dynamic contractions with age.
Aging is generally associated with a shift in fiber type toward slow-twitch oxidative fibers (61), which, conceptually, should result in improved muscle efficiency (18, 35) not a reduction in contractile efficiency, as reported here. However, it should be noted that conflicting results, again in the tibialis anterior, have both supported (80) and challenged (10) a role for fiber type in age-related changes in muscle efficiency. Therefore, because fiber type composition was not assessed in the present study, it is, at present, unclear to what extent a shift in fiber type may have influenced the current findings.
As previously discussed in more detail (58), to some extent, mechanical factors, for example, antagonist muscle coactivation, may have contributed to the greater ATP cost of contraction in the elderly. However, the use of a rather simple task (plantar flexion exercise) and experimental evidence questioning the importance of enhanced antagonist muscle coactivation during plantar flexion exercise in elderly subjects (70, 78) suggests that this effect may be minor.
The ATP cost of contraction may also increase in the elderly as a consequence of motor neuron loss with age (67, 75). However, this hypothesis does not seem to pertain to the soleus muscle (22, 23), and the dynamic process of fiber reinnervation, through collateral sprouting, appears to successfully compensate for neuronal loss in the early stage of aging (65, 81). Also, the age-related deficit in the ability to finely control force or movement during a motor task appears to be limited to low-intensity contraction and can be restored to the level of the young by a single-practice session (11). Together, it appears unlikely that age-related alteration in neural activation of agonist muscles and greater motor output variability contributed substantially to the greater ATP cost of contraction documented in the current study.
Experimental considerations.
Careful monitoring of the movement of the weight used for resistance during plantar flexion ensured that subjects exercised within the full range of motion throughout the exercise and maintained a consistent power output. However, the lack of a direct measurement of the power output is a limitation of the current study.
It could be expected that the skeletal muscle fat infiltration that occurs with aging (27) may affect the quantification of muscle volume using the current anthropometric approach. However, although this phenomenon is of clinical interest, it is unlikely that fat infiltration resulted in a significant error in our estimation of muscle volume. Indeed, the validity of this anthropometrically based method has been demonstrated multiple times in both young (46, 82, 83) and old subjects (6, 26), as well as in a wide spectrum of individuals including those with a spinal cord injury (59), a group recognized to exhibit significant muscle atrophy and fat infiltration. In addition, a recent MR imaging study comparing the dorsi- and plantar flexor muscles in two very distinct groups with regard to their anthropometric characteristics documented a limited increase in the relative proportion of the noncontractile tissues from ∼2–3% in normal-weight active young to ∼8–10% in overweight to obese old (31). Together, these findings therefore suggest that, in the current, normal-weight and activity-matched subjects, such a bias in terms of the estimation of muscle volume induced by the infiltration of fat into the muscle was likely negligible.
Perspectives and Significance
Here our findings indicate that the intrinsic ability of the skeletal muscle energy system to cope with a given ATP demand does not appear to be affected by chronological age but rather by changes in physical activity. We also identified contractile inefficiency as a key factor in the age-related reduction in exercise capacity, and this phenomenon should be the target for future intervention to reverse this source of debilitation in the elderly.
In conclusion, this study reveals that when young and old subjects are activity matched, apart from delayed glycolytic activation in the old, there is no evidence of age-related mitochondrial and glycolytic dysfunction. However, this study does reveal an abnormal elevation in exercise-induced skeletal muscle metabolic demand in the old (∼37%) that may contribute to the decline in exercise capacity with advancing age.
GRANTS
This work was funded in part by National Heart, Lung, and Blood Institute Grant PO1-HL-091830 and Veterans Affairs Merit Grant E6910R.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: G.L., S.-E.K., Y.L.F., E.-K.J., and R.S.R. conception and design of research; G.L., J.D.T., C.R.H., S.-E.K., H.J.G., J.R.S., and E.-K.J. performed experiments; G.L., J.D.T., C.R.H., Y.L.F., J.R.S., and R.S.R. analyzed data; G.L. interpreted results of experiments; G.L. prepared figures; G.L. drafted manuscript; G.L., J.D.T., C.R.H., S.-E.K., H.J.G., Y.L.F., J.R.S., E.-K.J., and R.S.R. edited and revised manuscript; G.L., J.D.T., C.R.H., S.-E.K., H.J.G., Y.L.F., J.R.S., E.-K.J., and R.S.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all of the subjects in this study for committed participation in this research.
Appendix
ATP production from PCr breakdown.
The rate of ATP production from the breakdown of PCr (ATPCK) through the creatine kinase (CK) reaction (mM/min) was calculated from the change in PCr for each time point of the exercise period (40):
ATP production from oxidative phosphorylation.
Based on the sigmoid relationship between the oxidative ATP production rate (ATPox, mM/min) and free cytosolic ADP concentration ([ADP]), the rate of mitochondrial ATP production was calculated as follows:
in which Km (the [ADP] at half-maximal oxidation rate) is ∼30 μM in skeletal muscle (40), 2.2 is the Hill coefficient for a sigmoid function (38), and Vmax is the inferred peak rate of mitochondrial respiration in vivo.
Vmax (in mM/min) was calculated using the initial rate of PCr resynthesis (ViPCr) during the recovery period, and [ADP] was measured at the end of exercise:
the ViPCr was calculated from the derivative of the next equation at time 0:
in which Δ[PCr] represents the amount of PCr resynthesized during the recovery, and the rate constant k = 1/τ (40). The first-order PCr recovery rate constant (k) was determined from a fitting of the PCr time-dependent changes during the recovery period to a single exponential curve described by the equation:
where Yend is the level of [PCr] measured at the end of exercise, and Yres refers to the amount of PCr resynthesized during the recovery.
Model variables were determined with an iterative process by minimizing the sum of squared residuals between the fitted function and the observed values. Goodness of fit was assessed by visual inspection of the residual plot, and the frequency plot distribution of the residuals, Chi square values, and the coefficient of determination (r2) were calculated as follows (68):
where SSreg is the sum of squares of the residuals from the fit, and SStot is the sum of squares of the residuals from the mean.
ATP production from anaerobic glycolysis.
Throughout the exercise period, glycogen breakdown to pyruvate and lactate, proton efflux, buffering capacity, protons produced by oxidative phosphorylation, and the consumption of protons by the CK reaction lead to changes in intramuscular pH (40). Assuming that the glycogenolytic production of 1 mol of H+, when coupled to ATP hydrolysis, yields 1.5 mol of ATP, ATP production from anaerobic glycolysis (ATPgly) can be deduced from the total number of protons (P) produced throughout exercise (32):
HCK+ (in mM/min) was calculated from the time-dependent changes in [PCr] and from the stoichiometric coefficient (γ):
where γ is the proton stoichiometric coefficient of the coupled Lohmann reaction as described previously (48).
Hβ+ (in mM/min) was calculated from the apparent buffering capacity βtotal (in Slykes, mmol acid added/unit change in pHi) and from the rate of pH changes:
where
where
in which βa was determined from the initial change in PCr (ΔPCri) and alkalinization of pH (ΔpHi) (14):
βPi and βPME were determined based on the dissociation constant of the buffer (K) according to the standard formula (16):
where X is either Pi or PME and K = 1.77 × 10−7 for Pi and 6.3 × 10−7 for PME.
In agreement with previous studies and assuming that muscle is a closed system during exercise (16, 42), βbicarbonate was set to zero.
Hox+ (in mM/min) was calculated from the factor m = 0.16/[1 + 10(6.1 − pH)], which accounts for the amount of protons produced through oxidative ATP production:
Hefflux+ (in mM/min) was calculated for each time point of exercise using the proportionality constant λ relating proton efflux rate to ΔpHi:
This proportionality constant λ (in mM·min−1·pH unit−1) was calculated during the recovery period:
During this period, PCr is regenerated throughout the CK reaction as the consequence of oxidative ATP production in mitochondria. Thus, Hefflux+ can be calculated from the rates of proton production from the CK reaction (HCK+, in mM/min) and mitochondrial ATP production (Hox+, in mM/min) on one side and the rate of pH changes on the other side. At this time, ATP production is exclusively aerobic, and lactate production is considered as negligible:
To improve precision, we use a modified version of this calculation (43) in which the total proton disappearance (i.e., ∫Edt) is estimated cumulatively from the start of recovery and then fitted to an exponential function to obtain the initial recovery rate E.
Total ATPase rate.
The total ATPase rate (ATPtot, mM/min) was calculated for each time point as:
Energy cost of contraction (in mM/W) was calculated as the ratio between total ATP production (ATPox + ATPCK + ATPgly) and power output.
REFERENCES
- 1.Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc Natl Acad Sci USA 104: 1057–1062, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boska M. ATP production rates as a function of force level in the human gastrocnemius/soleus using 31P MRS. Magn Reson Med 32: 1–10, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Callahan DM, Kent-Braun JA. Effect of old age on human skeletal muscle force-velocity and fatigue properties. J Appl Physiol 111: 1345–1352, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasiotis D, Bergstrom M, Hultman E. ATP utilization and force during intermittent and continuous muscle contractions. J Appl Physiol 63: 167–174, 1987. [DOI] [PubMed] [Google Scholar]
- 5.Cheetham ME, Boobis LH, Brooks S, Williams C. Human muscle metabolism during sprint running. J Appl Physiol 61: 54–60, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Chen BB, Shih TT, Hsu CY, Yu CW, Wei SY, Chen CY, Wu CH, Chen CY. Thigh muscle volume predicted by anthropometric measurements and correlated with physical function in the older adults. J Nutr Health Aging 15: 433–438, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Chilibeck PD, Paterson DH, Cunningham DA, Taylor AW, Noble EG. Muscle capillarization O2 diffusion distance, and V̇o2 kinetics in old and young individuals. J Appl Physiol 82: 63–69, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Chilibeck PD, Paterson DH, McCreary CR, Marsh GD, Cunningham DA, Thompson RT. The effects of age on kinetics of oxygen uptake and phosphocreatine in humans during exercise. Exp Physiol 83: 107–117, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Chilibeck PD, Paterson DH, Smith WD, Cunningham DA. Cardiorespiratory kinetics during exercise of different muscle groups and mass in old and young. J Appl Physiol 81: 1388–1394, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Christie AD, Tonson A, Larsen RG, DeBlois JP, Kent JA. Human skeletal muscle metabolic economy in vivo: effects of contraction intensity, age, and mobility impairment. Am J Physiol Regul Integr Comp Physiol 307: R1124–R1135, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christou EA. Aging and variability of voluntary contractions. Ex Sport Sci Rev 39: 77–84, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coen PM, Jubrias SA, Distefano G, Amati F, Mackey DC, Glynn NW, Manini TM, Wohlgemuth SE, Leeuwenburgh C, Cummings SR, Newman AB, Ferrucci L, Toledo FG, Shankland E, Conley KE, Goodpaster BH. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol 68: 447–455, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coggan AR, Abduljalil AM, Swanson SC, Earle MS, Farris JW, Mendenhall LA, Robitaille PM. Muscle metabolism during exercise in young and older untrained and endurance-trained men. J Appl Physiol 75: 2125–2133, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. Activation of glycolysis in human muscle in vivo. Am J Physiol Cell Physiol 273: C306–C315, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Conley KE, Jubrias SA, Cress ME, Esselman P. Exercise efficiency is reduced by mitochondrial uncoupling in the elderly. Exp Physiol 98: 768–777, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Conley KE, Kushmerick MJ, Jubrias SA. Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol 511: 935–945, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connett RJ. In vivo control of phosphofructokinase: system models suggest new experimental protocols. Am J Physiol Regul Integr Comp Physiol 257: R878–R888, 1989. [DOI] [PubMed] [Google Scholar]
- 18.Crow MT, Kushmerick MJ. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol 79: 147–166, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting skeletal muscle. I. Turning it on. Am J Physiol Endocrinol Metab 282: E67–E73, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Crowther GJ, Kemper WF, Carey MF, Conley KE. Control of glycolysis in contracting skeletal muscle. II. Turning it off. Am J Physiol Endocrinol Metab 282: E74–E79, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Dalton BH, Allen MD, Power GA, Vandervoort AA, Rice CL. The effect of knee joint angle on plantar flexor power in young and old men. Exp Gerontol 52: 70–76, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol 107: 1781–1788, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Dalton BH, McNeil CJ, Doherty TJ, Rice CL. Age-related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle Nerve 38: 1108–1115, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Dalton BH, Power GA, Vandervoort AA, Rice CL. Power loss is greater in old men than young men during fast plantar flexion contractions. J Appl Physiol 109: 1441–1447, 2010. [DOI] [PubMed] [Google Scholar]
- 25.DeLorey DS, Paterson DH, Kowalchuk JM. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metab 32: 1251–1262, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol 90: 2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Harridge S, Magnusson G, Saltin B. Life-long endurance-trained elderly men have high aerobic power, but have similar muscle strength to non-active elderly men. Aging 9: 80–87, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest 33: 109–120, 1974. [PubMed] [Google Scholar]
- 30.Hart CR, Layec G, Trinity JD, Liu X, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS. Evidence of preserved oxidative capacity and oxygen delivery in the plantar flexor muscles with age. J Gerontol In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasson CJ, Kent-Braun JA, Caldwell GE. Contractile and non-contractile tissue volume and distribution in ankle muscles of young and older adults. J Biomechan 44: 2299–2306, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hochachka PW, Mommsen TP. Protons and anaerobiosis. Science 219: 1391–1397, 1983. [DOI] [PubMed] [Google Scholar]
- 33.Horska A, Fishbein KW, Fleg JL, Spencer RG. The relationship between creatine kinase kinetics and exercise intensity in human forearm is unchanged by age. Am J Physiol Endocrinol Metab 279: E333–E339, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Hunter GR, Bamman MM, Larson-Meyer DE, Joanisse DR, McCarthy JP, Blaudeau TE, Newcomer BR. Inverse relationship between exercise economy and oxidative capacity in muscle. Eur J Appl Physiol 94: 558–568, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Hunter GR, Newcomer BR, Larson-Meyer DE, Bamman MM, Weinsier RL. Muscle metabolic economy is inversely related to exercise intensity and type II myofiber distribution. Muscle Nerve 24: 654–661, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Hunter SK, Thompson MW, Ruell PA, Harmer AR, Thom JM, Gwinn TH, Adams RD. Human skeletal sarcoplasmic reticulum Ca2+ uptake and muscle function with aging and strength training. J Appl Physiol 86: 1858–1865, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Jeneson JA, Westerhoff HV, Brown TR, Van Echteld CJ, Berger R. Quasi-linear relationship between Gibbs free energy of ATP hydrolysis and power output in human forearm muscle. Am J Physiol Cell Physiol 268: C1474–C1484, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Jeneson JA, Wiseman RW, Westerhoff HV, Kushmerick MJ. The signal transduction function for oxidative phosphorylation is at least second order in ADP. J Biol Chem 271: 27995–27998, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol 204: 63P–66P, 1969. [PubMed] [Google Scholar]
- 40.Kemp GJ, Radda GK. Quantitative interpretation of bioenergetic data from 31P and 1H magnetic resonance spectroscopic studies of skeletal muscle: an analytical review. Magn Reson Q 10: 43–63, 1994. [PubMed] [Google Scholar]
- 41.Kemp GJ, Roussel M, Bendahan D, Le Fur Y, Cozzone PJ. Interrelations of ATP synthesis and proton handling in ischaemically exercising human forearm muscle studied by 31P magnetic resonance spectroscopy. J Physiol 535: 901–928, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kemp GJ, Taylor DJ, Styles P, Radda GK. The production, buffering and efflux of protons in human skeletal muscle during exercise and recovery. NMR Biomed 6: 73–83, 1993. [DOI] [PubMed] [Google Scholar]
- 43.Kemp GJ, Thompson CH, Taylor DJ, Radda GK. Proton efflux in human skeletal muscle during recovery from exercise. Eur J Appl Physiol Occupat Physiol 76: 462–471, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Kent-Braun JA, Ng AV, Doyle JW, Towse TF. Human skeletal muscle responses vary with age and gender during fatigue due to incremental isometric exercise. J Appl Physiol 93: 1813–1823, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Klitgaard H, Ausoni S, Damiani E. Sarcoplasmic reticulum of human skeletal muscle: age-related changes and effect of training. Acta Physiol Scand 137: 23–31, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Knapik JJ, Staab JS, Harman EA. Validity of an anthropometric estimate of thigh muscle cross-sectional area. Med Sci Sports Exercise 28: 1523–1530, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Korzeniewski B, Liguzinski P. Theoretical studies on the regulation of anaerobic glycolysis and its influence on oxidative phosphorylation in skeletal muscle. Biophys Chem 110: 147–169, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Kushmerick MJ. Multiple equilibria of cations with metabolites in muscle bioenergetics. Am J Physiol Cell Physiol 272: C1739–C1747, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J Appl Physiol 99: 1736–1744, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Lanza IR, Larsen RG, Kent-Braun JA. Effects of old age on human skeletal muscle energetics during fatiguing contractions with and without blood flow. J Physiol 583: 1093–1105, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen RG, Callahan DM, Foulis SA, Kent-Braun JA. Age-related changes in oxidative capacity differ between locomotory muscles and are associated with physical activity behavior. Appl Physiol Nutr Metab 37: 88–99, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laughton CA, Slavin M, Katdare K, Nolan L, Bean JF, Kerrigan DC, Phillips E, Lipsitz LA, Collins JJ. Aging, muscle activity, and balance control: physiologic changes associated with balance impairment. Gait Posture 18: 101–108, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Layec G, Bringard A, Le Fur Y, Vilmen C, Micallef JP, Perrey S, Cozzone PJ, Bendahan D. Comparative determination of energy production rates and mitochondrial function using different 31P MRS quantitative methods in sedentary and trained subjects. NMR Biomed 24: 425–438, 2011. [DOI] [PubMed] [Google Scholar]
- 55.Layec G, Bringard A, Vilmen C, Micallef JP, Fur YL, Perrey S, Cozzone PJ, Bendahan D. Accurate work-rate measurements during in vivo MRS studies of exercising human quadriceps. Magma 21: 227–235, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Layec G, Hart CR, Trinity JD, Le Fur Y, Jeong EK, Richardson RS. Skeletal muscle work efficiency with age: the role of non-contractile processes. Clin Sci Lond 128: 213–223, 2015. [DOI] [PubMed] [Google Scholar]
- 57.Layec G, Hart CR, Trinity JD, Le Fur Y, Jeong EK, Richardson RS. Skeletal muscle work efficiency with age: the role of non-contractile processes. Clin Sci Lond In press. [DOI] [PubMed] [Google Scholar]
- 58.Layec G, Trinity JD, Hart CR, Kim SE, Groot HJ, Le Fur Y, Sorensen JR, Jeong EK, Richardson RS. In vivo evidence of an age-related increase in ATP cost of contraction in the plantar flexor muscles. Clin Sci Lond 126: 581–592, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Layec G, Venturelli M, Jeong EK, Richardson RS. The validity of anthropometric leg muscle volume estimation across a wide spectrum: From able-bodied adults to individuals with a spinal cord injury. J Appl Physiol 116: 1142–1147, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ, Kober F. Grid-free interactive and automated data processing for MR chemical shift imaging data. Magma 23: 23–30, 2010. [DOI] [PubMed] [Google Scholar]
- 61.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol 50: 11–16, 1995. [DOI] [PubMed] [Google Scholar]
- 62.Marinho-Carvalho MM, Costa-Mattos PV, Spitz GA, Zancan P, Sola-Penna M. Calmodulin upregulates skeletal muscle 6-phosphofructo-1-kinase reversing the inhibitory effects of allosteric modulators. Biochim Biophys Acta 1794: 1175–1180, 2009. [DOI] [PubMed] [Google Scholar]
- 63.Marinho-Carvalho MM, Zancan P, Sola-Penna M. Modulation of 6-phosphofructo-1-kinase oligomeric equilibrium by calmodulin: formation of active dimers. Mol Genet Metab 87: 253–261, 2006. [DOI] [PubMed] [Google Scholar]
- 64.McCully KK, Fielding RA, Evans WJ, Leigh JS Jr, Posner JD. Relationships between in vivo and in vitro measurements of metabolism in young and old human calf muscles. J Appl Physiol 75: 813–819, 1993. [DOI] [PubMed] [Google Scholar]
- 65.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve 31: 461–467, 2005. [DOI] [PubMed] [Google Scholar]
- 66.Miller MS, Toth MJ. Myofilament protein alterations promote physical disability in aging and disease. Ex Sport Sci Rev 41: 93–99, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mittal KR, Logmani FH. Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol 42: 8–10, 1987. [DOI] [PubMed] [Google Scholar]
- 68.Motulsky HJ, Christopoulos A. Fitting models to biological data using linear and nonlinear regression. A practical guide to curve fitting (Abstract). BMC Bioinform 7: 123, 2006. [Google Scholar]
- 69.Newcomer BR, Boska MD. Adenosine triphosphate production rates, metabolic economy calculations, pH, phosphomonoesters, phosphodiesters, and force output during short-duration maximal isometric plantar flexion exercises and repeated maximal isometric plantar flexion exercises. Muscle Nerve 20: 336–346, 1997. [DOI] [PubMed] [Google Scholar]
- 70.Ochala J, Lambertz D, Pousson M, Goubel F, Hoecke JV. Changes in mechanical properties of human plantar flexor muscles in ageing. Exp Gerontol 39: 349–358, 2004. [DOI] [PubMed] [Google Scholar]
- 71.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation 13: 315–327, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Experimental evidence against the mitochondrial theory of aging. A study of isolated human skeletal muscle mitochondria. Exp Gerontol 38: 877–886, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Ren JM, Hultman E. Regulation of phosphorylase a activity in human skeletal muscle. J Appl Physiol 69: 919–923, 1990. [DOI] [PubMed] [Google Scholar]
- 74.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve 22: 1094–1103, 1999. [DOI] [PubMed] [Google Scholar]
- 75.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PloS one 7: e29082, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santanasto AJ, Glynn NW, Jubrias SA, Conley KE, Boudreau RM, Amati F, Mackey DC, Simonsick EM, Strotmeyer ES, Coen PM, Goodpaster BH, Newman AB. Skeletal muscle mitochondrial function and fatigability in older adults. J Gerontol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmitz JP, Groenendaal W, Wessels B, Wiseman RW, Hilbers PA, Nicolay K, Prompers JJ, Jeneson JA, van Riel NA. Combined in vivo and in silico investigations of activation of glycolysis in contracting skeletal muscle. Am J Physiol Cell Physiol 304: C180–C193, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Simoneau EM, Billot M, Martin A, Van Hoecke J. Antagonist mechanical contribution to resultant maximal torque at the ankle joint in young and older men. J Electromyogr Kinesiol 19: e123–e131, 2009. [DOI] [PubMed] [Google Scholar]
- 79.Taylor DJ, Styles P, Matthews PM, Arnold DA, Gadian DG, Bore P, Radda GK. Energetics of human muscle: exercise-induced ATP depletion. Magn Reson Med 3: 44–54, 1986. [DOI] [PubMed] [Google Scholar]
- 80.Tevald MA, Foulis SA, Lanza IR, Kent-Braun J. A Lower energy cost of skeletal muscle contractions in older humans. Am J Physiol Regul Integr Comp Physiol 298: R729–R739, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson BJ, Ryan ED, Herda TJ, Costa PB, Herda AA, Cramer JT. Age-related changes in the rate of muscle activation and rapid force characteristics. Age 36: 839–849, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tonson A, Ratel S, Le Fur Y, Cozzone P, Bendahan D. Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc 40: 918–925, 2008. [DOI] [PubMed] [Google Scholar]
- 83.Tothill P, Stewart AD. Estimation of thigh muscle and adipose tissue volume using magnetic resonance imaging and anthropometry. J Sports Sci 20: 563–576, 2002. [DOI] [PubMed] [Google Scholar]
- 84.Tudor-Locke C, Craig CL, Brown WJ, Clemes SA, De Cocker K, Giles-Corti B, Hatano Y, Inoue S, Matsudo SM, Mutrie N, Oppert JM, Rowe DA, Schmidt MD, Schofield GM, Spence JC, Teixeira PJ, Tully MA, Blair SN. How many steps/day are enough? For adults (Abstract). Int J Behav Nutr Phys Activity 8: 79, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson 129: 35–43, 1997. [DOI] [PubMed] [Google Scholar]
- 86.Walter G, Vandenborne K, Elliott M, Leigh JS. In vivo ATP synthesis rates in single human muscles during high intensity exercise. J Physiol 519: 901–910, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wigmore DM, Damon BM, Pober DM, Kent-Braun J. A MRI measures of perfusion-related changes in human skeletal muscle during progressive contractions. J Appl Physiol 97: 2385–2394, 2004. [DOI] [PubMed] [Google Scholar]
- 88.Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil S, Carlier PG, Richardson RS. Multiparametric NMR-based assessment of skeletal muscle perfusion and metabolism during exercise in elderly persons: preliminary findings. J Gerontol 64: 968–974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]