Abstract
MicroRNAs are a family of small noncoding RNAs that regulate the expression of their target proteins at the posttranscriptional level. Their functions cover almost every aspect of cell physiology. However, the roles of microRNAs in fetal lung development are not completely understood. The objective of this study is to investigate the regulation and molecular mechanisms of alveolar epithelial cell maturation during fetal lung development by miR-124. We discovered that miR-124 was downregulated during rat fetal lung development and predominantly expressed in the epithelial cells at late stage of the lung development. Overexpression of miR-124 with an adenovirus vector led to the inhibition of epithelial maturation in rat fetal lung organ cultures and fetal alveolar epithelial type II cells, as demonstrated by a decrease in the type II cell marker expression and an increase in glycogen content. We further demonstrated by luciferase reporter assays that miR-124 inhibited the NF-κB, cAMP/PKA, and MAPK/ERK pathways. In addition, nuclear factor I/B (NFIB), a critical protein in fetal lung maturation, was validated as a direct target of miR-124. Furthermore, miR-124 expression was induced by the Wnt/β-catenin signaling pathway through a direct interaction of LEF1 and the miR-124 promoter region. We concluded that miR-124 downregulation is critical to fetal lung epithelial maturation and miR-124 inhibits this maturation process at least partially through the inhibition of NFIB.
Keywords: alveolar type II cells, fetal lung development, miR-124, Wnt signaling
the development of the rat lung can be divided into five stages: the embryonic stage (0–13 days), the glandular stage (13–18 days), the canalicular stage (18–20 days), the saccular stage (20 days to full term), and the alveolar stage (after birth). In the embryonic stage, a pair of lung rudiments arises from the primitive esophagus. In the glandular stage, the air passage tubes are formed by dichotomous branching of the terminal buds. At this time, the lung appears like a gland. During this stage, the epithelium transitions from pseudostratified cells to columnar cells. In the canalicular stage, epithelial cells become cuboidal, glycogen content in epithelial cells increases, the mesenchymal tissue thins out, and bronchioles appear. In the saccular stage, many cells are flattened and other cells remain cuboidal. The cuboidal cells lose glycogen and begin to synthesize surfactant. Alveolar ducts and air sacs form at this stage. Formation of true alveoli occurs in the alveolar stage after birth. Although the transcriptional control of fetal lung development has extensively been studied, the roles of microRNAs in the regulation of epithelial development and maturation are still largely unknown.
MicroRNAs are a group of small RNAs (∼22 nt) that regulate protein expression at the posttranscriptional level. They bind the mRNA of target genes directly and usually downregulate their expression by cleavage of the target mRNA, translational inhibition, or mRNA deadenylation (6, 17, 29, 86). According to a study in silico, most mammalian mRNAs are under selective pressure and are conserved targets of microRNAs (20). So far, the known functions of microRNAs have covered almost every aspect of cell physiology, including cell proliferation and differentiation, apoptosis, lipid and fat metabolism, cancer, diabetes, and many other diseases (2, 21, 22).
Several studies have described the roles of microRNAs in lung development. We have previously shown that the expression levels of 21 microRNAs are changed during different stages of fetal lung development and 1 of the changed microRNAs, miR-127, regulates branching morphogenesis (8). It has been reported that miR-130a and miR-221 control microvascular development and airway branching (60). miR-134 regulates lung septation by promoting cell proliferation and inhibiting cell apoptosis during mouse lung development (95). The miR-17-92 cluster promotes lung epithelial progenitor cell proliferation and inhibits their differentiation (51). Deletion of the miR-17-92 cluster leads to neonatal death with lung hypoplasia (80). Repression of three members of the miR-17 family, miR-17, miR-20a, and miR-106a, alters FGF10-induced branching morphogenesis by direct targeting of Stat3 and Mapk14 (11). In addition, the miR-302/367 cluster also regulates lung endoderm progenitor cell proliferation and differentiation (78). In the airway epithelium, five members of the miR-34/449 family (miR-34b/c and miR-449a/b/c) are enriched in the ciliated cells. miR-449 regulates airway epithelial multiciliogenesis through direct repression of the Delta/Notch pathway (56). Moreover, the functions of microRNAs in lung diseases have been extensively explored including lung cancers (28, 76), asthma (15), idiopathic pulmonary fibrosis (32, 62, 77), and pulmonary artery hypotension (36).
miR-124 is preferentially expressed in the brain (42). It has been reported that miR-124 drives the gene expression profile toward that of the brain (46). It helps to define and maintain the cell specificities in the brain. At least two mechanisms are involved in this regulation. First, miR-124 can directly target polypyrimidine tract-binding protein 1 (PTBP1), which is a global repressor of nervous system-specific pre-mRNA splicing. miR-124 inhibits the expression of PTBP1, leading to the differentiation of progenitor cells to neuronal cells (55). Another mechanism involves small COOH-terminal domain phosphatase 1 (SCP1), which is an anti-neural phosphatase. During neurogenesis, miR-124 represses the expression of SCP1 and antagonizes the REST/SCP1 pathway (82). In a recent study, miR-124 has been shown to control the choice between neuronal and astrocyte differentiation of mouse neural stem cells by targeting Ezh2 (61). In addition to its function in neuronal cell differentiation, miR-124 is a tumor suppressor. It inhibits proliferation, migration, and epithelial-mesenchymal transition. The involved targets of miR-124 include snail family zinc finger 2 (SNAI2), TEA domain 1 (TEAD1), MAPK14/38α, stress-associated endoplasmic reticulum protein 1 (SERP1), and ras-related C3 botulinum toxin substrate 1 (RAC1) (23, 45, 59). miR-124 is also involved in the pathogenesis of pulmonary vascular disorders such as pulmonary arterial hypertension. It inhibits proliferation of pulmonary artery smooth muscle cells and pulmonary vascular fibroblasts by suppression of the nuclear factor of the activated T cells (NFAT) pathway and inhibition of PTBP1 expression, respectively (37, 83).
WNT/β-catenin signaling pathway is essential to fetal lung development (88). Upon the binding of WNT3a to cell surface receptors, cytoplasmic β-catenin is translocated to the nucleus and forms complexes with LEF/TCF transcription factors. The complexes bind LEF/TCF response sequences and regulate target gene transcription. MicroRNAs control WNT/β-catenin signaling pathway (31). On the other hand, WNT/β-catenin signal also regulates microRNA expression (10, 31, 35). However, it is unknown whether WNT/β-catenin signaling controls miR-124 expression.
So far, no one has reported the functions of miR-124 in lung development. In this study, we investigated the role of miR-124 in fetal lung epithelial maturation and the regulation of miR-124.
MATERIALS AND METHODS
Real-time PCR.
Total RNA was isolated from cell lines and fetal rat lung explants using the mirVana miRNA isolation kit (Ambion, Austin, TX), following the manufacturer's instructions. It was then treated with TURBO DNA-free (Ambion) to eliminate any genomic DNA contamination. For each reverse transcription reaction, 1 μg of total RNA was reversed transcribed into cDNA using MMLV reverse transcriptase. Real-time PCR was performed using a SYBR Green master mix from Eurogentec (Seraing, Belgium) on an ABI 7500 fast system (Applied Biosystems, Foster City, CA). The primers are listed in Table 1.
Table 1.
Primers used for real-time PCR
| SP-A forward | GATCAAACATCAGATTCTGCAAACA |
| SP-A reverse | TCCTGCTCTGGTACACATCTCTTTA |
| SP-B forward | AATGACCTGTGCCAAGAGTGTG |
| SP-B reverse | AGGACCAGCTTGTTCAGCAGAG |
| SP-C forward | AGCTCCAGGAACCTACTGCTACAT |
| SP-C reverse | AGGACTTGGCCTGGAAGTTCTT |
| T1α forward | GCCATCGGTGCGCTAGAAGATGATCTT |
| T1α reverse | GTGATCGTGGTCGGAGGTTCCTGAGGT |
| CCSP forward | CTAATTATGAGGCAGCCCTGAAG |
| CCSP reverse | GTCTCCTGTGGGAGGGTATCC |
| FAS forward | TCAGAGGTTACACTGTGTTAGGTGTTG |
| FAS reverse | CCCATCCCTGAGCAGATGAA |
| NFIB forward | GCAGGGAAATCCATGTCACAA |
| NFIB reverse | ACCCTGATGGCACTTCTCTGA |
| 18S rRNA forward | TCCCAGTAAGTGCGGGTCATA |
| 18S rRNA reverse | CGAGGGCCTCACTAAACCATC |
SP, surfactant protein; FAS, fatty acid synthase; NFIB, nuclear factor I/B.
The expression of miR-124 was determined with TaqMan MicroRNA Assays (Applied Biosystems), following the instructions of the manufacturer. In each reverse transcription reaction, 75 ng of total RNA were used as a template for cDNA synthesis. The reactions were kept on ice for 5 min and then incubated at 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. The PCR reactions were kept at 95°C for 10 min and then 40 cycles of 95°C for 15 s and 60°C for 60 s. The expression of 18S rRNA was also determined as an internal control. The relative expression of miR-124 was calculated with the formula, 2−(CTmiRNA−CT18s).
In situ hybridization.
In situ hybridization of miR-124 was performed with 5′-DIG-labeled LNA probes from Exiqon (Woburn, MA), following the instruction of the manufacturer. A probe with a scrambled sequence (GTGTAACACGTCTATACGCCCA) was used as a negative control. Paraffin sections of rat fetal lung tissues were dewaxed in xylene and rehydrated. The sections were then permeabilized with 10 μg/ml of proteinase K at 37°C for 5 min, followed by incubation in 0.2% glycine to stop the reaction. The sections were then fixed in 4% paraformaldehyde and prehybridized in a hybridization buffer for 2 h. After that, the sections were hybridized with 20-nM probes in a hybridization buffer at 60°C overnight. After stringent washes, the sections were blocked in a blocking buffer for 1 h and then incubated with anti-DIG-AP Fab fragments (1:2,000; Roche Applied Sciences, Indianapolis, IN) at 4°C overnight. After being washed with PBST and AP buffer, the sections were incubated in 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitro blue tetrazolium (NBT) color solution (Roche Applied Sciences) until the desired blue color developed.
Adenovirus construction.
The construction of miR-124 overexpression adenoviral vector (Ad-miR-124) was performed as previously described (85). The mature sequence of miR-124, with flanking sequences at both the 5′- and the 3′-ends, was PCR-amplified from human genomic DNA. The primers used were forward primer CACCTCGAGCACACGCACCGTCTACACTTC and reverse primer GAGAATTCTATTTGCACAGGCGGGAACTAC. This fragment was then inserted into the pENTR vector (Invitrogen, Carlsbad, CA), downstream of the CMV-GFP, and switched to the adenoviral vector plasmid with the LR recombination. This plasmid was digested by Pac I and transfected into HEK 293A cells to produce adenovirus.
Fetal lung organ culture.
Fetal lung organ culture was performed as previously described (87). For timed pregnancy, the day on which vaginal plugs were discovered was denoted as gestational day 0. On gestational day 15, pregnant Sprague-Dawley rats were euthanized with CO2. Fetal lungs were dissected from the fetuses without the surrounding tissues. They were then transferred to the surface of Transwell inserts (Millipore, Billerica, MA) in six-well plates. Each well contained 1.5 ml of culture medium. The culture medium consisted of BGJb medium supplemented with 0.2 mg/ml of ascorbic acid, 50 U/ml of penicillin, and 50 μg/ml of streptomycin. Three hours later, the medium was removed and replaced with fresh culture medium. Adenovirus (108 pfu) was incubated with 0.1 mg/ml of protamine for 15 min. The total volume of the mixture was 40 μl. After the incubation, the mixture was added to each of the fetal lung explants. This day was denoted as day 0 (D0). Culture medium was changed every day until sample collection (D5). The same virus treatment was given to each fetal lung explant on D2. The protocols used with the animal experiments in this study were approved by the Oklahoma State University Animal Care and Use Committee.
Fetal alveolar epithelial type II cells isolation and culture in Matrigel.
Fetal alveolar epithelial type II cells (fAEC II) was isolated as previously described (87). The purity of fAEC II was 90%. The pregnant Sprague-Dawley rats on gestational day 18 (E18) were euthanized with CO2. Fetal lungs were dissected from the fetuses and chopped into 1-mm3 pieces. Cells were dissociated by digestion with a solution consisting of 1 mg/ml collagenase, 1 mg/ml trypsin, and 0.4 mg/ml DNase I in minimum essential media (MEM) for 10 min, four times. The resulting cell suspension was filtered through 160- and 37-μm of nylon filters in sequence. Cells were then seeded in a 20-cm plastic dish and incubated for 45 min, four times to remove fibroblasts. The cell suspension was then filtered through a 15-μm nylon filter. Two million of the E18 fAEC II were cultured on 500 μl of Matrigel (BD Matrigel Basement Membrane Matrix High Concentration, Growth Factor Reduced; cat. no. 354263; lot no. 99301; 18.7 mg/ml; 1:1 dilution) in each well of a six-well plate and infected by adenovirus. The medium used on day 0 was MEM, supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). On the second day, the medium was changed to the defined medium, which consisted of DMEM + 1% mix 1 + 0.25% mix 2 + 1% nonessential amino acid (NEAA) + 1% P/S + 0.01% Na selenite. Mix 1 contained d-biotin, ethanolamine, phosphoethanolamine, putrescine/bitane-1,4-diamile, and transferrin, and mix 2 contained CuSO4, FeCl3, ZnCl2, and MnCl2. The cells were then cultured on Matrigel for 2 days. After that, Matrigel was resolved by incubation with 1 ml of dispase for 1 h. The cells were then collected for RNA extraction.
Anthrone assay.
Glycogen determination was performed by anthrone assay as described (72). Cultured fetal lung explants were briefly boiled in 100 μl of 30% KOH for 30 min. The resulting slurry was diluted with 1.5 ml of H2O and mixed completely. A 400-μl aliquot of this diluted sample was then mixed with 800 μl of 0.2% anthrone reagent in 95% H2SO4 in a cold water bath. The mixture was boiled for 10 min and cooled down to room temperature in a cold water bath. The optical density was read at 620 nm with a spectrophotometer.
Pathway screening.
Pathway analysis was performed with Cignal Reporter Assays (SABiosciences, Frederick, MD). In brief, HEK 293T cells were transfected with 50 ng of a pathway reporter vector and 100 ng of microRNA overexpression plasmid or control plasmid for 24 h and incubated with or without a corresponding stimulus for another 24 h. The cells were then harvested and the firefly and Renilla luciferase activities were detected using the Dual Luciferase Reporter Assay System (Promega, Madison, WI) and measured by the FLUOstar OPTIMA microplate fluorometer (BMG LABTECH, Offenburg, Germany). Firefly signals were normalized with Renilla signals.
Western blotting.
Thirty-five micrograms of protein were loaded into each well and run on SDS-PAGE. The proteins were transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies at 4°C overnight. After being washed with Tris-buffered saline and Tween 20 (TBS-T), the membranes were then incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (1:2,000; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h. The following primary antibodies were used in Western blotting: mouse monoclonal anti-PIK3C2A (phosphatidylinositol-4-phosphate 3-kinase, catalytic subunit type 2 alpha; sc-136298; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-son of sevenless homolog 1 (SOS1; sc-256; 1:200; Santa Cruz Biotechnology), rabbit polyclonal anti-nuclear factor I/B (NFIB; 39091; 1:1000; Active Motif, Carlsbad, CA), rabbit polyclonal anti-SP-C (sc-13979; 1:500; Santa Cruz Biotechnology), and rabbit polyclonal anti-β-actin from Sigma (A-2066; 1:2,000). The blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and visualized and quantitated with Molecular Imager VersaDoc MP 5000 System (Bio-Rad, Hercules, CA).
Construction of promoter and 3′-UTR luciferase reporter vectors.
To construct miR-124 promoter reporter vectors, the 5′-flanking regions of miR-124-1 (−1,753 to +36), miR-124-2 (−2,146 to +46), and miR-124-3 (−1,770 to +36) were amplified by PCR using specific primers and human genomic DNA. The start site of the stem-loop pre-miRNA was nominated as position +1. The primers were as follows: miR-124-1: sense 5′-ATAAGCGCTGAACCCAGAACACGCGTG-3′ and antisense 5′-AAACTCGAGAATCAAGGTCCGCTGTGAACAC-3′; miR-124-2: sense 5′-ATAACGCGTTCCGGAATGGCATTCGTGATAAG-3′ and antisense: 5′-AAACTCGAGATCAAGGTCCGCTGTGAACACG-3′, and miR-124-3: sense, 5′-ATACGCGTCGCTCCTTGCAGTCTAAACAAAG-3′ and antisense: 5′-AACAGATCTATCAAGGTCCGCTGTGAACACG-3′. The miR-124-1 or miR-124-2 fragment was inserted into the upstream of the firefly luciferase gene using the promoterless luciferase reporter vector, pGL3-Basic (Promega) at the MluI and XhoI sites. The miR-124-3 fragment was inserted into the pGL3-Basic at the MluI and Bgl II sites. All plasmid constructs were confirmed by DNA sequencing.
To identify a necessary region in the miR-124-2 promoter for β-catenin regulation, a series of truncated miR-124-2 promoter constructs, including −1,970/+46, −1,834/+46, −1,076/+46, −593/+46, and −529/+46 were created by PCR using the −2,146 to +46 fragment of miR-124-2 promoter as a template. The products were inserted into the Mlu I and Xhol I site of the pGL3-Basic vector.
The 3′-UTRs of rat guanine nucleotide binding protein, alpha-inhibiting activity polypeptide 3 (GNAI3), SOS1, neuroblastoma RAS viral oncogene homolog (NRAS), v-akt murine thymoma viral oncogene homolog 2 (AKT2), growth factor receptor-bound protein 2 (GRB2), PIK3C2A, NFIB, and adenylate cyclase 9 (ADCY9) were PCR amplified from rat genomic DNA and cloned into the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). For the NFIB 3′-UTR mutations, the 5′-fragments were amplified by PCR with NFIB forward and NFIB_Mut1/2 reverse, using the miRGlo-NFIB 3′-UTR plasmid as the template. The 3′-fragments were amplified with NFIB_Mut1/2 forward and NFIB reverse, using the same plasmid as the template. Then, the whole mutated 3′-UTRs were created by PCR with NFIB forward and NFIB reverse, using the mixture of the abovementioned 5′-fragments and 3′-fragments as the template. The mutated NFIB 3′-UTRs were then inserted into the pmiRGlo vector. The primers used were listed in Table 2.
Table 2.
Primers used for amplifying 3′-UTR
| GNAI3 forward | TCAGCTAGCTGTGGCCTTTTTTGCTAGGAGAC |
| GNAI3 reverse | TCAGTCGACGACAATCTTCAGACAGCTTTGG |
| SOS1 forward | TCTGCTAGCGACCTAAGCTGAGCCAAGAGAATAC |
| SOS1 reverse | TCAGTCGACTGTGGGCTATATAAGGCATTTT |
| NRAS forward | TCAGCTAGCAAGGACCCTTTAAAAGTTCTGT |
| NRAS reverse | TCAGTCGACGGTTTGAAGAATCATTAATCAC |
| AKT2 forward | ATAGCTAGCTCTGCCACCACAGGACACAGCAT |
| AKT2 reverse | TCAGTCGACCTAAGGCTTCCTTGTTCCACAC |
| GRB2 forward | TCAGCTAGCTTAAAGAAAGTGAAAAGTTGAG |
| GRB2 reverse | TCAGTCGACTTATTCACAGTTAATCACTACC |
| PIK3C2A forward | TATGCTAGCTGTTGACTCCTACCAATTCCAA |
| PIK3C2A reverse | ATAGTCGACGAAGTCACATTCAATTCACTGA |
| NFIB forward | TCAGCTAGCTGGTTCCTTTTCAAGTGTCAAA |
| NFIB reverse | TCAGTCGACGGTCAATTAAAACAAACAAACA |
| ADCY9 forward | TCAGAGCTCCTCTGCTTGTCCAAACACAATA |
| ADCY9 reverse | TCTGTCGACTCAGCTGTGTCCTTTGCAAACT |
| NFIB_Mut1 forward | CTAGAACGGAATTTATTTGCATGATAAGCT |
| NFIB_Mut1 reverse | GCAAATAAATTCCGTTCTAGAAAGTCAGTT |
| NFIB_Mut2 forward | CCACATTACCACGGAAGCCTTGAAATCTAA |
| NFIB_Mut2 reverse | CAAGGCTTCCGTGGTAATGTGGACATTATA |
GNAI3, alpha-inhibiting activity polypeptide 3; SOS1, son of sevenless homolog 1; NRAS, neuroblastoma RAS viral oncogene homolog; AKT2, v-akt murine thymoma viral oncogene homolog 2; GRB2, growth factor receptor-bound protein 2; ADCY9, adenylate cyclase 9.
Luciferase assay.
For promoter activity assay, MLE15 cells were seeded onto a 96-well plate at a density of 2 × 104 cells per well and transfected with 50 ng of miR-124 promoter reporter plasmid, 20 ng of mouse ΔGSK-β-catenin expression vector (7) or human LEF1 expression vector, and 2 ng of the pRL-TK plasmid (Promega). The pRL-TK plasmid, which expresses Renilla luciferase, was used as an internal control for normalizing luciferase activity. The empty pCMV vector was used to keep the total transfected DNA at a fixed amount. Cells were collected 48 h after transfection and the firefly and Renilla luciferase activities were measured as described above.
For 3′-UTR activity assay, 50 ng of the microRNA overexpression plasmid pENTR-miR-124 were transfected into 293T cells using Lipofectamine 2000 (Invitrogen) together with 5 ng of the pmirGLO reporter construct. The cells were harvested 48 h posttransfection, and the firefly and Renilla luciferase activities were measured.
Chromatin immunoprecipitation.
The chromatin immunoprecipitation (ChIP) was performed using EZ ChIP kit according to the instruction manual of the manufacturer. In brief, rat fAEC II was cross linked with 1% formaldehyde at room temperature for 10 min. The reaction was stopped by adding glycine to a final concentration of 125 mM. The cells were harvested by centrifugation, lysed, and sonicated to achieve an average size of 500 bp. Diluted chromatin suspensions were precleared with sonicated salmon sperm DNA and protein G agarose slurry (50%) for 2 h at 4°C. Supernatants were collected and were then incubated with 2 μg of mouse monoclonal anti-β-catenin (no. 610154; 1:2,000) from BD (Franklin Lakes, NJ), goat anti-LEF-1 antibody (no. sc-8591,1:1,000) from Santa Cruz Biotechnology, or normal IgG as the negative control at 4°C overnight. The salmon sperm DNA/protein G agarose slurry were added and incubated for 1 h. The precipitates were collected, washed, and eluted. The cross links were reversed at 65°C for 7 h. The chromatins were then digested with proteinase K at 45°C for 2 h. Chromatin DNAs were purified using spin columns. The DNAs were amplified by PCR using GoTaq polymerase (Promega). The putative LEF1 binding regions and primers used for amplification are listed in Table 3 and Table 4. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) promoter, which does not have LEF1 binding site, was employed as a negative control (Table 4).
Table 3.
Potential LEF1 binding sites in human and rat miR-124 promoters
| Binding Site | Promoter/Position in Human | Sequence | Promoter/Position in Rat |
|---|---|---|---|
| LBE1 | hsa-miR-124-1: −240-232 | CTTTGGAA | |
| LBE2 | hsa-miR-124-1: −251-244 | GAACAAAG | rno-miR-124-1: −350-343 |
| LBE3 | hsa-miR-124-1: −289-282 | CTTTGCAG | rno-miR-124-1: −388-381 |
| LBE4 | hsa-miR-124-1: −860-853 | TGACAAAG | |
| LBE5 | hsa-miR-124-1: −1,793-1,786 | GGACAAAG | rno-miR-124-1: −1,825-1,818 |
| LBE6 | hsa-miR-124-1: −1,881-1,874 | CTTTGGTT | |
| LBE1 | hsa-miR-124-2: −559-552 | TATCAAAG | rno-miR-124-2: −557-550 |
| LBE2 | hsa-miR-124-2: −634-627 | TTACAAAG | rno-miR-124-2: −628-621 |
| LBE3 | hsa-miR-124-2: −1,109-1,102 | CTTTGATT | rno-miR-124-2: −1,098-1,091 |
| LBE4 | hsa-miR-124-2: −1,941-1,934 | CTTTGGGA | rno-miR-124-2: −1,877-1,870 |
| LBE5 | hsa-miR-124-2: −1,975-1,968 | CTTTGGTT | rno-miR-124-2: −1,911-1,904 |
| LBE1 | hsa-miR-124-3: −458-451 | CTTTGAGC | |
| LBE2 | hsa-miR-124-3: −1,755-1,748 | AAACAAAG | rno-miR-124-2 −1,673-1,666 |
Table 4.
Primers used for ChIP-PCR
| Primer | Sequence | |
|---|---|---|
| rno-miR-124-1-forward-365 | CGAAAGGATGSGGGAGAACAAAG | S = G, C |
| rno-miR-124-1-reverse-214 | TGCGTGCGCACTGACAGCAGRG | R = A, G |
| rno-miR-124-1-forward-516 | CATTGTCTGGARCTGCAGGGGAG | R = A, G |
| rno-miR-124-1-reverse-373 | GCTTTTTCCTGCAAAGSGGAGG | S = G, C |
| rno-miR-124-1-forward-1912 | TTGGTTTGGACTGGCTGARACG | R = A, G |
| rno-miR-124-1-reverse-1752 | CCAAGACGCTTTGTCCCGGAR | R = A, G |
| rno-miR-124-2-forward-606 | CGGCCTGGGATTCTGATCTTTTC | |
| rno-miR-124-2-reverse-512 | TCATGCGTGTTTGTWTGTCCGAC | W = A, T |
| rno-miR-124-2-forward-699 | ATCATAGCCAGACCAGATGGGCC | |
| rno-miR-124-2-reverse-588 | AGATCAGAATCCCAGGCCGACC | |
| rno-miR-124-2-forward-1185 | GGTCACCTYGGCTCCCTAGAGTG | Y = C, T |
| rno-miR-124-2-reverse-1002 | GACTGCGCTCGCYTGAAGGAAG | Y = C, T |
| rno-miR-124-2-forward-1901 | AAGGACACAGCGAGCCGTTCAG | |
| rno-miR-124-2-reverse-1724 | GATCGTAGCGGTCCGGTCTTTC | |
| rno-miR-124-2-forward-2066 | TGCTGTGGGGAAGTCTAGGGAG | |
| rno-miR-124-2-reverse-1886 | GGCTCGCTGTGTCCTTGAAACC | |
| rno-miR-124-3-forward-1756 | CAGGTGGTCAAGGGCTGGKTTG | K = G, T |
| rno-miR-124-3-reverse-1584 | GGGTGACTACCTTGATCTGGGYC | Y = C, T |
| GAPDH-forward | GTGCAAAAGACCCTGAACAATG | |
| GAPDH-reverse | GAAGCTATTCTAGTCTGATAACCTCC |
ChIP, chromatin immunoprecipitation.
Bioinformatics analysis.
The chromosome localization and sequence conservation were analyzed using the Genome browser (http://genome.ucsc.edu). Prediction of the putative LEF1 binding site was performed using version 8.3 of TRANSFAC software (http://alggen.lsi.upc.es/).
RESULTS
miR-124 is downregulated during fetal lung development.
We measured the expression levels of miR-124 at different stages of rat fetal lung development, including gestational days 16, 19, and 21 (E16, E19, and E21); postnatal days 0, 6, and 14 (P0, P6, and P14); and 2 mo after birth (AD), with real-time PCR (Fig. 1A). The expression of miR-124 was remarkably decreased from E16 to E19 and continued to decrease gradually from E19 to adult. The localization of miR-124 in E16 fetal lungs was determined by microRNA in situ hybridization. As shown in Fig. 1B, miR-124 was mainly expressed in the epithelial cells of E16 lungs. The negative control performed on E16 lungs using a scrambled probe did not show any signals.
Fig. 1.
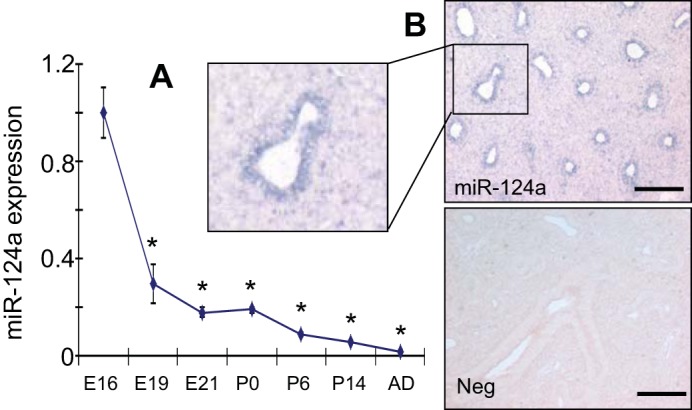
Expression of miR-124 during rat fetal lung development. A: miR-124 expression at different stages of fetal lung development [embroynic days E16, E19, and E21; postnatal days P0, P6, and P14; and after birth (AD)] was determined using real-time PCR. Relative expression against 18S rRNA was calculated using ΔCt formula: 2−(CtmiRNA−Ct18S) and then normalized to E16. Data shown are means ± SE from 3 independent preparations. *P < 0.05 vs. E16 (Student t-test). B: in situ hybridization for miR-124 in E16 fetal lung. Neg: negative control on E16 fetal lung using a probe with a scrambled sequence. The images are a representative of 2 repetitions. Scale bar = 100 μm.
miR-124 inhibits AEC II cell marker expression in fetal lung organ culture.
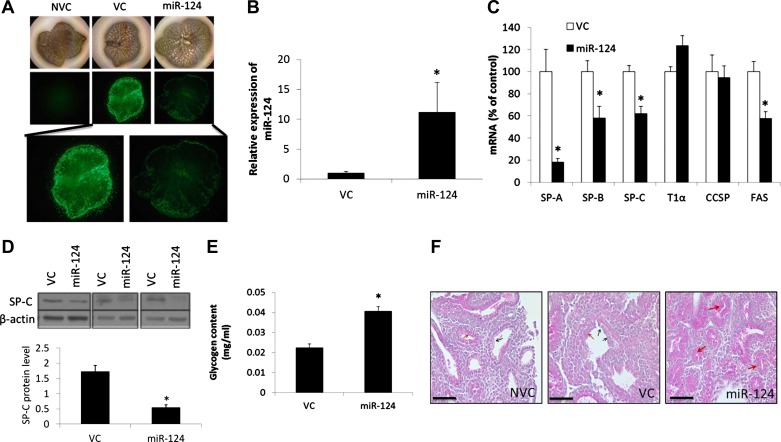
To identify the functions of miR-124 in fetal lung development, we overexpressed miR-124 ectopically with an adenovirus in rat fetal lung organ culture. The miR-124 adenovirus contains a GFP marker for monitoring transduction efficiency. The virus control contains GFP but not any microRNA sequences. To improve the transduction efficiency, we preincubated adenovirus with protamine, which neutralizes the charges on the viruses and cells and has been shown to improve the adenoviral transduction efficiency (47). Fetal lungs on gestational day 15 were transduced with miR-124 overexpression adenovirus (Ad-miR-124) or virus control together with 0.1 mg/ml of protamine and cultured for 5 days. There was GFP expression in fetal lungs transduced with miR-124 virus or control virus (VC) at day 5 (Fig. 2A), indicating an efficient transduction. There was no background signal in the fetal lungs without transduction (NVC). The weak GFP signal in the miR-124-transduced lungs compared with VC-transduced lungs is likely due to instability after miR-124 processing since miR-124 and GFP were under the control of the same promoter. The overexpression virus increased the expression of miR-124 >10-fold (Fig. 2B).
Fig. 2.
miR-124 overexpression and alveolar epithelial cell maturation. Fetal lungs on gestation day 15 were transduced with miR-124 overexpression virus with a GPF marker, virus control (VC) containing an empty vector with GFP, but not any microRNA sequences, or nothing (NVC) and were cultured for 5 days. A: brightfield and GPF images. B and C: expression levels of miR-124 and cell marker genes was determined using real-time PCR. Relative expression against 18S rRNA was calculated using ΔCt formula: 2−(CtmiRNA−Ct18S). Data are expressed as a ratio to VC or a percentage of VC. D: protein levels of surfactant protein-C (SP-C) were determined using Western blotting. SP-C protein levels were normalized to β-actin. E: glycogen content was detected with anthrone reagent. F: periodic acid-Schiff staining. Red and black arrows indicate positive and negative staining. Scale bar = 50 μm. Data shown are means ± SE from at least 3 independent replicates. *P < 0.01 vs. VC (Student t-test).
To determine whether miR-124 influences the alveolar epithelial cell differentiation, we determined the mRNA expression of several cell marker genes. The expression levels of surfactant proteins A, B, C, and D [SP-A, SP-B, SP-C, SP-D, alveolar epithelial type II cell (AEC II) markers], and fatty acid synthase (FAS, an important enzyme in surfactant synthesis) were significantly decreased in the miR-124-treated lungs (Fig. 2C). However, the alveolar epithelial type I cell (AEC I) marker T1α and the Clara cell marker CCSP were not significantly changed. Western blots revealed that the SP-C protein was also decreased by miR-124 overexpression (Fig. 2D).
During AEC II maturation, glycogen disappears from the precursor AEC II. We performed an anthrone assay to measure the glycogen content in the fetal lung organ culture. The glycogen content was higher in the Ad-miR-124-transduced fetal lungs (Fig. 2E). In addition, the glycogen was also detected with periodic acid-Schiff staining. The amount of glycogen was significantly increased in the Ad-miR-124-treated lungs (Fig. 2F). Taken together, these results indicate that miR-124 inhibited AEC II marker expression but not AEC I and Clara cell marker expression in fetal lung organ culture.
miR-124 inhibits AEC II cell marker expression in fAEC II culture.
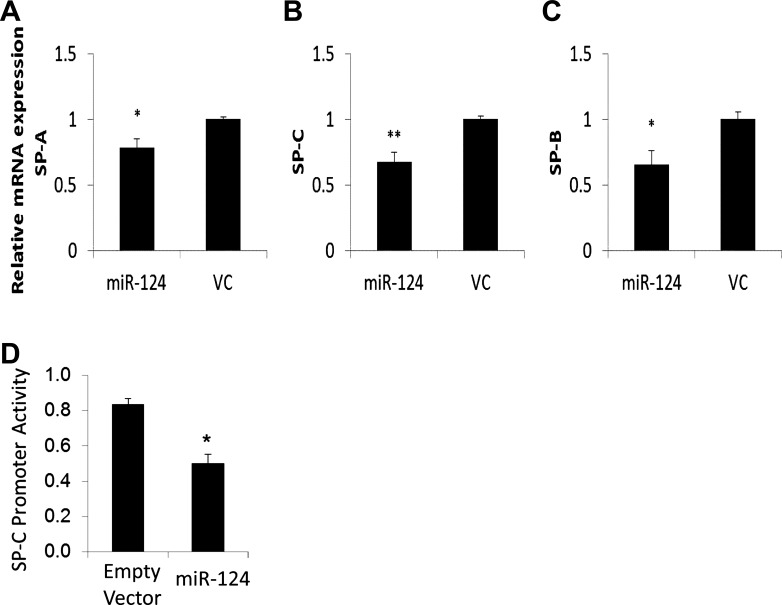
To determine whether miR-124 inhibits AEC II maturation directly, we isolated fAEC II cells from pregnant rats on gestational day 18 and transduced them with Ad-miR-124 or control virus in the presence of 0.1 mg/ml of protamine. It has been reported that isolated rat fAEC II can differentiate in defined medium on a basement membrane matrix, with formation of lamellar bodies and the expression of surfactant proteins (19). The isolated fAEC II were cultured on Matrigel for 2 days, and the expression of SP-A, SP-B, and SP-C was detected with real-time PCR (Fig. 3, A–C). Consistent with the results from fetal lung organ culture, the expression of SP-A, SP-B, and SP-C was significantly decreased. We also constructed a human SP-C promoter-driven firefly luciferase gene reporter vector. We used MLE-15 cells, which have SP-B and SP-C expression (89). We cotransfected miR-124 into MLE-15 cells with the SP-C reporter plasmid. miR-124 inhibited the SP-C promoter activity (Fig. 3D). However, the empty vector had no effects. These results indicate that miR-124 can inhibit fAEC II maturation.
Fig. 3.
Expression of surfactant proteins in cultured fetal alveolar epithelial type II (fAEC II). E18 fAEC II were infected with Ad-miR-124 or VC containing an empty vector (multiplicity of infection = 100) and cultured on Matrigel for 2 days. The mRNA expression of SP-A (A), SP-B (B), and SP-C (C) was determined using real-time PCR. Relative expression against 18S rRNA was calculated using ΔCt formula: 2−(CtmiRNA−Ct18S) and then normalized to VC. Data shown are means ± SE from 3 independent cell preparations. *P < 0.05 vs. VC; **P < 0.005 vs. VC. D: MLE-15 cells were transfected with the SP-C promoter reporter (5 ng) pRL-TK (0.5 ng), empty vector, and miR-124 expression vector (195 ng). Forty-eight hours posttransfection, dual luciferase activities were measured. The promoter activity was expressed as a ratio of firefly over Renillia luciferase activities (means ± SE). *P < 0.05 vs. empty vector; n = 3.
miR-124 inhibits NF-κB, cAMP/PKA, and MAPK/ERK pathways.
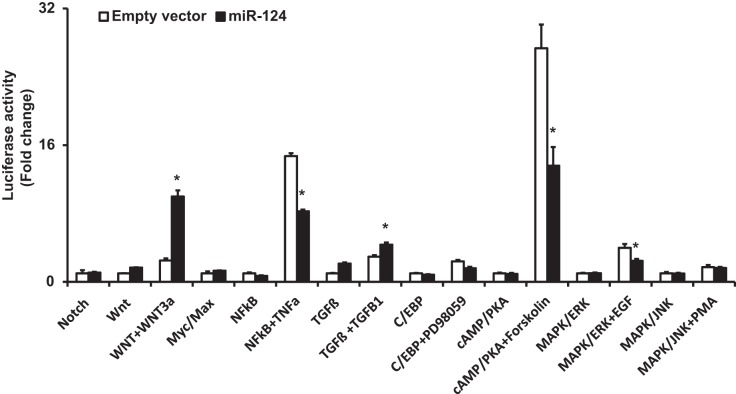
To determine how miR-124 regulates epithelium maturation in fetal lung development, we screened nine developmental pathways with a luciferase reporter assay to identify which pathways are regulated by miR-124. We transfected a luciferase pathway reporter along with miR-124 expression vector or empty vector control into HEK 293 cells and stimulated the cells with 50% WNT3a conditioned medium (WNT3a-CM) for Wnt pathway, tumor necrosis factor-α (TNF-α, 0.01 ng/μl) for NF-κB pathway, transforming growth factor-β (TGF-β; 0.005 ng/μl) for TGF-β pathway, PD98059 (2.67 ng/μl) for C/EBP pathway, forskolin (1 ng/μl) for cAMP/PKA pathway, epidermal growth factor (EGF; 0.1 ng/μl) for MAPK/ERK pathway, and phorbol 12-myristate 13-acetate (PMA; 0.01 ng/μl) for MAPK/JNK pathway. WNT3a-CM was produced from L WNT3a cells, which are a stable clone of L-cells transfected with WNT3a and secrete biologically active WNT3a protein. The results revealed that the NF-κB, cAMP/PKA, and MAPK/ERK pathways under stimulated conditions were significantly repressed by miR-124 (Fig. 4).
Fig. 4.
Luciferase reporter assays for screening of pathways affected by miR-124. The activities of 9 developmental pathways after miR-124 overexpression with or without stimuli were determined using luciferase reporter assays in HEK 293T cells. Open bars represent empty vector controls and solid bars represent miR-124 vectors. The fold changes were relative to the unstimulated empty vector-transfected cells for each reporter. Data shown are means ± SE. *P < 0.005 (Student t-test); n = 6.
NFIB is identified as a target of miR-124.
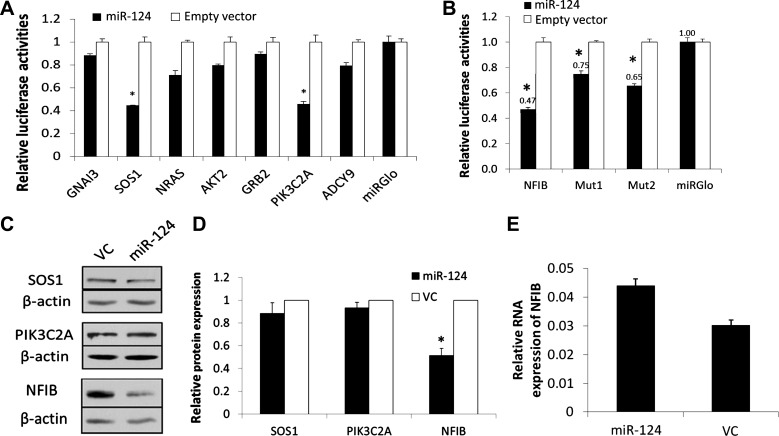
To identify potential targets of miR-124 in these pathways, we performed target prediction with TargetScan (http://www.targetscan.org/) and pathway analysis with DAVID (http://david.abcc.ncifcrf.gov/). Several predicted targets of miR-124 were nested in the miR-124-repressed pathways, including GNAI3 and ADCY9 in the cAMP/PKA pathway; SOS1, NRAS, and GRB2 in the MAPK/ERK pathway; and AKT2 and PIK3C2A in the upstream of the NF-κB pathway. In addition, it has been reported that NFIB increases the transcription of insulin-like growth factor-binding protein-5 (IGFBP-5), which activates the MAPK/ERK signaling pathway (41, 66). Furthermore, NFIB can regulate epithelium maturation in fetal lung development (30). We thus also included NFIB in our target verification. The 3′-UTR dual luciferase assays revealed that SOS1, PIK3C2A, and NFIB were significantly inhibited by miR-124 and all others were not affected (Fig. 5, A and B).
Fig. 5.
Verification of predicted targets. A and B: 3′-UTR luciferase reporter assays. HEK 293T cells were transfected with miRGlo luciferase reporter vectors containing the 3′-UTR of 7 predicted miR-124 predicted targets (A) or containing nuclear factor I/B 3′-UTR (NFIB), mutated 3′-UTR at the 1st or 2nd binding sites (Mut1 and Mut2; B), together with the miR-124 expression pENTR plasmid or empty vector without any microRNA sequences (empty vector). miRGlo was the empty vector without any 3′-UTR sequences. Luciferase activities were determined 48 h posttransfection. C–E: MLE15 cells were transduced with Ad-miR-124 or VC and were collected 72 h after transduction. The protein levels of SOS1, PIK3C2A, and NFIB were measured with Western blotting. Representatives of the blots are shown in C. Quantitation of the blot are shown in D and were normalized to β-actin. mRNA expression of NFIB was measured with real-time PCR (E). Relative expression of NFIB mRNA against 18S rRNA was calculated using ΔCt formula: 2−(CtmiRNA−Ct18S). Data shown were means ± SE from 3–6 experiments and are expressed as fractions of empty vector or VC (A, B, and D). *P < 0.001 vs. empty vector or VC.
TargetScan predicts two conserved miR-124 binding sites in the 3′-UTR of NFIB at positions of 214–418 (site 1) and 1,481-1,487 (site 2). When binding site 1 or site 2 was mutated, the luciferase activity was only decreased 25 and 35%, respectively, compared with 50% decrease for the wild-type 3′-UTR reporter (Fig. 5B). This result suggests that both binding sites are functional in the inhibition of NFIB expression.
To examine whether miR-124 reduces protein levels of the potential targets in lung epithelial cells, we overexpressed miR-124 using an adenovirus expressing miR-124 in MLE15 cells and determined the protein levels of SOS1, PIK3C2A, and NFIB by Western blotting. As shown in Fig. 5, C and D, miR-124 reduced the NFIB protein level by 50% but had no effects on the protein levels of SOS1 and PIK3C2A. However, miR-124 cannot decrease the mRNA level of NFIB (Fig. 5E), which suggests that the inhibition of NFIB expression by miR-124 is at the posttranscriptional level. Taken together, miR-124 directly targets NFIB and thus inhibits MAPK/ERK signaling.
Activation of Wnt/β-catenin signaling increases miR-124 expression.
Wnt/β-catenin signaling has been shown to regulate the expression of microRNAs (10, 31, 35). However, it is not known whether Wnt/β-catenin signaling can modulate miR-124 expression. To determine the effect of Wnt/β-catenin signaling on miR-124, we treated primary fAEC II with 50% WNT3a-CM. WNT3a-CM significantly induced the expression of miR-124 in fAEC II and E10 cells (Fig. 6A).
Fig. 6.
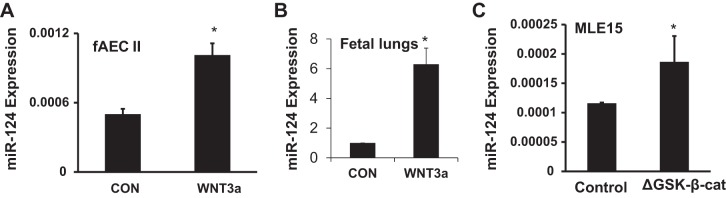
WNT/β-catenin signaling increases the expression of miR-124. Real-time PCR analysis of miR-124 levels in fAEC II (A) or E16 fetal lung slice (B) treated with 50% WNT3a conditioned medium or control L-cell conditioned medium (CON) for 24 h (A) or 5 days (B). Real-time PCR analysis of miR-124 in MLE15 cells with transient transfection of ΔGSK-β-catenin or control for 24 h (C). The expression level of miR-124 was calculated using ΔCt formula: 2−(CtmiRNA−Ct18S) (A and C). Data was further expressed as a ratio to CON in B. Results are presented as means ± SD from 3 independent experiments. *P < 0.05 vs. CON or control (Student t-test).
We also used fetal lung slice culture to study the regulation of miR-124 by WNT/β-catenin signaling during fetal lung development. This model has been used to study the regulation of surfactant proteins and other genes (9, 24, 38, 57, 79, 81, 92). Fetal lungs were isolated from timed-pregnant Sprague-Dawley rats on gestational day 16 (E16) and minced into 1-mm cubes. The slices were cultured for 5 days in Millicell inserts with 2 ml of DMEM/F12 (1:1) medium or with medium DMEM/F12 containing WNT3a outside of the inserts on a rocking platform. WNT3a increased miR-124 expression by approximately sixfold (Fig. 6B).
Furthermore, miR-124 was significantly induced by constitutively active mouse β-catenin ΔGSK-β-catenin in MLE15 cells (Fig. 6C). ΔGSK-β-catenin has four point mutations in the GSK-3β phosphorylation sites of β-catenin (S/T > A) and thus cannot be phosphorylated by GSK-3β. The mutated protein was under the control of CMV promoter using pUDH10-3 vector (7). These data indicate that the activation of Wnt/β-catenin signaling enhances miR-124 expression.
Wnt/β-catenin-TCF/LEF1 signaling activates miR-124 promoter transcription.
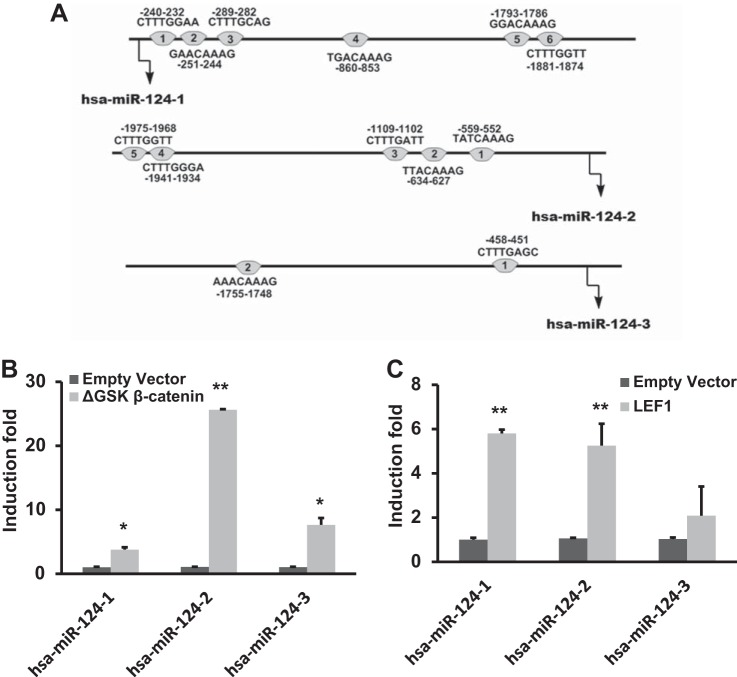
By use of the miRBase (http://microrna.sanger.ac.uk), three miR-124 isoforms were found in different species, including human, rat, and mouse, miR-124-1, miR-124-2, and miR-124-3. These isoforms are located at three separate loci on the genome. Human miR-124-1, miR-124-2, and miR-124-3 are encoded on chromosome 8p23.1, 8q12.3, and 20q13.33, respectively. To identify whether human miR-124-1, miR-124-2, and miR-124-3 promoters contain putative LEF1 binding sites, upstream genomic DNA from the start site of stem loop of precursor miRNA-124-1, miRNA-124-2, or miRNA-124-3 were downloaded from the University of California, Santa Cruz genome browser (http://genome.ucsc.edu). The 2-kb DNA was applied to prediction analysis, using version 8.3 of TRANSFAC software. The results indicated that miR-124-1, miR-124-2, and miR-124-3 promoters contain six, five, and two putative LEF1 binding sites (Fig. 7A). To investigate whether β-catenin/LEF1 affects miR-124 promoter activity, we constructed miR-124-1, miR-124-2, and miR-124-3 promoter luciferase reporters by inserting 5′-flanking regions of miR-124-1 (−1,753 to +36), miR-124-2 (−2,146 to +46), and miR-124-3 (−1,770 to +36) into the upstream of the firefly luciferase gene, using the pGL3-Basic vector. The transcription activities of miR-124-1, miR-124-2, and miR-124-3 promoters in MLE15 cells were significantly enhanced by the activation of the Wnt signal pathway, with a constitutively activated ΔGSK-β-catenin (Fig. 7B). The overexpression of LEF1, a cotranscriptional activator of β-catenin, also induced all three miR-124 promoter transcription activities (Fig. 7C). Both ΔGSK-β-catenin and LEF1 ΔGSK-β-catenin had no effects on the control SV40 promoter (pGL3; data not shown).
Fig. 7.
Effect of ΔGSK β-catenin and LEF1 overexpression on miR-124 promoter activities in MLE15 cells. A: putative LEF1 binding sites in human miR-124 promoters identified by TRANSFAC software (version 8.3). miR-124-1 is transcribed from the minus strand of genomic DNA. B and C: MLE15 cells were cotransfected with human miR-124-1 (−1,753 to +36), miR-124-2 (−2,146 to +46), or miR-124-3 (−1,770 to +36) luciferase promoter construct (50 ng/well) and ΔGSK β-catenin expression plasmid (20 ng/well), LEF1 expression plasmid (20 ng/well) or empty vector. Dual luciferase activities were expressed as ratios of firefly to Renilla luciferase activities. Data shown are induction fold over respective control empty vector. Results are presented as means ± SD from 3 independent transfection experiments performed in triplicate. *P < 0.05 vs. empty vector, **P < 0.01 vs. empty vector (Student t-test).
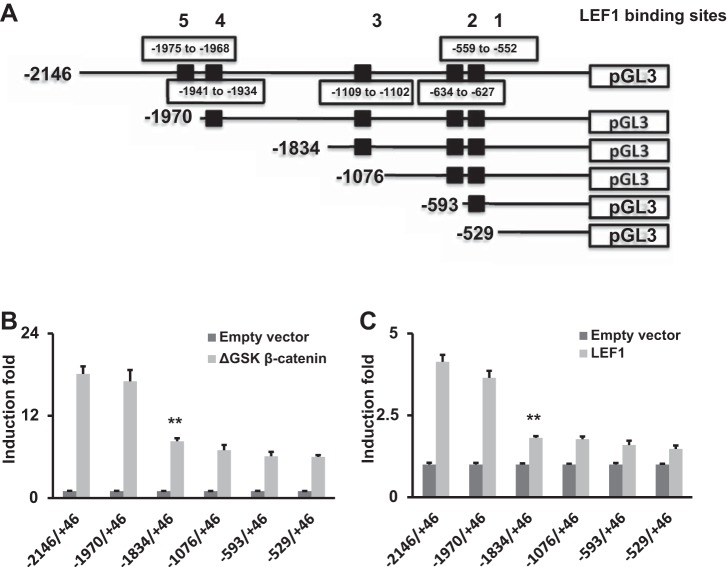
Among all miR-124 isoforms, miR-124-2 promoter activity was the highest induced by ΔGSK-β-catenin, and thus miR-124-2 promoter was selected for further studies. To determine which region of the miR-124-2 promoter is responsible for WNT/β-catenin-LEF/TCF signaling-mediated activation in MLE15 cells, we created a series of truncated miR-124-2 promoter constructs (Fig. 8A). Each truncated construct, in combination with either the ΔGSK β-catenin or LEF1 expression vector, was cotransfected into MLE15 cells. As shown in Fig. 8, B and C, compared with −2,146/+46 miR-124-2 promoter, ΔGSK β-catenin- or LEF1- mediated activation of −1,970/+46 miR-124-2 promoter activity did not significantly change, but the induction of −1,834/+46 miR-124-2 promoter activity was significantly reduced compared with −2,146/+46 miR-124-2 promoter activity. The deletion analysis indicated that the miR-124-2 promoter region, located between positions −1,970 and −1,834 containing the LEF1 binding site CTTTGGGA (−1,941 to −1,934), is necessary for its induction by Wnt/β-catenin-TCF/LEF1 signaling in MLE15 cells. There are no predicted LEF1 binding sites on the −529/+46 construct. However, this construct can still be activated by ΔGSK-β-catenin and to a less extent by LEF1. The possible reason is that ΔGSK-β-catenin works independent of LEF1 and/or there are weak LEF1 binding sites that are not identified by TRANSFAC software.
Fig. 8.
Deletion analysis identifies LEF1-binding region in human miR-124-2 promoter. A: schematic representation of has-miR-124-2 promoter showing putative LEF1 binding sites and deletion constructs. B and C: MLE15 cells were cotransfected luciferase reporter constructs containing truncated segments of has-miR-124-2 promoter and ΔGSK β-catenin expression plasmid, LEF1 expression plasmid or empty vector. Dual luciferase activities were expressed as ratios of firefly to Renilla luciferase activities. Data shown are induction fold over empty vector. Results are presented as means ± SD from 3 independent transfection experiments performed in triplicate. **P < 0.01 vs. 2,146/+46 construct (Student t-test).
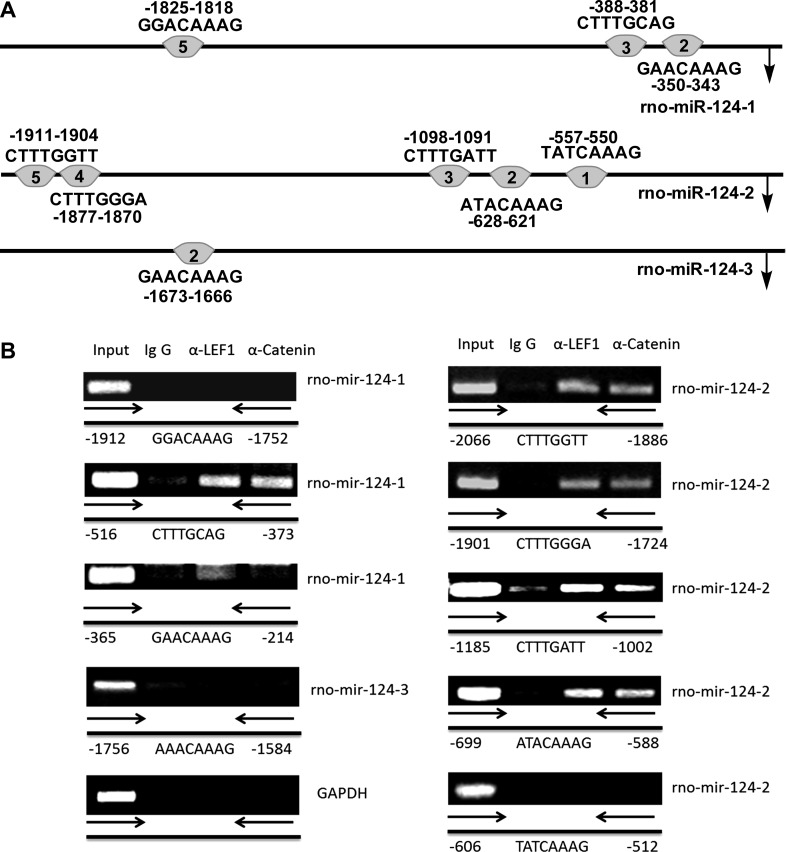
Direct interaction of LEF1 with chromatin DNA in the miR-124 promoter region.
To determine whether LEF1 and the endogenous miR-124 promoter interact in rat fAEC II cells, ChIP assay was performed. fAEC II were cross linked with 1% formaldehyde and sonicated. The chromatin was immunoprecipiated with anti-LEF1 or anti-β-catenin antibodies and amplified using the primers for putative LEF1 binding sites and their flanking sequences. Figure 9A shows the putative LEF1 binding sites in rat miR-124 promoters with the same numbering system of the binding sites in human miR-124 promoters (Fig. 7A). The rat and human miR-124-2 promoters had the same number of the LEF1 binding sites. However, rat miR-124-1 and miR-124-3 promoters had less number of the LEF1 binding sites compared with the human promoter (Table 3). As shown in Fig. 9B, binding site 3 (−516 to −373), to a less extent, binding site 2 (−365 to −214), but not binding site 5 (−1,912 to −1,810) in the miR-124-1 promoter; binding site 5 (−2,066 to −1,886), binding site 4 (−1,901 to −1,724), binding site 3 (−1,185 to −1,002), binding site 2 (−699 to −588), but not binding site 1 (−606 to −512) in the miR-124-2 promoter; and not binding site 2 (−1,756 to −1,584) in the miR-124-3 promoter were amplified from the LEF1- and β-catenin-pulled down DNAs. IgG negative control did not generate any PCR products. LEF1 or β-catenin did not bind the promoter of GADPH that does not contain the LEF1 binding sites. The results suggest that LEF1 can directly bind to two and four of the LEF1 binding sites on the miR-124-1 and miR-124-2 promoters, respectively.
Fig. 9.
Chromatin immunoprecipitation (ChIP) assay to detect LEF1 binding to the endogenous miR-124 promoter region containing putative LEF1 binding element. A: putative LEF1 binding sites in rat miR-124 promoters using version 8.3 of TRANSFAC software. B: rat fAEC II were cross linked and immunoprecipitated with anti-LEF1 antibody, anti-β-catenin antibody, or control IgG. All of the potential LEF1 binding regions in the miR-124-1, miR-124-2, and miR-124-3 promoters were amplified by PCR. The upstream region of the GAPDH promoter was selected as a negative control.
DISCUSSION
In this study, we found that miR-124 was predominantly expressed in the epithelial cells of the fetal lung on gestation day 16 and was downregulated during fetal lung development. Overexpression of miR-124 inhibits pulmonary epithelium maturation as indicated by the decrease in surfactant proteins and FAS and increase in glycogen content. In addition, we identified NFIB as a direct target of miR-124 and that the activation of WNT/β-catenin signaling induced miR-124 expression.
To our knowledge, this is the first report studying the function of miR-124 in fetal lung development. In addition, we are the first to identify NFIB as a direct target of miR-124. miR-124 is preferentially expressed in brain and retina (5, 43, 73). Most of the studies on miR-124 focused on the neural system. It has been reported that miR-124 plays important roles in neurogenesis, neuronal differentiation, plasticity, and microglia quiescence (12, 13, 49, 50, 53–55, 65, 68, 69, 71, 94). miR-124 is also involved in brain cancers, including medulloblastoma and glioblastoma (18, 67). In addition, miR-124 has been shown to be involved in intracellular signaling in pancreatic beta-cells, hepatocellular carcinoma, hematological malignancies, cervical cancer, oral squamous cell carcinoma, acute lymphoblastic leukemia, and gastrulation of embryonic stem cells (1, 4, 33, 44, 90, 91, 96). Many proteins have been identified as targets of miR-124, including Foxa2, CDK6, ROCK2, EZH2, CREB, ITGB, NeuroD1, C/EBP-α, Sox9, Lhx2, JAG1, IκBζ, SLUG, and IQGAP1 (4, 33, 44, 48–50, 67–69, 71). In this study, we added NFIB to this growing list of miR-124 targets.
Lung epithelial cell differentiation becomes more evident at the saccular stage of lung development. At this stage, cuboidal epithelial cells begin to synthesize pulmonary surfactant. The increase of surfactant protein expression corresponds with the decrease of miR-124 at this period. As indicated by in situ hybridization, miR-124 is mainly expressed in epithelial cells in E16 lungs. This suggests that the disappearance of miR-124 from epithelial cells may be necessary for epithelial cell maturation. Our data support this assumption, as ectopic expression of miR-124 in fetal lung organ culture inhibits lung epithelial maturation. The decrease of miR-124 and synthesis of surfactant also correspond with the increase of NFIB in the epithelial cells (30). The decrease of miR-124 in epithelial cells is critical for the increase of NFIB and thus epithelial cell maturation.
Nuclear factor I (NFI) is a family of transcription factors that bind to the consensus palindromic binding site, TTGGCN5GCCAA, in double-stranded DNA as homo- and heterodimers (25, 40, 58). In vertebrates, there are four NFI genes (Nfia, Nfib, Nfic, and Nfix) (39, 70). These genes have unique but overlapping expression patterns. Depending on the cellular context and alternative splicing of NFI mRNAs, NFI can activate or repress the expression of target genes (26). The importance of NFI proteins in development has been demonstrated with knockout mice. Loss of Nfia results in perinatal lethality and defects in brain development (16). Nfib-deficient mice die early after birth with severe lung hypoplasia and defects in lung maturation (27, 75). These mice also show defects in brain development similar to Nfia-deficient mice. NFIB is expressed predominantly in mesenchymal cells at early stages of fetal lung development and is expressed in both epithelium and mesenchyme at late stages. Loss of Nfib affects the expression of a number of genes involved in extracellular matrix and cell adhesion. Loss of Nfib also leads to a decreased expression of pulmonary surfactant proteins, including SP-A, SP-B, and SP-C, and the increase of fibroblast growth factor 10 (Fgf10) (30, 75). Fgf10 is expressed in mesenchymal cells and is known to regulate lung epithelial cell proliferation and differentiation. Overexpression of Fgf10 in the embryonic lung results in interruption of fetal lung branching morphogenesis and distal epithelial cell differentiation (14, 63). Nfic null mice showed defects in tooth root development (74).
miR-124 has been shown to regulate the Notch signaling pathway (50). From the results of our developmental pathway study, it is clear that miR-124 has important roles in development. It dramatically affects activity of the NF-κB, cAMP/PKA, and MAPK/ERK signaling pathways. However, no components in the NF-κB, cAMP/PKA, or MAPK/ERK pathways can be confirmed as direct targets of miR-124. It is possible that miR-124 regulates these pathways indirectly or by actions on unidentified targets. For example, NFIB activates IGFBP-5 transcription in osteoblasts (66) and IGFBP-5 activates the MAPK/ERK signaling pathway by Ras-dependent induction of p38 MAP kinase phosphorylation in human intestinal smooth muscle cells (41). Thus NFIB may activate the MAPK/ERK signaling pathway through the activation of IGFBP-5 and miR-124 may inhibit the MAPK/ERK signaling pathway by NFIB repression.
The regulation of miR-124 has been studied in the nervous system and other tissues. Hypermethylation of promoter (52, 84), ephrin-B1 reverse signaling (3), and cocaine (12) regulates miR-124 expression. miR-124-3 is downregulated in lung cancer (93, 96) and in mouse lung exposed to environmental cigarette smoke (34). miR-124-2 is preferentially expressed in the brain and lung at E14.5 to E18.5 (4). In this study, we demonstrated that WNT/β-catenin signaling regulates miR-124 expression. Mature miR-124 is processed from miR-124-1, miR-124-2, and miR-124-3. The upstream DNA of human pre-miR-124-1, 2, and 3 was cloned and was confirmed to have promoter activities by luciferase assay. WNT/β-catenin-LEF/TCF signaling regulates its target gene transcription by binding a conserved DNA region in the promoter with a flexible consensus binding sequence (T/A) (T/A) CAAAG. We analyzed the promoters of miR124-1, miR124-2, and miR124-3 and identified LEF/TCF binding sites in these regions. The physiological interaction of LEF1 and endogenous miR-124 promoters in rat fAEC II cells was further confirmed by ChIP assay. In addition, the miR-124 promoter was inducible under Wnt/beta-catenin-LEF/TCF signaling of MLE15 cells.
The WNT/β-catenin signaling pathway has been shown to be involved in lung development. However, it is unknown whether WNT/β-catenin can regulate lung development via modifying microRNA expression. We found that the expression of miR-124 remarkably decreased from E16 to E19. WNT/β-catenin signaling was active at the early stages of lung development, E10.5–12.5, and was reduced at E13.5-E18.5 (64). A decrease in WNT/β-catenin signaling during fetal lung development may contribute to the decrease in miR-124 expression.
In summary, we demonstrated that miR-124 was downregulated during the later stages of fetal lung development and NFIB was a direct target of miR-124. miR-124 inhibits fetal lung epithelial maturation, and WNT/β-catenin induces miR-124 expression. These results will help us to understand fetal lung development from a new perspective and may provide potential targets for therapeutic intervention to promote epithelial cell maturation in premature infants.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-071628 and R01-HL-116876 and National Institute of General Medical Sciences Grant P20-GM-103648 (Molecular Biology Core).
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.W., C.H., and L.L. conception and design of research; Y.W., C.H., N.R.C., D.X., and T.W. performed experiments; Y.W., C.H., and L.L. analyzed data; Y.W., C.H., and L.L. interpreted results of experiments; Y.W. and C.H. prepared figures; Y.W. and C.H. drafted manuscript; Y.W., C.H., N.R.C., D.X., and L.L. approved final version of manuscript; L.L. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank Dr. Angela Barth (Stanford University) and Dr. Joseph Alcorn (University of Texas Medical Center at Houston) for kindly providing ΔGSK-β-catenin and MLE-15 cells, respectively.
REFERENCES
- 1.Agirre X, Vilas-Zornoza A, Jimenez-Velasco A, Martin-Subero JI, Cordeu L, Garate L, San Jose-Eneriz E, Abizanda G, Rodriguez-Otero P, Fortes P, Rifon J, Bandres E, Calasanz MJ, Martin V, Heiniger A, Torres A, Siebert R, Roman-Gomez J, Prosper F. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res 69: 4443–4453, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. The functions of animal microRNAs. Nature 431: 350–355, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Arvanitis DN, Jungas T, Behar A, Davy A. Ephrin-B1 reverse signaling controls a posttranscriptional feedback mechanism via miR-124. Mol Cell Biol 30: 2508–2517, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baroukh N, Ravier MA, Loder MK, Hill EV, Bounacer A, Scharfmann R, Rutter GA, Van OE. MicroRNA-124a regulates Foxa2 expression and intracellular signaling in pancreatic beta-cell lines. J Biol Chem 282: 19575–19588, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Baroukh NN, Van OE. Function of microRNA-375 and microRNA-124a in pancreas and brain. FEBS J 276: 6509–6521, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics DP, biogenesis, mechanism, function. Cell 116: 281–297, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Barth AI, Stewart DB, Nelson WJ. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant beta-catenin. Proc Natl Acad Sci USA 96: 4947–4952, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaskaran M, Wang Y, Zhang H, Weng T, Baviskar P, Guo Y, Gou D, Liu L. MicroRNA-127 modulates fetal lung development. Physiol Genomics 37: 268–278, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogue CW, Jacobs HC, Dynia DW, Wilson CM, Gross I. Retinoic acid increases surfactant protein mRNA in fetal rat lung in culture. Am J Physiol Lung Cell Mol Physiol 271: L862–L868, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Cai WY, Wei TZ, Luo QC, Wu QW, Liu QF, Yang M, Ye GD, Wu JF, Chen YY, Sun GB, Liu YJ, Zhao WX, Zhang ZM, Li BA. The Wnt-beta-catenin pathway represses let-7 microRNA expression through transactivation of Lin28 to augment breast cancer stem cell expansion. J Cell Sci 126: 2877–2889, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Carraro G, El-Hashash A, Guidolin D, Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W, Parnigotto PP, Warburton D. miR-17 family of microRNAs controls FGF10-mediated embryonic lung epithelial branching morphogenesis through MAPK14 and STAT3 regulation of E-cadherin distribution. Dev Biol 333: 238–250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci 42: 350–362, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 12: 399–408, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JC, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT, Whitsett JA. FGF-10 disrupts lung morphogenesis and causes pulmonary adenomas in vivo. Am J Physiol Lung Cell Mol Physiol 280: L705–L715, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Comer BS, Camoretti-Mercado B, Kogut PC, Halayko AJ, Solway J, Gerthoffer WT. MicroRNA-146a and microRNA-146b expression and anti-inflammatory function in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 307: L727–L734, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.das NL, Duchala CS, Tolentino-Silva F, Haxhiu MA, Colmenares C, Macklin WB, Campbell CE, Butz KG, Gronostajski RM. Disruption of the murine nuclear factor I-A gene (Nfia) results in perinatal lethality, hydrocephalus, and agenesis of the corpus callosum. Proc Natl Acad Sci USA 96: 11946–11951, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9: 102–114, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Fowler A, Thomson D, Giles K, Maleki S, Mreich E, Wheeler H, Leedman P, Biggs M, Cook R, Little N, Robinson B, McDonald K. miR-124a is frequently down-regulated in glioblastoma and is involved in migration and invasion. Eur J Cancer 47: 953–963, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Fraslon C, Lacaze-Masmonteil T, Zupan V, Chailley-Heu B, Bourbon JR. Fetal rat lung type II cell differentiation in serum-free isolated cell culture: modulation and inhibition. Am J Physiol Lung Cell Mol Physiol 264: L504–L516, 1993. [DOI] [PubMed] [Google Scholar]
- 20.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol 10: 116–125, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med 60: 167–179, 2009. [DOI] [PubMed] [Google Scholar]
- 23.Geng S, Zhang X, Chen J, Liu X, Zhang H, Xu X, Ma Y, Li B, Zhang Y, Bi Z, Yang C. The tumor suppressor role of miR-124 in osteosarcoma. PLoS One 9: e91566, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Glumoff V, Vayrynen O, Kangas T, Hallman M. Degree of lung maturity determines the direction of the interleukin-1-induced effect on the expression of surfactant proteins. Am J Respir Cell Mol Biol 22: 280–288, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Gronostajski RM. Analysis of nuclear factor I binding to DNA using degenerate oligonucleotides. Nucleic Acids Res 14: 9117–9132, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gronostajski RM. Roles of the NFI/CTF gene family in transcription and development. Gene 249: 31–45, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Grunder A, Ebel TT, Mallo M, Schwarzkopf G, Shimizu T, Sippel AE, Schrewe H. Nuclear factor I-B (Nfib) deficient mice have severe lung hypoplasia. Mech Dev 112: 69–77, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res 65: 9628–9632, 2005. [DOI] [PubMed] [Google Scholar]
- 29.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5: 522–531, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Hsu YC, Osinski J, Campbell CE, Litwack ED, Wang D, Liu S, Bachurski CJ, Gronostajski RM. Mesenchymal nuclear factor I B regulates cell proliferation and epithelial differentiation during lung maturation. Dev Biol 354: 242–252, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang K, Zhang JX, Han L, You YP, Jiang T, Pu PY, Kang CS. MicroRNA roles in beta-catenin pathway. Mol Cancer 9: 252, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huleihel L, Ben-Yehudah A, Milosevic J, Yu G, Pandit K, Sakamoto K, Yousef H, LeJeune M, Coon TA, Redinger CJ, Chensny L, Manor E, Schatten G, Kaminski N. Let-7d microRNA affects mesenchymal phenotypic properties of lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 306: L534–L542, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hunt S, Jones AV, Hinsley EE, Whawell SA, Lambert DW. MicroRNA-124 suppresses oral squamous cell carcinoma motility by targeting ITGB1. FEBS Lett 585: 187–192, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Izzotti A, Calin GA, Steele VE, Croce CM, De FS. Relationships of microRNA expression in mouse lung with age and exposure to cigarette smoke and light. FASEB J 23: 3243–3250, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji J, Yamashita T, Wang XW. Wnt/beta-catenin signaling activates microRNA-181 expression in hepatocellular carcinoma. Cell Biosci 1: 4, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin Y, Jin Y, Chen B, Tipple TE, Nelin LD. Arginase II is a target of miR-17-5p and regulates miR-17-5p expression in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 307: L197–L204, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang K, Peng X, Zhang X, Wang Y, Zhang L, Gao L, Weng T, Zhang H, Ramchandran R, Raj JU, Gou D, Liu L. MicroRNA-124 suppresses the transactivation of nuclear factor of activated t cells by targeting multiple genes and inhibits the proliferation of pulmonary artery smooth muscle cells. J Biol Chem 288: 25414–25427, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein JM, McCarthy TA, Dagle JM, Snyder JM. Antisense inhibition of surfactant protein A decreases tubular myelin formation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol 282: L386–L393, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Kruse U, Qian F, Sippel AE. Identification of a fourth nuclear factor I gene in chicken by cDNA cloning: NFI-X. Nucleic Acids Res 19: 6641, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kruse U, Sippel AE. Transcription factor nuclear factor I proteins form stable homo- and heterodimers. FEBS Lett 348: 46–50, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Kuemmerle JF, Zhou H. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates growth and IGF-I secretion in human intestinal smooth muscle by Ras-dependent activation of p38 MAP kinase and Erk1/2 pathways. J Biol Chem 277: 20563–20571, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol 12: 735–739, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foa R, Schliwka J, Fuchs U, Novosel A, Muller RU, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De VG, Frezzetti D, Trompeter HI, Hornung V, Teng G, Hartmann G, Palkovits M, Di LR, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129: 1401–1414, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MR, Kim JS, Kim KS. miR-124a is important for migratory cell fate transition during gastrulation of human embryonic stem cells. Stem Cells 28: 1550–1559, 2010. [DOI] [PubMed] [Google Scholar]
- 45.Liang YJ, Wang QY, Zhou CX, Yin QQ, He M, Yu XT, Cao DX, Chen GQ, He JR, Zhao Q. MiR-124 targets Slug to regulate epithelial-mesenchymal transition and metastasis of breast cancer. Carcinogenesis 34: 713–722, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 433: 769–773, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Lin T, Gu J, Zhang L, Davis JJ, Huang X, Cabbini G, Ji L, Fang B. Enhancing adenovirus-mediated gene transfer in vitro and in vivo by addition of protamine and hydrocortisone. J Gene Med 5: 868–875, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Lindenblatt C, Schulze-Osthoff K, Totzke G. IkappaBzeta expression is regulated by miR-124a. Cell Cycle 8: 2019–2023, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Liu K, Liu Y, Mo W, Qiu R, Wang X, Wu JY, He R. MiR-124 regulates early neurogenesis in the optic vesicle and forebrain, targeting NeuroD1. Nucleic Acids Res 39: 2869–2879, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6: e23461, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol 310: 442–453, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setien F, Casado S, Suarez-Gauthier A, Sanchez-Cespedes M, Git A, Spiteri I, Das PP, Caldas C, Miska E, Esteller M. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res 67: 1424–1429, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Maiorano NA, Mallamaci A. Promotion of embryonic cortico-cerebral neuronogenesis by miR-124. Neural Dev 4: 40, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maiorano NA, Mallamaci A. The pro-differentiating role of miR-124: indicating the road to become a neuron. RNA Biol 7: 528–533, 2010. [DOI] [PubMed] [Google Scholar]
- 55.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27: 435–448, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marcet B, Chevalier B, Luxardi G, Coraux C, Zaragosi LE, Cibois M, Robbe-Sermesant K, Jolly T, Cardinaud B, Moreilhon C, Giovannini-Chami L, Nawrocki-Raby B, Birembaut P, Waldmann R, Kodjabachian L, Barbry P. Control of vertebrate multiciliogenesis by miR-449 through direct repression of the Delta/Notch pathway. Nat Cell Biol 13: 693–699, 2011. [DOI] [PubMed] [Google Scholar]
- 57.McGowan SE, Doro MM, Jackson SK. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol Lung Cell Mol Physiol 273: L410–L416, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Meisterernst M, Gander I, Rogge L, Winnacker EL. A quantitative analysis of nuclear factor I/DNA interactions. Nucleic Acids Res 16: 4419–4435, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mucaj V, Lee SS, Skuli N, Giannoukos DN, Qiu B, Eisinger-Mathason TS, Nakazawa MS, Shay JE, Gopal PP, Venneti S, Lal P, Minn AJ, Simon MC, Mathew LK. MicroRNA-124 expression counteracts pro-survival stress responses in glioblastoma. Oncogene 34: 2204–2214, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mujahid S, Nielsen HC, Volpe MV. MiR-221 and miR-130a regulate lung airway and vascular development. PLoS One 8: e55911, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neo WH, Yap K, Lee SH, Looi LS, Khandelia P, Neo SX, Makeyev V, Su IH. MicroRNA miR-124 controls the choice between neuronal and astrocyte differentiation by fine-tuning Ezh2 expression. J Biol Chem 289: 20788–20801, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nho RS, Im J, Ho YY, Hergert P. MicroRNA-96 inhibits FoxO3a function in IPF fibroblasts on type I collagen matrix. Am J Physiol Lung Cell Mol Physiol 307: L632–L642, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol 8: 2, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okubo T, Hogan BL. Hyperactive Wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol 3: 11, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papagiannakopoulos T, Kosik KS. MicroRNA-124: micromanager of neurogenesis. Cell Stem Cell 4: 375–376, 2009. [DOI] [PubMed] [Google Scholar]
- 66.Perez-Casellas LA, Wang X, Howard KD, Rehage MW, Strong DD, Linkhart TA. Nuclear factor I transcription factors regulate IGF binding protein 5 gene transcription in human osteoblasts. Biochim Biophys Acta 1789: 78–87, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pierson J, Hostager B, Fan R, Vibhakar R. Regulation of cyclin dependent kinase 6 by microRNA 124 in medulloblastoma. J Neurooncol 90: 1–7, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU1 pathway. Nat Med 17: 64–70, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T, Kandel E. Characterization of small RNAs in aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63: 803–817, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rupp RA, Kruse U, Multhaup G, Gobel U, Beyreuther K, Sippel AE. Chicken NFI/TGGCA proteins are encoded by at least three independent genes: NFI-A, NFI-B and NFI-C with homologues in mammalian genomes. Nucleic Acids Res 18: 2607–2616, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanuki R, Onishi A, Koike C, Muramatsu R, Watanabe S, Muranishi Y, Irie S, Uneo S, Koyasu T, Matsui R, Cherasse Y, Urade Y, Watanabe D, Kondo M, Yamashita T, Furukawa T. miR-124a is required for hippocampal axogenesis and retinal cone survival through Lhx2 suppression. Nat Neurosci 14: 1125–1134, 2011. [DOI] [PubMed] [Google Scholar]
- 72.Seifter S, Dayton S. The estimation of glycogen with the reagent. Arch Biochem 25: 191–200, 1950. [PubMed] [Google Scholar]
- 73.Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 5: R13, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steele-Perkins G, Butz KG, Lyons GE, Zeichner-David M, Kim HJ, Cho MI, Gronostajski RM. Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol Cell Biol 23: 1075–1084, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steele-Perkins G, Plachez C, Butz KG, Yang G, Bachurski CJ, Kinsman SL, Litwack ED, Richards LJ, Gronostajski RM. The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol Cell Biol 25: 685–698, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takamizawa J, Konishi H, Yanagisawa K, Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y, Mitsudomi T, Takahashi T. Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res 64: 3753–3756, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Thannickal VJ. Mechanistic links between aging and lung fibrosis. Biogerontology 14: 609–615, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tian Y, Zhang Y, Hurd L, Hannenhalli S, Liu F, Lu MM, Morrisey EE. Regulation of lung endoderm progenitor cell behavior by miR302/367. Development 138: 1235–1245, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van de Wetering JK, Elfring RH, Oosterlaken-Dijksterhuis MA, Mol JA, Haagsman HP, Batenburg JJ. Perinatal expression of IGFBPs in rat lung and its hormonal regulation in fetal lung explants. Am J Physiol Lung Cell Mol Physiol 273: L1174–L1181, 1997. [DOI] [PubMed] [Google Scholar]
- 80.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132: 875–886, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Villanueva D, McCants D, Nielsen HC. Effects of epidermal growth factor (EGF) on the development of EGF-receptor (EGF-R) binding in fetal rabbit lung organ culture. Pediatr Pulmonol 29: 27–33, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev 21: 744–749, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang D, Zhang H, Li M, Frid MG, Flockton AR, McKeon BA, Yeager ME, Fini MA, Morrell NW, Pullamsetti SS, Velegala S, Seeger W, McKinsey TA, Sucharov CC, Stenmark KR. MicroRNA-124 controls the proliferative, migratory, and inflammatory phenotype of pulmonary vascular fibroblasts. Circ Res 114: 67–78, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang P, Chen L, Zhang J, Chen H, Fan J, Wang K, Luo J, Chen Z, Meng Z, Liu L. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene 33: 514–524, 2014. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Huang C, Reddy CN, Bhaskaran M, Weng T, Guo Y, Xiao X, Liu L. miR-375 regulates rat alveolar epithelial cell trans-differentiation by inhibiting Wnt/beta-catenin pathway. Nucleic Acids Res 41: 3833–3844, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Stricker HM, Gou D, Liu L. MicroRNA: past and present. Front Biosci 12: 2316–2329, 2007. [DOI] [PubMed] [Google Scholar]
- 87.Weng T, Gao L, Bhaskaran M, Guo Y, Gou D, Narayanaperumal J, Chintagari NR, Zhang K, Liu L. Pleiotrophin regulates lung epithelial cell proliferation and differentiation during fetal lung development via beta-catenin and Dlk1. J Biol Chem 284: 28021–28032, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weng T, Liu L. The role of pleiotrophin and beta-catenin in fetal lung development. Respir Res 11: 80, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 90: 11029–11033, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilting SM, van Boerdonk RA, Henken FE, Meijer CJ, Diosdado B, Meijer GA, le SC, Agami R, Snijders PJ, Steenbergen RD. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer 9: 167, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wong KY, So CC, Loong F, Chung LP, Lam WW, Liang R, Li GK, Jin DY, Chim CS. Epigenetic inactivation of the miR-124-1 in haematological malignancies. PLoS One 6: e19027, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu ZX, Rooney SA. Glucocorticoids increase fatty-acid synthase mRNA stability in fetal rat lung. Am J Physiol Lung Cell Mol Physiol 272: L860–L864, 1997. [DOI] [PubMed] [Google Scholar]
- 93.Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, Calin GA, Liu CG, Croce CM, Harris CC. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell 9: 189–198, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Yu JY, Chung KH, Deo M, Thompson RC, Turner DL. MicroRNA miR-124 regulates neurite outgrowth during neuronal differentiation. Exp Cell Res 314: 2618–2633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Wang H, Zhang S, Song J, Zhang Y, Wei X, Feng Z. MiR-134 functions as a regulator of cell proliferation, apoptosis, and migration involving lung septation. In Vitro Cell Dev Biol Anim 48: 131–136, 2012. [DOI] [PubMed] [Google Scholar]
- 96.Zheng F, Liao YJ, Cai MY, Liu YH, Liu TH, Chen SP, Bian XW, Guan XY, Lin MC, Zeng YX, Kung HF, Xie D. The putative tumour suppressor microRNA-124 modulates hepatocellular carcinoma cell aggressiveness by repressing ROCK2 and EZH2. Gut 61: 278–289, 2012. [DOI] [PubMed] [Google Scholar]