Abstract
Stress- and anxiety-related disorders are on the rise in both military and general populations. Over the next decade, it is predicted that treatment of these conditions, in particular, posttraumatic stress disorder (PTSD), along with its associated long-term comorbidities, will challenge the health care system. Multiple organ systems are adversely affected by PTSD, and PTSD is linked to cancer, arthritis, digestive disease, and cardiovascular disease. Evidence for a strong link between PTSD and cardiovascular disease is compelling, and this review describes current clinical data linking PTSD to cardiovascular disease, via inflammation, autonomic dysfunction, and the renin-angiotensin system. Recent clinical and preclinical evidence regarding the role of the renin-angiotensin system in the extinction of fear memory and relevance in PTSD-related immune and autonomic dysfunction is also addressed.
Keywords: posttraumatic stress disorder, cardiovascular disease, renin-angiotensin system
posttraumatic stress disorder (PTSD) is a psychiatric illness characterized by persistent emotional and mental stress following a traumatic event. Symptoms of PTSD include hyperarousal, flashbacks, intrusive thoughts, or nightmares, and avoidance of activities that trigger memories of the traumatic event. The health consequences of PTSD are substantial, affecting multiple organ systems, with evidence linking PTSD to diseases such as cancer, arthritis, digestive disease, and cardiovascular disease (CVD) (13, 14, 112). The evidence demonstrating increased risk for CVD in PTSD (9, 15, 19, 49, 57, 58) is compelling, and several excellent recent review articles have highlighted this association (20, 23, 52, 63, 112). While this association could certainly be due, in part, to related unhealthy behaviors, such as increased prevalence of smoking, poor diet, and physical inactivity (46, 119). Yet even after adjustments for lifestyle, comorbid conditions, and combat engagements in multivariate models, PTSD remains a significant and independent risk factor for the development of CVD and CVD-related mortality (15).
Increased CVD risk in PTSD has been demonstrated in both military (21) and civilian populations (44, 82). A co-twin study design (monozygotic and dizygotic), which controlled for genetic and familial confounders, demonstrated that the incidence of coronary heart disease was more than double in Vietnam War veteran twins with PTSD (22.6%) compared with those without PTSD (8.9%) (106). Most recently, one of the largest longitudinal studies examining the association between PTSD and heart failure was completed, and veterans with PTSD were shown to be nearly 50% more likely to develop heart failure than veterans without PTSD (91). This remained significant after adjustments for age, sex, diabetes, hyperlipidemia, hypertension, body mass index, combat, and military service. Civilian PTSD populations are also at greater risk for CVD. Following life-threatening traumatic events such as earthquakes (82), the 9–11 World Trade Center attack (45), and living in urban distressed neighborhoods (111), those diagnosed with PTSD have a higher incidence of CVD and related metabolic syndrome. Moreover, in the Framingham Coronary Heart Disease study, patients with PTSD were found to have increased Framingham risk scores for CVD (40). To date, there have been six PTSD-CVD prospective studies completed, following participants from 1 to 30 years, which have demonstrated consistent associations between PTSD and CVD after adjusting for demographic, clinical, and psychosocial factors, including depression (15, 44, 57, 58, 89, 96).
There are multiple risk factors (stroke, hypertension, atherosclerosis, and obesity metabolic syndrome) for the development of CVD, and increases in the incidence of these risk factors are often associated with PTSD (1, 22, 30, 51, 111). Data from the U.S. National Comorbidity Survey showed that people with PTSD had a 2.9-fold greater risk for developing hypertension (51). In a sample of more than 300,000 veterans of the Iraq and Afghanistan wars, those with PTSD had a 59% higher chance of developing hypertension compared with those without PTSD (19). In addition to hypertension, there is evidence of increased atherosclerosis in PTSD. Comparing Veterans with PTSD to those without, Ahmadi et al. (3) showed that the PTSD group had increased coronary calcium scores. Similarly, a nonmilitary PTSD population had greater arterial stiffness and vascular dysfunction (109), indicating increased atherosclerosis compared with a non-PTSD population. Furthermore, studies have demonstrated that CVD risk increases incrementally with worsening of PTSD symptoms. In a 14-year prospective study of more than 1,900 patients, men had an increased risk for both nonfatal myocardial infarction and fatal coronary heart disease with every SD increase in symptom level; similarly, women with five or more PTSD symptoms had over three times the risk of incidence of CVD (57, 58). It is also worth noting that clinically significant PTSD symptoms can be induced by cardiovascular related events, and these individuals are more likely to have recurrent major adverse coronary events (24, 59).
In summary, these studies provide compelling evidence for the association between PTSD and increased CVD risk and mortality, with some evidence pointing to a causal relationship. The mechanisms underlying these clinical findings are clearly complex and as pointed out in other reviews (52), the etiology is multifactorial, likely involving autonomic, immune, and neuroendocrine disturbances, resulting from the traumatic event(s). Subsequent sections will expand on these mechanisms and discuss the relevance of the renin-angiotensin system (RAS) in PTSD and its potential role in the link to CVD.
The Renin-Angiotensin System: Beyond Blood Pressure Control
It is well known that the RAS plays a fundamental role in blood pressure and fluid and electrolyte homeostasis. This neurohormonal system also promotes cardiovascular injury (i.e., vasoconstriction, inflammation, hypertrophy, and fibrosis) and is activated in response to psychological stress (42, 94). In response to increased psychological stress, sympathetic activation leads to renin release from the juxtaglomerular cells of the kidney through noradrenergic activation of β1 receptors, increasing ANG II synthesis through downstream processes (42, 56). In turn, ANG II causes its effects by binding to its two receptor subtypes: the angiotensin type 1 receptor (AT1R) and the angiotensin type 2 receptor (AT2R). The des Asp1 ANG II metabolite (ANG III) is also a full agonist of the AT1R and AT2R. Other metabolites of ANG II; des-Asp1,des-Arg2 ANG II (ANG IV) and des Phe8 ANG II (ANG 1–7) are also physiologically active, binding to the angiotensin type 4 receptor (AT4R) and mas, respectively, [see review (102)]. The AT1R is expressed across many organs, including the heart, kidney, vasculature, and brain (71, 116). The major cardiovascular effects, including elevation in blood pressure, increased sympathetic activity, and altered electrolyte balance, as well as proliferative, hypertrophic, and proinflammatory effects, are mediated by AT1R signaling. Inhibition of the RAS with angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs), which selectively block the AT1R, are, therefore, widely used for the treatment of hypertension and CVD. There is a growing body of evidence, however, demonstrating that the therapeutic actions of both ACE-Is and ARBs extend beyond blood pressure reduction (11, 92, 108) and may have beneficial effects on stress-related pathologies, such as PTSD and panic disorder (43, 50).
Chronic stress, as manifested with PTSD, induces activation of the RAS, leading to increased ANG II synthesis (86). Experimental studies have shown that immobilization stress in rats increases circulating levels of ANG II (18) and binding of brain AT1R. Inhibition of the RAS using ACE-Is and ARBs can reverse these affects (6, 17, 62, 94). Additional studies provide evidence that blockade of the RAS has protective effects against stress, anxiety, brain inflammation, and neurodegeneration (5, 10, 93, 95). Other studies have shown that increased circulating and brain components of RAS correlate with increased hypothalamic-pituitary-adrenal axis (HPA) stimulation and increased corticotrophin-releasing hormone (CRH), a critical stress hormone (2, 6, 84). Supporting these data, Krause et al. (55) demonstrated that knockdown of AT1R with siRNA (synthetic RNA) within the brain (hypothalamic region), attenuates the HPA neuroendocrine and anxiety stress response in rats. Chronic stress can lead to a breakdown of the negative feedback systems of the HPA axis, promoting reduced CRH and cortisol, which profoundly affect the long-term psychological state of affected individuals. This is endorsed by a meta-analysis showing decreased CRH and cortisol levels in individuals with psychiatric illness, including PTSD (76). Although it is clear that long-term elevations in HPA activity contribute to psychopathology, the associated increases in ANG II production and the influence of ANG II on psychiatric disorders, such as PTSD, are unknown. Further understanding the role of ANG II and the RAS in psychiatric disease is important, as current treatment strategies are limited. Recent clinical and preclinical data presented in the next section support a major role of the RAS in PTSD.
In a highly traumatized inner-city population, Khoury et al. (50), reported that individuals taking ARBs and ACE-Is had fewer PTSD symptoms. Notably, this reduction in PTSD symptoms was not observed with other classes of blood pressure medications, including β-blockers, calcium channel blockers, and diuretics. These clinical data support a role for the RAS in the regulation of stress response in individuals exposed to traumatic stress, and this finding was recently replicated (80). In this same study, genetic evidence for a single nucleotide polymorphism (SNP) in the ACE gene in this PTSD cohort was determined. Differential responses to ACE-Is and ARBs were found in those with the ACE intronic SNP (rs4311), as well as greater symptom improvements in PTSD among those with the CC genotype (80). Although speculative, it is possible that ACE genetic polymorphisms such as SNP (rs4311) may explain the varying individual propensities to develop CVD in this PTSD population.
Extending these clinical observations, recent studies using an animal model of PTSD have examined the acute and chronic effects of AT1R inhibition on fear memory (68). Patients with PTSD and other anxiety disorders are thought to have deficits in extinction of aversive memories (12, 113). Similarly, rodents with anxiety-like behavior or trauma exposure demonstrate a deficit in extinction of conditioned fear (4, 41). Using Pavlovian fear conditioning (pairing of auditory cues with footshocks), we examined the acute and chronic effects of the ARB losartan on extinction. While no effect of losartan was observed on extinction training, there was a marked reduction in fear, as measured by freezing behavior to an auditory cue when tested the following day (extinction retention). Similarly, when losartan was given chronically, animals exhibited reduced freezing during extinction. Importantly, losartan had no significant effects on locomotion, baseline anxiety, or blood pressure measures. Gene expression changes in the brain were also altered in mice with chronic ARB treatment, in particular, reduced mRNA levels of AT1R in the amygdala and c-Fos in the bed nucleus of the stria terminalis (68).
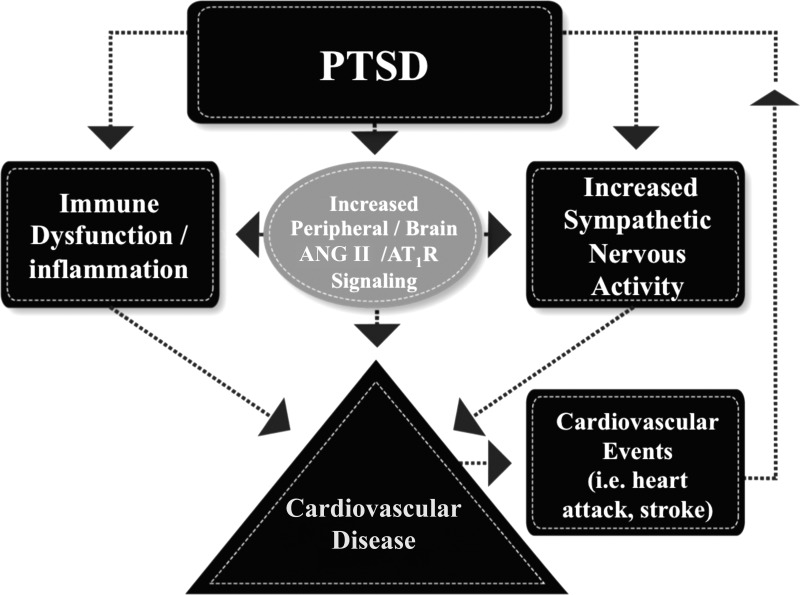
The amygdala is an integral part of the fear circuitry (47, 60), and key inputs to the amygdala from the medial prefrontal cortex are thought to be required for the extinction of fear (64, 65). It is, therefore, plausible that brain AT1R signaling may be an important factor in the fear circuit pathway contributing to extinction and fear-related behaviors. A recent study by Marinzalda et al. (67), showing that AT1R in the central amygdala modulates fear-potentiated behavior, provides further support for this hypothesis. Currently, the mechanisms for ANG II-mediated effects on fear memory are unknown; however, it is possible that brain ANG II modulates local excitatory and inhibitory neurons and CRF production (2, 6, 39), influencing the neural networks involved in fear memory formation (97). Aside from its unknown role in amygdala/fear-dependent neural pathways, the autonomic and proinflammatory effects of ANG II may also play a direct or indirect role in promoting CVD in the setting of PTSD (Fig. 1).
Fig. 1.
Pathways involved in posttraumatic stress disorder (PTSD)-associated cardiovascular disease development and PTSD induced from cardiovascular related events. Consequences of PTSD can lead to increased immune cell dysfunction/inflammation, heightened sympathetic nerve activity (hyperarousal), and activation of the renin-angiotensin system (gray circle). We propose that renin-angiotensin system activation further promotes autonomic/immune disturbances in the setting of PTSD, and these changes ultimately contribute to the culmination of increased cardiovascular disease risk. PTSD symptoms can also be induced (upward-pointing arrow) by a single cardiovascular related event such as a stroke or heart attack, thus putting these individuals at greater risk for future adverse cardiovascular events.
Autonomic Dysfunction in PTSD
The sympathoexcitatory effects of ANG II are well recognized (69, 87), and previous studies in both animals and humans with a variety of chronic diseases, such as obesity, heart failure, and chronic kidney disease, have shown that blockade of the RAS reduces sympathetic nervous system (SNS) activity and improves baroreceptor sensitivity (BRS) (25, 34, 53, 105, 110, 118). One of the hallmark symptoms of PTSD is hyperarousal, and therefore, it is not surprising that basal overactivation of the SNS is present. For example, studies investigating indirect markers of sympathetic activity show that PTSD patients have higher resting heart rates and blood pressure, decreased heart rate variability, and increased plasma and urine catecholamine levels, suggesting a state of SNS overactivity (9, 16). In addition, failure of blood pressure (BP) to decrease by ≥10% nocturnally (nondipping), which is thought to be mediated by SNS overactivity, is associated with increased CVD risk and is more prevalent in young African-Americans with PTSD (72).
One potential mechanism underlying SNS overactivity in PTSD is decreased baroreceptor sensitivity (BRS). Arterial baroreceptors, located in the carotid arteries and aortic arch, are sensory nerve endings that function as arterial pressure sensors that connect to sympathetic control centers in the brain. The baroreceptors are important in the moment-to-moment control of BP and buffering of acute fluctuations in arterial BP during postural and volume changes, as well as physiological or mental stress, thereby minimizing BP variability. Decreased BRS contributes to the pathogenesis of many conditions characterized by SNS overactivity, including chronic heart failure (32), obesity (33), and hypertension (70, 79). In PTSD, there is evidence for dysfunctional baroreflexes as well. For example, Gulf War veterans with comorbid PTSD and chronic fatigue syndrome have greater hypotensive responses to orthostatic stress (85), and female PTSD patients have blunted changes in heart rate interbeat interval in relation to BP at rest (38). Further evidence of autonomic dysfunction in PTSD has emerged from studies examining disrupted sleep patterns (trauma-related nightmares, insomnia) in PTSD (81, 90). In particular, blunted day-to-night reduction (8, 72, 73) and elevation of nocturnal SNS arousal or SNS dominance over parasympathetic nervous system (PNS) function have been observed in PTSD (77, 115). Moreover, young adult African-Americans with PTSD had a lower PNS activation indexed by heart rate variability during sleep at home compared with those who were resilient to trauma (i.e., individuals who had never experienced significant PTSD symptoms despite exposure to a high-impact trauma) (54). Interestingly, diminished SNS activation with increased sleep duration was observed in resilient individuals, but not those with PTSD (54). These observations of elevated nocturnal SNS arousal and dissociations between sleep duration and SNS activity suggest the possibility that sleep disturbance in PTSD is a likely contributor to decreased BRS, autonomic dysfunction, and increased CVD risk. Despite these data, the mechanisms contributing to SNS overactivity, BRS dysfunction, and associated consequences in PTSD are unclear.
Autonomic-Immune Dysfunction in PTSD
The autonomic nervous system has extensive communication with the immune system (78). Thus, dysregulation of the immune system leading to inflammation is a likely consequence of autonomic dysfunction in PTSD. Chronic elevations in catecholamines increases cytokine production via stimulation of β2-adrenergic receptors on immune cells (78), which may contribute to the numerous lines of evidence showing increased levels of inflammatory markers in PTSD (48). For example, PTSD patients exhibit increases in blood levels of proinflammatory cytokines including IL-1β, IL-2, IL-6, the IL-6 receptor, and TNF-α (26–28, 35, 37, 66, 104) and decreases in anti-inflammatory cytokines such as IL-4 (48, 98). Moreover, peripheral blood mononuclear cells produced more IL-1β, IL-6, and TNF-α in those with PTSD than in healthy controls (31). Overall, these studies demonstrate a shift to an increase in proinflammatory cytokines and a decrease in anti-inflammatory cytokines, which increases inflammation in PTSD. C-reactive protein (CRP), a biomarker of inflammation that is also predictive of cardiovascular disease (88), is also increased and frequently associated with PTSD in many (36, 74, 103), but not all studies (7, 101).
It is likely that dysregulation of the HPA axis, as discussed above, contribute to heightened immune responses and inflammation in PTSD. Further evidence of altered immune function in PTSD has been demonstrated in studies showing that the numbers of leukocytes and lymphocytes, as well as early activation markers of T lymphocytes such as CD45RA, are elevated in PTSD (29, 61, 107). Moreover, numbers of T regulatory cells, which are involved in suppressing inflammatory T lymphocyte responses, are significantly reduced (99). Taken together, these alterations in T lymphocytes can cause immunodysfunction, thus contributing to CVD progression and/or other inflammatory diseases. Overall, studies of inflammatory markers in PTSD have generally been cross-sectional and included a small sample size and failed to correct for potential confounding factors (such as depression, known cardiovascular disease, or cardiovascular risk factors) that increase levels of peripheral inflammatory cytokines. Despite these limitations and inconsistencies, the majority of evidence supports an association between PTSD and heightened immune function and inflammation.
When considering inflammation as a contributing factor or consequence of PTSD, it is important to keep in mind that inflammation and cytokines may have secondary effects on neurotransmitters, which could contribute to the impairments in fear memory and extinction processes found in PTSD patients and other psychiatric diseases (75). For example, under stress, microglia, endothelial cells, and macrophages release IL-1β, and many IL-1β receptors are concentrated near the hippocampus and amygdala. These may alter fear memory (100, 114) by influencing synaptic strength and signaling between neurons. Both IL-1β and nitric oxide are thought to play a role in long-term potentiation (increased postsynaptic response to a fixed stimulus), which can serve to enhance learning and memory (83, 100). Interestingly, IL-1β has been found to promote fear memory and the conditioned fear response. Following footshock, rats receiving an intracerebroventricular infusion of IL-1β showed a heightened fear memory (100), while blocking the IL-1 receptor in mice decreased perceived anxiety-type behavior, thus emphasizing the importance of this cytokine in fear memory (114). These and other data suggest that the development of PTSD may involve a failure of immune system homeostasis, such that inflammation and cytokines can disrupt neuronal signaling, promote injury, and impair circuits involved in fear memory extinction. Moreover, as described earlier in The Renin-Angiotensin System: Beyond Blood Pressure Control, ANG II is an important mediator of brain inflammation and activates microglia (117, 123). Therefore, it is possible that ANG II may directly or indirectly affect central signals involved in fear memory formation. Overall, we hypothesize that following a traumatic event, increases in peripheral and central ANG II lead to enhanced autonomic and proinflammatory effects that alter fear extinction mechanisms, and in time, position these individuals at greater risk for CVD-related mortality (Fig. 1). Other factors such as PTSD-associated sleep disturbances may accelerate the development of CVD.
Summary and Conclusion
In summary, there are accumulating epidemiological studies demonstrating a link between PTSD and CVD and evidence that the RAS, sympathetic overactivation, and inflammation contribute to compromised cardiovascular health in PTSD. Furthering the understanding of these neurobiological/immunological pathways and the role of the brain and peripheral RAS will be critical to identifying new treatment and prevention strategies and importantly identifying PTSD patients at risk for the development of CVD. Continued multidisciplinary research will be essential to identifying unique biological and genetic markers in these patients with the goal of improved clinical outcomes in PTSD and other stress-related illnesses.
GRANTS
Support was provided by National Institutes of Health Grant R00 HL-107675-03.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.B., J.P., J.W., I.K., T.A.M., and P.J.M. drafted manuscript; C.B., J.P., J.W., I.K., T.A.M., and P.J.M. edited and revised manuscript; J.P., J.W., I.K., T.A.M., and P.J.M. approved final version of manuscript; P.J.M. prepared figures.
ACKNOWLEDGMENTS
The authors would like to thank Nicole Gouws for her work in designing Fig. 1 and Colin N. Young and Robert C. Speth for their comments on this manuscript.
REFERENCES
- 1.Abouzeid M, Kelsall HL, Forbes AB, Sim MR, Creamer MC. Posttraumatic stress disorder and hypertension in Australian veterans of the 1991 Gulf War. J Psychosom Res 72: 33–38, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Aguilera G, Young WS, Kiss A, Bathia A. Direct regulation of hypothalamic corticotropin-releasing-hormone neurons by angiotensin II. Neuroendocrinology 61: 437–444, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol 108: 29–33, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8 dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am J Psychiatry 168: 163–172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson C. More indirect evidence of potential neuroprotective benefits of angiotensin receptor blockers. J Hypertens 28: 429, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Armando I, Volpi S, Aguilera G, Saavedra JM. Angiotensin II AT1 receptor blockade prevents the hypothalamic corticotropin-releasing factor response to isolation stress. Brain Res 1142: 92–99, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumert J, Lukaschek K, Kruse J, Emeny RT, Koenig W, Känel von R, Ladwig KH, KORA Investigators. No evidence for an association of posttraumatic stress disorder with circulating levels of CRP and IL-18 in a population-based study. Cytokine 63: 201–208, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Beckham JC, Feldman ME, Barefoot JC, Fairbank JA, Helms MJ, Haney TL, Hertzberg MA, Moore SD, Davidson JR. Ambulatory cardiovascular activity in Vietnam combat veterans with and without posttraumatic stress disorder. J Consult Clin Psychol 68: 269–276, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Bedi US, Arora R. Cardiovascular manifestations of posttraumatic stress disorder. J Natl Med Assoc 99: 642–649, 2007. [PMC free article] [PubMed] [Google Scholar]
- 10.Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36: 857–870, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2: 247–257, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 45: 2019–2033, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Boscarino JA. Diseases among men 20 years after exposure to severe stress: implications for clinical research and medical care. Psychosom Med 59: 605–614, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Boscarino JA. External-cause mortality after psychologic trauma: the effects of stress exposure and predisposition. Compr Psychiatry 47: 503–514, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med 70: 668–676, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckley TC, Kaloupek DG. A meta-analytic examination of basal cardiovascular activity in posttraumatic stress disorder. Psychosom Med 63: 585–594, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Castren E, Saavedra JM. Repeated stress increases the density of angiotensin II binding sites in rat paraventricular nucleus and subfornical organ. Endocrinology 122: 370–372, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Chung IM, Kim YM, Yoo MH, Shin MK, Kim CK, Suh SH. Immobilization stress induces endothelial dysfunction by oxidative stress via the activation of the angiotensin II/its type I receptor pathway. Atherosclerosis 213: 109–114, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA 302: 489–492, 2009. [DOI] [PubMed] [Google Scholar]
- 20.Coughlin SS. Post-traumatic stress disorder and cardiovascular disease. Open Cardiovasc Med J 5: 164–170, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crum-Cianflone NF, Bagnell ME, Schaller E, Boyko EJ, Smith B, Maynard C, Ulmer CS, Vernalis M, Smith TC. Impact of combat deployment and posttraumatic stress disorder on newly reported coronary heart disease among US active duty and reserve forces. Circulation 129: 1813–1820, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Dedert EA, Calhoun PS, Watkins LL, Sherwood A, Beckham JC. Posttraumatic stress disorder, cardiovascular, and metabolic disease: a review of the evidence. Ann Behav Med 39: 61–78, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis 55: 548–556, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One 7: e38915, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eguchi K, Shimizu M, Hoshide S, Shimada K, Kario K. A bedtime dose of ARB was better than a morning dose in improving baroreflex sensitivity and urinary albumin excretion—the J-TOP study. Clin Exp Hypertens 34: 488–492, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun 27: 8–12, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill J, Luckenbaugh D, Charney D, Vythilingam M. Sustained elevation of serum interleukin-6 and relative insensitivity to hydrocortisone differentiates posttraumatic stress disorder with and without depression. Biol Psychiatry 68: 999–1006, 2010. [DOI] [PubMed] [Google Scholar]
- 28.Gill J, Vythilingam M, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF-α, and IL-6 in women with PTSD. J Traum Stress 21: 530–539, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care 45: 262–277, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Goetz M, Shah A, Goldberg J, Cheema F, Shallenberger L, Murrah NV, Bremner JD, Vaccarino V. Posttraumatic stress disorder, combat exposure, and carotid intima-media thickness in male twins. Am J Epidemiol 180: 989–996, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, Groettrup M, Elbert T, Kolassa IT. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry 13: 40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92: 3206–3211, 1995. [DOI] [PubMed] [Google Scholar]
- 33.Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, Mancia G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 97: 2037–2042, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Grassi G, Seravalle G, Dell'Oro R, Trevano FQ, Bombelli M, Scopelliti F, Facchini A, Mancia G. Comparative effects of candesartan and hydrochlorothiazide on blood pressure, insulin sensitivity, and sympathetic drive in obese hypertensive individuals: results of the CROSS study. J Hypertens 21: 1761–1769, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Guo M, Liu T, Guo JC, Jiang XL, Chen F, Gao YS. Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pac J Trop Med 5: 323–325, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Heath NM, Chesney SA, Gerhart JI, Goldsmith RE, Luborsky JL, Stevens NR, Hobfoll SE. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine 63: 172–178, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 26: 447–455, 2009. [DOI] [PubMed] [Google Scholar]
- 38.Hughes JW, Dennis MF, Beckham JC. Baroreceptor sensitivity at rest and during stress in women with posttraumatic stress disorder or major depressive disorder. J Traum Stress 20: 667–676, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Hurt RC, Garrett JC, Keifer OP, Linarea A, Couling L, Speth RC, Milner T, Ressler KJ, Marvar PJ. Angiotensin II receptor type 1 knockout from corticotropin-releasing factor neurons reduces conditioned fear expression in mice FASEB J. 29: Suppl 840.5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin H, Folsom D, Sasaki A, Mudaliar S, Henry R, Torres M, Golshan S, Glorioso DK, Jeste D. Increased Framingham 10-year risk of coronary heart disease in middle-aged and older patients with psychotic symptoms. Schizophr Res 125: 295–299, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansen JP, Tarpley JW, LeDoux JE, Blair HT. Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci 13: 979–986, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol 57 Suppl 11: 5–29, 2006. [PubMed] [Google Scholar]
- 43.Johnson PL, Sajdyk TJ, Fitz SD, Hale MW, Lowry CA, Hay-Schmidt A, Shekhar A. Angiotensin II's role in sodium lactate-induced panic-like responses in rats with repeated urocortin 1 injections into the basolateral amygdala: amygdalar angiotensin receptors and panic. Prog Neuro-Psychopharmacol Biol Psychiatry 44: 248–256, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan HT, Miller-Archie SA, Cone JE, Morabia A, Stellman SD. Heart disease among adults exposed to the September 11, 2001 World Trade Center disaster: results from the World Trade Center Health Registry. Prev Med 53: 370–376, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Jordan HT, Stellman SD, Morabia A, Miller-Archie SA, Alper H, Laskaris Z, Brackbill RM, Cone JE. Cardiovascular disease hospitalizations in relation to exposure to the September 11, 2001 World Trade Center disaster and posttraumatic stress disorder. J Am Heart Assoc 2: e000431–e000431, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joseph AM, McFall M, Saxon AJ, Chow BK, Leskela J, Dieperink ME, Carmody TP, Beckham JC. Smoking intensity and severity of specific symptom clusters in posttraumatic stress disorder. J Traum Stress 25: 10–16, 2012. [DOI] [PubMed] [Google Scholar]
- 47.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167: 648–662, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanel von R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, Schnyder U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 41: 744–752, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Kang H, Bullman T, Taylor J. Risk of selected cardiovascular diseases and posttraumatic stress disorder among former World War II prisoners of war. Annals of Epidemiology 16: 381–386, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, Kramer M, Ressler KJ. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry 73: 849–855, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kibler JL, Joshi K, Ma M. Hypertension in relation to posttraumatic stress disorder and depression in the US National Comorbidity Survey. Behav Med 34: 125–132, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Kibler JL. Metabolic, autonomic and immune markers for cardiovascular disease in posttraumatic stress disorder. World J Cardiol 6: 455, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Enalapril and losartan reduce sympathetic hyperactivity in patients with chronic renal failure. J Am Soc Nephrol 14: 425–430, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi I, Lavela J, Mellman TA. Nocturnal autonomic balance and sleep in PTSD and resilience. J Traum Stress 27: 712–716, 2014. [DOI] [PubMed] [Google Scholar]
- 55.Krause EG, de Kloet AD, Scott KA, Flak JN, Jones K, Smeltzer MD, Ulrich-Lai YM, Woods SC, Wilson SP, Reagan LP, Herman JP, Sakai RR. Blood-borne angiotensin II acts in the brain to influence behavioral and endocrine responses to psychogenic stress. J Neurosci 31: 15,009–15,015, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubzansky LD, Adler GK. Aldosterone: a forgotten mediator of the relationship between psychological stress and heart disease. Neurosci Biobehav Rev 34: 80–86, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol 28: 125–130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kubzansky LD, Koenen KC, Spiro A, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry 64: 109–116, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Ladwig KH, Baumert J, Marten-Mittag B, Kolb C, Zrenner B, Schmitt C. Posttraumatic stress symptoms and predicted mortality in patients with implantable cardioverter-defibrillators: results from the prospective living with an implanted cardioverter-defibrillator study. Arch Gen Psychiatry 65: 1324–1330, 2008. [DOI] [PubMed] [Google Scholar]
- 60.LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol 23: 727–738, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lemieux A, Coe CL, Carnes M. Symptom severity predicts degree of T-cell activation in adult women following childhood maltreatment. Brain Behav Immun 22: 994–1003, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leong DS, Terrón JA, Falcón-Neri A, Armando I, Ito T, Jöhren O, Tonelli LH, Hoe KL, Saavedra JM. Restraint stress modulates brain, pituitary and adrenal expression of angiotensin II AT(1A), AT(1B), and AT(2) receptors. Neuroendocrinology 75: 227–240, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Levine AB, Levine LM, Levine TB. Posttraumatic stress disorder and cardiometabolic disease. Cardiology 127: 1–19, 2014. [DOI] [PubMed] [Google Scholar]
- 64.Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J Neurosci 25: 7429–7437, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature 454: 642–645, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maes M, Lin AH, Delmeire L, Van Gastel A, Kenis G, De Jongh R, Bosmans E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol Psychiatry 45: 833–839, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Marinzalda M de los A, Pérez PA, Gargiulo PA, Casarsa BS, Bregonzio C, Baiardi G. Fear-potentiated behaviour is modulated by central amygdala angiotensin II AT1 receptors stimulation. Biomed Res Int 2014: 183248, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marvar PJ, Goodman J, Fuchs S, Choi DC, Banerjee S, Ressler KJ. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol Psychiatry 75: 864–872, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand 141: 157–165, 1991. [DOI] [PubMed] [Google Scholar]
- 70.Matsukawa T, Gotoh E, Uneda S, Miyajima E, Shionoiri H, Tochikubo O, Ishii M. Augmented sympathetic nerve activity in response to stressors in young borderline hypertensive men. Acta Physiol Scand 141: 157–165, 1991. [DOI] [PubMed] [Google Scholar]
- 71.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol 292: C82–C97, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Mellman TA, Brown DD, Jenifer ES, Hipolito MM, Randall OS. Posttraumatic stress disorder and nocturnal blood pressure dipping in young adult African Americans. Psychosom Med 71: 627–630, 2009. [DOI] [PubMed] [Google Scholar]
- 73.Mellman TA, Brown TSH, Kobayashi I, Abu-Bader SH, Lavela J, Altaee D, McLaughlin L, Randall OS. Blood pressure dipping and urban stressors in young adult African Americans. Ann Behav Med (January 27, 2015). doi: 10.1007/s12160-014-9684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry 172: 353–362, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30: 297–306, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev 32: 301–315, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muraoka MY, Carlson JG, Chemtob CM. Twenty-four-hour ambulatory blood pressure and heart rate monitoring in combat-related posttraumatic stress disorder. J Traum Stress 11: 473–484, 1998. [DOI] [PubMed] [Google Scholar]
- 78.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav Immun 21: 736–745, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nicholls JG, Paton JFR. Brainstem: neural networks vital for life. Philos Trans R Soc B Biol Sci 364: 2447–2451, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, Schwartz AC, Habib L, Gamwell KL, Marvar PJ, Bradley B, Ressler KJ. An angiotensin-converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin-pathway medications on posttraumatic stress symptoms. Am J Med Genet B Neuropsychiatr Genet 168B: 307–315, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry 41: 469–478, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Onose T, Nochioka K, Sakata Y, Miura M, Tadaki S, Ushigome R, Yamauchi T, Sato K, Tsuji K, Abe R, Miyata S, Takahashi J, Shimokawa H, CHART2 Investigators. Predictors and prognostic impact of post-traumatic stress disorder after the great East Japan earthquake in patients with cardiovascular disease. Circ J 79: 664–667, 2015. [DOI] [PubMed] [Google Scholar]
- 83.Oosthuizen F, Wegener G, Harvey BH. Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr Dis Treat 1: 109–123, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pavlatou MG, Mastorakos G, Lekakis I, Liatis S, Vamvakou G, Zoumakis E, Papassotiriou I, Rabavilas AD, Katsilambros N, Chrousos GP. Chronic administration of an angiotensin II receptor antagonist resets the hypothalamic-pituitary-adrenal (HPA) axis and improves the affect of patients with diabetes mellitus type 2: Preliminary results. Stress 11: 62–72, 2008. [DOI] [PubMed] [Google Scholar]
- 85.Peckerman A, Dahl K, Chemitiganti R, LaManca JJ, Ottenweller JE, Natelson BH. Effects of posttraumatic stress disorder on cardiovascular stress responses in Gulf War veterans with fatiguing illness. Auton Neurosci Basic Clin 108: 63–72, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Porter JP. Effect of stress on the control of renin release in spontaneously hypertensive rats. Hypertension 15: 310–317, 1990. [DOI] [PubMed] [Google Scholar]
- 87.Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol Endocrinol Metab 262: E763–E778, 1992. [DOI] [PubMed] [Google Scholar]
- 88.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 107: 363–369, 2003. [DOI] [PubMed] [Google Scholar]
- 89.Roberts AL, Agnew-Blais JC, Spiegelman D, Kubzansky LD, Mason SM, Galea S, Hu FB, Rich-Edwards JW, Koenen KC. Posttraumatic stress disorder and incidence of type 2 diabetes mellitus in a sample of women: a 22-year longitudinal study. JAMA Psychiatry 72: 203–210, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roszell DK, McFall ME, Malas KL. Frequency of symptoms and concurrent psychiatric disorder in Vietnam veterans with chronic PTSD. Hosp Community Psychiatry 42: 293–296, 1991. [DOI] [PubMed] [Google Scholar]
- 91.Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. Am J Public Health 105: 757–763, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept 128: 227–238, 2005. [DOI] [PubMed] [Google Scholar]
- 93.Saavedra JM, Ando H, Armando I, Baiardi G, Bregonzio C, Juorio A, Macova M. Anti-stress and anti-anxiety effects of centrally acting angiotensin II AT1 receptor antagonists. Regul Pept 128: 227–238, 2005. [DOI] [PubMed] [Google Scholar]
- 94.Saavedra JM, Benicky J. Brain and peripheral angiotensin II play a major role in stress. Stress 10: 185–193, 2007. [DOI] [PubMed] [Google Scholar]
- 95.Saavedra JM. Angiotensin II AT1 receptor blockers ameliorate inflammatory stress: a beneficial effect for the treatment of brain disorders. Cell Mol Neurobiol 32: 667–681, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scherrer JF, Chrusciel T, Zeringue A, Garfield LD, Hauptman PJ, Lustman PJ, Freedland KE, Carney RM, Bucholz KK, Owen R, True WR. Anxiety disorders increase risk for incident myocardial infarction in depressed and nondepressed Veterans Administration patients. Am Heart J 159: 772–779, 2010. [DOI] [PubMed] [Google Scholar]
- 97.Shekhar A. Angiotensin type 1 receptor antagonists-a novel approach to augmenting posttraumatic stress disorder and phobia therapies? Biol Psychiatry 75: 836–837, 2014. [DOI] [PubMed] [Google Scholar]
- 98.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, Tang Y, Gillespie CF, Cubells JF, Ressler KJ. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet 156: 700–708, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg EM, Elbert T, Groettrup M, Kolassa IT. Substantial reduction of naïve and regulatory T cells following traumatic stress. Brain Behav Immun 23: 1117–1124, 2009. [DOI] [PubMed] [Google Scholar]
- 100.Song C, Phillips AG, Leonard B. Interleukin 1β enhances conditioned fear memory in rats: possible involvement of glucocorticoids. Eur J Neurosci 18: 1739–1743, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Söndergaard HP, Hansson LO, Theorell T. The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta 342: 93–98, 2004. [DOI] [PubMed] [Google Scholar]
- 102.Speth RC, Giese MJ. Update on the renin-angiotensin system. J Pharmacol Clin Toxicol 1: 1004, 2013. [Google Scholar]
- 103.Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, Löwe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psychiatr Res 44: 15–21, 2010. [DOI] [PubMed] [Google Scholar]
- 104.Spivak B, Shohat B, Mester R, Avraham S, Gil-Ad I, Bleich A, Valevski A, Weizman A. Elevated levels of serum interleukin-1β in combat-related posttraumatic stress disorder. Biol Psychiatry 42: 345–348, 1997. [DOI] [PubMed] [Google Scholar]
- 105.Sueta D, Koibuchi N, Hasegawa Y, Toyama K, Uekawa K, Katayama T, Ma M, Nakagawa T, Ogawa H, Kim-Mitsuyama S. Telmisartan exerts sustained blood pressure control and reduces blood pressure variability in metabolic syndrome by inhibiting sympathetic activity. Am J Hypertension 27: 1464–1471, 2014. [DOI] [PubMed] [Google Scholar]
- 106.Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J Am Coll Cardiol 62: 970–978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vidović A, Vilibić M, Sabioncello A, Gotovac K, Rabatić S, Folnegović-Smalc V, Dekaris D. Circulating lymphocyte subsets, natural killer cell cytotoxicity, and components of hypothalamic-pituitary-adrenal axis in Croatian war veterans with posttraumatic stress disorder: cross-sectional study. Croat Med J 48: 198–206, 2007. [PMC free article] [PubMed] [Google Scholar]
- 108.Villapol S, Yaszemski AK, Logan TT, Sánchez-Lemus E, Saavedra JM, Symes AJ. Candesartan, an angiotensin II AT1-receptor blocker and PPAR-γ agonist, reduces lesion volume and improves motor and memory function after traumatic brain injury in mice. Neuropsychopharmacology 37: 2817–2829, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walczewska J, Rutkowski K, Wizner B, Cwynar M, Grodzicki T. Stiffness of large arteries and cardiovascular risk in patients with post-traumatic stress disorder. Eur Heart J 32: 730–736, 2011. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Seto SW, Golledge J. Angiotensin II, sympathetic nerve activity and chronic heart failure. Heart Fail Rev 19: 187–198, 2014. [DOI] [PubMed] [Google Scholar]
- 111.Weiss T, Skelton K, Phifer J, Jovanovic T, Gillespie CF, Smith A, Umpierrez G, Bradley B, Ressler KJ. Posttraumatic stress disorder is a risk factor for metabolic syndrome in an impoverished urban population. Gen Hosp Psychiatry 33: 135–142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wentworth BA, Stein MB, Redwine LS, Xue Y, Taub PR, Clopton P, Nayak KR, Maisel AS. Post-traumatic stress disorder: a fast track to premature cardiovascular disease? Cardiol Rev 21: 16–22, 2013. [DOI] [PubMed] [Google Scholar]
- 113.Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am J Psychiatry 164: 1684–1692, 2007. [DOI] [PubMed] [Google Scholar]
- 114.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J Neurosci 34: 2583–2591, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woodward SH, Arsenault NJ, Voelker K, Nguyen T, Lynch J, Skultety K, Mozer E, Leskin GA, Sheikh JI. Autonomic activation during sleep in posttraumatic stress disorder and panic: a mattress actigraphic study. Biol Psychiatry 66: 41–46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wright JW, Harding JW. Brain angiotensin receptor subtypes AT1, AT2, and AT4 and their functions. Regul Pept 59: 269–295, 1995. [DOI] [PubMed] [Google Scholar]
- 117.Wu CY, Zha H, Xia QQ, Yuan Y, Liang XY, Li JH, Guo ZY, Li JJ. Expression of angiotensin II and its receptors in activated microglia in experimentally induced cerebral ischemia in the adult rats. Mol Cell Biochem 382: 47–58, 2013. [DOI] [PubMed] [Google Scholar]
- 118.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53: 210–216, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zen AL, Whooley MA, Zhao S, Cohen BE. Post-traumatic stress disorder is associated with poor health behaviors: findings from the heart and soul study. Health Psychol 31: 194–201, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]