Abstract
Traditional motor learning studies focus on highly goal-oriented, volitional tasks that often do not readily generalize to real-world movements. The goal of this study was to investigate how different perturbation paradigms alter error-based learning outcomes in a highly automated task. Swallowing was perturbed with neck surface electrical stimulation that opposes hyo-laryngeal elevation in 25 healthy adults (30 swallows: 10 preperturbation, 10 perturbation, and 10 postperturbation). The four study conditions were gradual-masked, gradual-unmasked, abrupt-masked, and abrupt-unmasked. Gradual perturbations increasingly intensified overtime, while abrupt perturbations were sustained at the same high intensity. The masked conditions reduced cues about the presence/absence of the perturbation (pre- and postperturbation periods had low stimulation), but unmasked conditions did not (pre- and postperturbation periods had no stimulation). Only hyo-laryngeal range of motion measures had significant outcomes; no timing measure demonstrated learning. Systematic-error reduction occurred only during the abrupt-masked and abrupt-unmasked perturbations. Only the abrupt-masked perturbation caused aftereffects. In this highly automated task, gradual perturbations did not induce learning similarly to findings of some volitional, goal-oriented adaptation task studies. Furthermore, our subtle and brief adjustment of the stimulation paradigm (masked vs. unmasked) determined whether aftereffects were present. This suggests that, in the unmasked group, sensory predictions of a motor plan were quickly and efficiently modified to disengage error-based learning behaviors.
Keywords: adaptation, deglutition, learning
an important challenge in dysphagia research is understanding the processes by which rehabilitation is made optimal. Rehabilitation research in limb systems for tasks such as locomotion and reaching have applied the principles of motor adaptation (often referred to as error-based learning) to learn about improving function by exploiting the central nervous system (1, 33). Specifically, error-based learning is a form of motor learning that involves improving movements in a trial-by-trial fashion (through error reduction) in the presence of a perturbation or disturbance to a movement goal. Error-based learning suggests that a feed-forward mechanism enables planning of the next movement before the movement itself has begun (29). Thus error-based learning occurs when movements gradually compensate to overcome the effect of the perturbation and improve the movement outcome. Error-based learning has the potential to help us to understand how a normal system adapts to reduce the effects of a perturbation and whether a disordered system might have impaired capabilities to do the same. Impaired adaptation (or impaired error-based learning) could lead to the inability to effectively overcome perturbations caused by an underlying disease or impairment.
Motor adaptation paradigms have involved a reductionist approach by examining movement patterns of isolated physiologic components of reaching or visuomotor tasks rather than the complete function (i.e., isolated arm reaching without whole body movements) (29, 32). Although outcomes from these types of studies have offered substantial gains in knowledge of motor learning, their application to functional tasks, especially in a rehabilitation domain, does not translate directly. Unlike limb movements, oropharyngeal swallowing is a task that is difficult to reduce to subcomponents of the entire behavior. That is, within the context of a normal swallow, it is not possible to produce laryngeal function in isolation. Other co-occurring swallowing events, such as pharyngeal contraction, upper-esophageal sphincter opening, posterior lingual propulsion, and velar elevation are unavoidable. Therefore, we posit that studies of error-based learning in swallowing could provide significant insight to the field of motor learning as the function of the whole system must be studied together (12).
The process of swallowing is initiated by an ordered series of complex events. Oropharyngeal swallowing is unique in that it involves central nervous system control of co-occurring volitional (oral) and reflexive (laryngeal and pharyngeal) swallowing events. Swallowing is rapid and completed in ∼1 s in healthy individuals (18). Movements of the larynx and hyoid bone (hyo-larynx) contribute to laryngeal vestibule closure, which is critical for preventing ingested material from entering the trachea (5-7). Disordered timing or extent of airway protection can, therefore, lead to aspiration (ingested material enters the trachea) (28). Consequently, the many peripheral (e.g., hyo-laryngeal excursion) and central (e.g., brainstem, cerebellum, cortical activation) processes that must make precise calculations to allow for safe swallowing are significant. Unfortunately, because of this complexity, they are also poorly understood.
Studies of swallowing physiology are not prevalent in current motor learning research, yet recent studies suggest that the principles of motor learning have the potential to optimize dysphagia (swallowing impairment) rehabilitation (12). Humbert et al. (11) demonstrated that motor perturbations that oppose hyo-laryngeal elevation lead to error-based learning, characterized by gradually increasing hyo-laryngeal elevation to preperturbation levels (11). This suggests that the intact swallowing system has the capability to reduce swallowing errors; thus adaptation is a feasible model to investigate in swallowing research, with the potential of enhancing rehabilitation outcomes.
Research in motor adaptation has tested more than one form of a motor perturbation to determine whether error-based learning outcomes differ. For example, differential outcomes on error-based learning occur depending on whether a perturbation is introduced abruptly or gradually. In healthy young adults, Kargerer et al. (17a) showed that gradually increasing a perturbation (visual feedback rotation) leads to more “complete” adaptation than sudden (abrupt) perturbation, characterized by little disruption in performance over the range of the perturbation. Furthermore, gradual perturbations caused larger aftereffects, which these authors believed supported their hypothesis as aftereffects are an “indicator of the degree to which the internal model has been updated during adaptation.” These findings (17a) have spawned several additional studies comparing gradual and abrupt perturbations that examine retention, generalization, and outcomes in patients (2, 3, 19).
Thus far, varied forms of motor perturbation have been tested in a swallowing error-based paradigm in only one study to our knowledge (10). In this study, error-based learning of swallowing hyo-laryngeal movements were only evident in healthy adults who were continuously perturbed (during both swallowing and interswallowing intervals) but not in participants who were intermittently perturbed (during swallowing only, not interswallow intervals). However, no studies have examined whether gradual vs. abrupt perturbations can vary the error-based learning outcomes in swallowing. This area will offer relevant information for clinical management of dysphagia because swallowing movement goals are frequently disrupted in an abrupt manner following stroke and other acute events. Not only specific to patients, healthy adults also experience abrupt disruptions during a choking or aspiration event. Conversely, gradually worsening swallowing impairments occur in cases of neurodegeneration, muscular degeneration, age-related frailty, and tumor growth in the oropharynx.
In the current study, the primary aim was to compare the effects of gradual vs. abrupt swallowing perturbations on error-based learning during hyo-laryngeal range of motion. Our secondary aim was to investigate timing of hyo-laryngeal, bolus flow, and upper-esophageal function. We have built on previous findings (11) by restricting hyo-laryngeal elevation with surface electrical stimulation to the anterior neck by either gradually increasing the perturbation to a high intensity or by abruptly stimulating at the same high intensity. We hypothesized that, consistent with previous findings, abrupt perturbations would lead to progressively increased hyo-laryngeal elevation over several swallows and significant aftereffects (11). This hypothesis is based on our assumption that swallowing is highly responsive to errors that impact airway function, especially when the disruption is sudden. Conversely, we hypothesized that gradual perturbations would incrementally reduce peak hyo-laryngeal elevation. This is in contrast to outcomes in limb studies where incremental increases in a perturbation result in minimal disruptions to performance (17a). Unlike volitional arm tasks with explicit, precise movement goals, swallowing does not involve specific movement targets for hyo-laryngeal function (i.e., achieve 15 mm of hyoid elevation). In fact, the normal range of hyo-laryngeal swallowing movements has significant inter- and intraperson variability (24, 25, 27). Thus we hypothesized that incremental increases in a perturbation would gradually reduce hyo-laryngeal peak levels until an error signal is triggered (perhaps when peaks are out of normal range), to which participants will learn to resist. Based in the secondary aim previously described, we hypothesized motor adaptation would not be evident as shown in Humbert et al. (22). Our rationale is that when hyo-laryngeal range of motion is perturbed, healthy adults are able to prevent timing abnormalities to maintain safe swallowing.
The current study investigates the effects of masking the onset and offset of a perturbation on hyo-laryngeal error-based learning. In Humbert et al. (10), it was notable that when initiation and removal of the perturbation were unmasked (i.e., easily identified), aftereffects were not present. Aftereffects are exaggerated movements in the opposite direction of the perturbation effect, exceeding preperturbation levels (1). Thus we compared masked and unmasked onset and offset of both gradual and abrupt perturbations. We hypothesized that, consistent with Humbert et al. (10), masking the presence of the perturbation would lead to aftereffects because the stimulation is unexpectedly removed, leading the participant to execute a movement plan that accommodates the perturbation. On the other hand, we predicted that unmasking the presence of the perturbation would not lead to aftereffects due to the perceived sensory discrepancy between the two states and explicit cues of the perturbation removal. Examining masked and unmasked conditions in addition to abrupt and gradual perturbations provides further insight into the ideal characteristics of perturbation for enhancing motor adaptation.
Our conceptual framework is based on studies showing that motor adaptation involves recalibrating a motor plan to prevent errors before they occur (1, 8, 9, 16, 20). Thus sensory predictions are made before each movement attempt to shift the preferred outcome of a movement closer to the actual outcome (29, 30). We hypothesize that we can manipulate the sensory predictions made for hyo-laryngeal motor adaptation during swallowing with masking and with a gradual vs. abrupt perturbation paradigm.
METHODS AND ANALYSIS
Methods
Participants.
Twenty-five participants (12 female) ages 19–45 yr (mean 26 yr) were consented according to the study protocol approved by the Institutional Review Board at Johns Hopkins University. Participants were required to complete a health screening before participation and were self-reported healthy subjects with no history of neurological deficits, speech, or swallowing dysfunction. While participants were made aware that they would be swallowing with electrical stimulation, they were blinded to the specific purpose and methods during participation.
General procedures.
VIDEOFLUOROSCOPY.
Imaging was obtained through videofluorographic recording. Studies were recorded in the sagittal plane in full resolution, continuous, digital real time (30 frames/s). Imaging studies had a superimposed timestamp, giving each frame a unique identifier. Imaging parameters were set to include the oral cavity, the posterior pharyngeal wall, and immediately below the upper esophageal sphincter. All swallows were 5-ml thin liquid barium (Varibar) to standardize swallows throughout.
ELECTROMYOGRAPHY.
Submental surface electromyography was used to confirm the presence of electrical stimulation throughout the study by observing stimulation artifact. Bipolar ssEMG electrodes (Motion Lab Systems) were placed on the left and right sides of the submental muscle group, approximately halfway between the hyoid bone and the mentalis of the mandible. Submental surface EMG was recorded throughout each study at a rate of 1,000 Hz.
ELECTRICAL STIMULATION.
Surface electrical stimulation (Ministim; Life-Tech, Stafford, TX) placement parameters were identical to Humbert et al. (10, 11) with application on the skin of the anterior neck on either side of the thyroid laminae, overlying infrahyoid muscles bilaterally (omohyoid, sternohyoid, sternothyroid, and thyrohyoid). The Ministim has an output current range of 0 to 50 mA, stimulus pulse of 220 μm, square wave monophasic, and participants were stimulated at 100 Hz. Sensory-motor stimulation was set at a relatively high intensity to induce hyo-laryngeal descent at rest. Sensory-only stimulation was set at a relatively low intensity that induced the characteristic prickly or tingly sensation of the skin but without causing hyo-laryngeal movement at rest.
Study design.
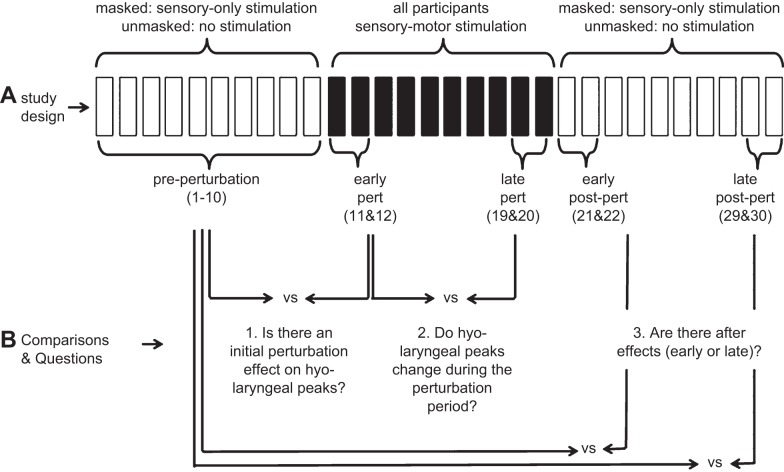
Participants were alternately assigned to one of two groups (Fig. 1A): the masked (n = 12) or the unmasked group (n = 13). Within each group, participants completed an abrupt and gradual perturbation study in random order, separated by 14 days. All participants swallowed 30 times throughout each study, with 10 swallows in each of 3 phases (preperturbation, perturbation, and postperturbation).
Fig. 1.
Study design (A) and comparisons and research questions (B) for the study. White bars: unperturbed swallows (masked: sensory-only stimulation; unmasked: no stimulation). Black bars: perturbed swallows (sensory-motor stimulation).
Each study began by establishing the sensory-motor stimulation levels that would perturb swallowing. It has been shown that surface electrical stimulation on the anterior neck leads to significant hyo-laryngeal descent at rest and reduced hyo-laryngeal peak elevation levels during swallowing (14, 21). Thus perturbation in our study was achieved by having the participant determine the maximum tolerated stimulation level that did not induce pain. Then, stimulation at this level was administered during videofluoroscopy during rest to observe whether hyo-laryngeal descent occurred. The maximum tolerated stimulation level that also caused hyo-laryngeal descent was deemed the maximum perturbation level. The minimum perturbation level was found by establishing the lowest stimulation level that caused hyo-laryngeal descent at rest during videofluoroscopy. In abrupt perturbation studies, the maximum perturbation level of stimulation was applied for all 10 swallows during the perturbation phase. In gradual perturbation studies, the stimulation was evenly distributed from minimum to maximum perturbation throughout the 10 swallows of the perturbation phase. Whenever stimulation was applied at any level, it was always continuously administered during both swallowing and interswallow intervals, i.e., perturbation was continuous throughout the perturbation phase.
UNMASKED GROUP PROCEDURES.
The unmasked group experienced sensory-motor stimulation during perturbation trial swallows (whether gradual or abrupt) and no stimulation during the preperturbation or postperturbation trials. The onset and offset of perturbation was perceivable by both a strong prickly cutaneous sensation of sensory-motor stimulation as well as resistance to hyo-laryngeal elevation.
MASKED GROUP PROCEDURES.
The masked group also experienced sensory-motor stimulation only during perturbation trial swallows (whether gradual or abrupt). They also received sensory-only stimulation during the preperturbation and postperturbation trials. Thus the onset and offset of perturbation was perceivable only by resistance to hyo-laryngeal elevation. Sensory-only stimulation is defined as low stimulation levels where the participant felt a prickly sensation but no hyo-laryngeal movement was observed at rest on videofluoroscopy.
Analysis
Hyoid and laryngeal peak range of motion (primary outcome variable).
Videofluoroscopic recordings were digitized using Peak Motus version 9 while blinded to the study group and to the presence of perturbation. Three points were digitized for each swallow, including the anterior-inferior point of the hyoid bone (during both superior and anterior peak excursion), the subglottal air column for laryngeal position (superior peak), and a cervical vertebra used as a reference point to compare to hyoid position and to account for whole body movement during swallowing. Peak excursion (elevation or anterior) is thus defined as the maximum hyoid or laryngeal axis value compared with the vertebra. Similar to previous studies [Humbert et al. (11)] peak hyo-laryngeal measures were adjusted for individual differences in range of hyo-laryngeal movement during swallowing to normalize differences due to variance across participants (26). Data were scaled ranging from 0 (lowest among 30 swallows) to 1 (highest among 30 swallows) individually for each participant.
Timing measures (secondary outcome variable).
We derived five timing measures from our data, including 1) swallowing delay time (time between the 1st frame that the bolus passes the ramus of the mandible and hyoid burst onset); 2) duration to maximum hyoid elevation (time between hyoid burst and first frame of hyoid at maximum superior position); 3) duration to upper esophageal sphincter opening (time between hyoid burst and first frame of upper esophageal sphincter opening); 4) duration of laryngeal vestibule closure (time between first frame of laryngeal vestibule closure and reopening); and 5) laryngeal vestibule closure delay time (time between hyoid burst and the first frame of laryngeal vestibule closure).
Comparisons.
Building on previous experiments showing adaptation of hyo-laryngeal movement during sensory motor stimulation, this study aimed to examine evidence of error-based learning described within the adaptation literature. We derived means for five periods within the study (Fig. 1B). Given that the 30 swallows within the study are evenly divided among three phases (swallows 1–10 = preperturbation; swallows 11–20 = perturbation; and swallows 21–30 = postperturbation), we derived the following five means: 1) preperturbation (all 10 swallows); 2) early perturbation (swallows 11 and 12); 3) late perturbation (swallows 19 and 20); 4) early postperturbation (swallows 21 and 22); and 5) late postperturbation (swallows 29 and 30). To answer questions about error-based learning we considered three comparisons (Fig. 1B).
1) Is there an initial perturbation effect on range of motion or timing measures? This is important because the onset of perturbation differs depending on whether it is gradually or abruptly introduced and whether the preperturbation period was masked or unmasked. This question was answered by comparing preperturbation means to early perturbation means. Our outcome measure for the initial perturbation effect is significantly reduced hyo-laryngeal peak elevation during the early phase of the perturbation period. No changes to timing are expected.
2) Do swallowing kinematics change during the perturbation period? It has been established that error-based learning occurs when the error is reduced (movements approach baseline levels during the perturbation period). We compared the early and late perturbation periods to answer this question. Our outcome measure for learning during the perturbation period is a significant increase in peak hyoid and laryngeal elevation during late perturbation compared with early perturbation. No changes to timing are expected.
3) Are there aftereffects (early or late)? Error-based learning is evident when aftereffects, an overcompensation that shows preparation for the perturbation, are observed upon unexpected perturbation removal. This question was answered by comparing the preperturbation means to the early post perturbation means for early aftereffects and to the late perturbation means for late aftereffects. Our outcome measure for learning during the postperturbation period is a significant increase in peak hyoid and laryngeal elevation during the postperturbation period compared with the preperturbation period. No changes in timing are expected.
Statistical analysis.
This study used a linear mixed models analysis (SPSS version 22). Subject was used as random effects to control for heterogeneity among individuals. There was a fixed effect of condition (masked abrupt, masked gradual, unmasked abrupt, and unmasked gradual), period (prepert, early pert, late pert, early postpert, and late postpert), and a covariate of trial and order of stimulation type (abrupt vs. gradual). When fixed effects were significant, pairwise comparisons were Sidak corrected for multiple comparisons. To test the reliability of measurement on 15% of the data (inter- and intrarater), intraclass correlation coefficients were computed. The intraclass correlation coefficient represents the proportion of total variation, including between-subject variability and measurement variability. Values near 1 suggest nearly all variability is essentially biological, whereas values near 0 indicate variability is primarily a result of measurement problems.
RESULTS
Stimulation Levels and Reliability
The masked group had an average sensory-only stimulation of 4 mA (range 2–5 mA; Fig. 2). The average minimum perturbation levels during gradual perturbation studies were 7 mA in masked (range 5–11 mA) and 7 mA in unmasked (range 5–8 mA). The average maximum perturbation levels during gradual and abrupt perturbation studies were 11 mA (range 8–15) in masked and unmasked. Intrarater and interrater reliability were excellent (≤0.98).
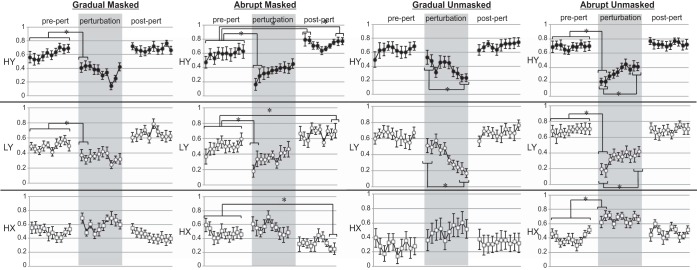
Fig. 2.
Hyoid peak elevation (HY), larynx peak elevation (LY), and hyoid peak anterior (HX) movements for each of the 4 conditions. Gray shaded areas indicate perturbation periods. Black circles: hyoid peak elevation; white circles: laryngeal peak elevation; white squares: hyoid peak anterior.
Fixed Effects
Range of motion.
Statistically significant fixed effects on peak extent of hyo-laryngeal movements were found for the following conditions across the five periods: 1) gradual-masked: hyoid superior (P < 0.001, F = 14), larynx superior (P < 0.001, F = 10.5), and hyoid anterior (P = 0.015, F = 3.17); 2) abrupt-masked: hyoid superior (P < 0.001, F = 20.1), larynx superior (P < 0.001, F = 9), and hyoid anterior (P < 0.001, F = 5.4); 3) gradual-unmasked: hyoid superior (P < 0.001, F = 13.1) and larynx superior (P < 0.001, F = 16.2); and 4) abrupt-unmasked: hyoid superior (P < 0.001, F = 35.1), larynx superior (P < 0.001, F = 35.5), and hyoid anterior (P < 0.001, F = 12.7).
Timing.
Statistically significant fixed effects were only found for the duration to maximum hyoid elevation measure for two conditions across the five periods: 1) abrupt-masked (P = 0.014, F = 3.66); and 2) abrupt-unmasked (P = 0.042, F = 2.78).
Pairwise Comparisons
Outcomes from pairwise comparisons provided answers to our three research questions and are discussed below (P values in Table 1).
Table 1.
Pairwise comparisons
| Masked |
Unmasked |
|||||||
|---|---|---|---|---|---|---|---|---|
| Gradual |
Abrupt |
Gradual |
Abrupt |
|||||
| P value | Cohen's d | P value | Cohen's d | P value | Cohen's d | P value | Cohen's d | |
| Question 1: Is there an initial perturbation effect? | ||||||||
| HY | P = 0.003 | 0.781* | P < 0.001 | 1.29* | P = 0.385 | 0.464 | P < 0.001 | 2.26* |
| HX | P = 0.708 | 0.382 | P = 0.703 | 0.338 | NA | 0.294 | P < 0.001 | 1.05* |
| LY | P = 0.026 | 0.670* | P = 0.002 | 0.871* | P = 0.283 | 0.511 | P < 0.001 | 2.24* |
| Question 2: Do hyo-laryngeal peaks change during the perturbation period? | ||||||||
| HY | P = 0.895 | 0.394 | P = 0.093 | 0.771 | P = 0.009 | 1.14* | P = 0.006 | 0.980* |
| HX | P = 1.000 | 0.156 | P = 0.872 | 0.348 | NA | 0.343 | P = 1.000 | 0.124 |
| LY | P = 1.000 | 0.088 | P = 0.192 | 0.653 | P = 0.001 | 1.27* | P = 0.009 | 0.885* |
| Question 3A: Are there aftereffects (early)? | ||||||||
| HY | P = 0.663 | 0.378 | P < 0.001 | 0.836* | P = 1.000 | 0.082 | P = 0.928 | 0.260 |
| HX | P = 1.000 | 0.076 | P = 0.212 | 0.546 | NA | 0.124 | P = 0.166 | 0.484 |
| LY | P = 0.101 | 0.67 | P = 0.194 | 0.533 | P = 1.000 | 0.000 | P = 1.000 | 0.017 |
| Question 3B: Are there aftereffects (late)? | ||||||||
| HY | P = 0.375 | 0.460 | P = 0.018 | 0.855* | P = 0.542 | 0.443 | P = 1.000 | 0.108 |
| HX | P = 0.490 | 0.421 | P = 0.005 | 0.025* | NA | 0.13 | P = 0.728 | 0.399 |
| LY | P = 0.092 | 0.813 | P = 0.024 | 0.741* | P = 0.747 | 0.356 | P = 1.000 | 0.094 |
| df = 211 | df = 192 | df = 173 | df = 223 | |||||
Statistical significance (P values) and effect size (Cohen's d) for each research question and outcome measure across study group (HY, hyoid vertical movement; HX, hyoid horizontal movement; LY, laryngeal vertical movement) are shown. NA, not applicable.
1) Is there an initial perturbation effect on hyo-laryngeal peaks (range of motion)? Yes.
Initial perturbation effects were significant for the gradual-masked, abrupt-masked, and abrupt-unmasked conditions. In the gradual-masked and abrupt-masked conditions, the perturbation onset significantly reduced hyoid superior and larynx superior peaks, but no effects on hyoid anterior peaks were found. In the abrupt-unmasked condition, the onset of the perturbation significantly reduced hyoid superior and larynx superior peaks as well as hyoid anterior peaks. No initial perturbation effects were found in the gradual-unmasked condition.
Is there an initial perturbation effect on duration to maximum hyoid elevation? Yes.
In the abrupt-masked group, the hyoid bone reached its maximum elevation position significantly faster during the preperturbation swallows (112 ms ± 8.8 SE) compared with the early perturbation swallows (185 ms ± 20.1 SE). No pairwise comparisons were significant for the abrupt-unmasked group.
2) Does the magnitude of hyo-laryngeal excursion change during the perturbation period? Yes.
In gradual-unmasked trials, the late perturbation period had significantly lower peaks than the early perturbation period for both hyoid and laryngeal superior movement. The abrupt-unmasked condition had the opposite effect, where the early perturbation period had significantly lower peaks than the late perturbation period for both hyoid and laryngeal superior movement. No other significant differences were found.
Is there an initial perturbation effect on duration to maximum hyoid elevation? No pairwise comparisons were significant for this measure.
3) Are there aftereffects (early or late)? Yes.
Only the abrupt-masked group had aftereffects. Early aftereffects were significant only for hyoid superior peaks, where early postperturbation peaks were significantly higher than preperturbation peaks. During the late postperturbation period, both hyoid and laryngeal superior peaks were significantly more superior compared with preperturbation peaks. Hyoid anterior peaks were significantly more anterior during late postperturbation than preperturbation. Also, for this question about the presence of aftereffects, there was a significant order effect in the abrupt-masked group and in the abrupt-unmasked group. In both groups, hyoid anterior peaks (HX) were more anterior in the late perturbation phase when the abrupt condition occurred on the second visit compared with when it occurred on the first visit. This suggests that when the abrupt condition was experienced in visit 1, followed by the gradual condition in visit 2, hyoid anterior peaks remained more anterior for a longer period of time after the perturbation was removed.
Are there aftereffects (early or late) for duration to maximum hyoid elevation? No.
No pairwise comparisons were significant for this measure.
DISCUSSION
Swallowing is a highly complex process that engages the central nervous system involving the cerebral cortex, cerebellum, and central pattern generators of the brainstem (4, 15, 17). It is also believed to be highly dependent on sensory input to plan motor function (31). Given the importance of sensory predictions for feed-forward processing, it is possible that manipulating sensory stimuli can impact error-based learning (1). We probed this possibility in swallowing through varied perturbation paradigms. We have shown that manipulating the perturbation paradigm can modify the outcome of error-based learning on hyo-laryngeal movements during swallowing. Our data indicate that awareness of the onset and offset of the perturbation (masking) as well as the structure of the perturbation (abrupt vs. gradual) both impact learning outcomes. These findings could potentially have direct clinical relevance on swallowing rehabilitation programs that challenge movements and aim to generalize improvements to contexts without a perturbation. Our outcomes also prompt further inquiry into the underlying central nervous system mechanisms that might have been modulated to alter feed-forward processing in our particular paradigm, besides sensory input (masking).
Timing Outcomes
There was no evidence of learning in any of the timing measures that were considered in this study. Only duration to maximum peak elevation was longer when the perturbation began in the abrupt-masked group. This longer ramp time to the peak elevation position likely occurred in the abrupt-masked group because the stimulation onset was masked and because the stimulation intensity was suddenly high. Thus participants required more time to elevate the hyoid bone when surface electrical stimulation caused resistance to this movement. As expected, there was no other change in any other timing measurement. Motor adaptation of a swallowing timing measure (duration of laryngeal vestibule closure) has only previously been shown by Humbert et al. (10), when the perturbation (also neck surface electrical stimulation) was administered intermittently (only during the perturbation, not during the 10-s interswallow intervals) (10). The current study included a continuous perturbation paradigm (perturbation during both swallowing and the interswallow interval). Timing changes may not have occurred with the continuous perturbation paradigm because healthy adults were able to keep timing variables stable in the presence of changing range of motion variables. This is both interesting and important because timing of swallowing events are critical for adequate and timely protection of the airway, which is the primary aim of swallowing function. This also suggests that timing adjustments are not strictly subject to adjustments in hyo-laryngeal range of motion in a normal swallowing system that has been perturbed.
Initial Perturbation Effect
All conditions, except the gradual-unmasked condition, had initial perturbation effects. A significant perturbation effect on hyo-laryngeal elevation has been shown with abrupt perturbation paradigms, whether the perturbation onset was masked or not (10, 11). Therefore, it was expected that abrupt perturbation would lead to similar outcomes in the current study. This is the first error-based learning study differentiating gradual and abrupt perturbations on hyo-laryngeal range of motion. Our data suggest that even minimum perturbation intensities can significantly lower the magnitude of hyo-laryngeal excursion, but only when the perturbation onset is masked. The distinction in onset effects between masked and unmasked gradual perturbation may have occurred because the gradual-unmasked group had greater awareness about perturbation onset (sensory = prickly; motor = resistance), therefore responding by immediately resisting the effects of the perturbation. The gradual-masked group may have had less cues to resist during the early period because subtle changes in perturbation onset may not have been recognized.
Overcoming the Effect of the Perturbation
Differential early and late perturbation effects were only evident for hyo-laryngeal magnitude during the unmasked perturbation paradigms. This means that when participants were provided simultaneous sensory and motor cues about the perturbation onset, the effects of early and late perturbation significantly differed. The perturbation effect with gradual-unmasked stimulation led to significantly lower magnitude of hyo-laryngeal excursion overtime, while the abrupt-unmasked stimulation led to significantly higher magnitude over time.
Gradual perturbation.
In both gradual perturbation paradigms, learning, characterized by incrementally overcoming the effects of the perturbation, is not evident. This may have occurred because no error to swallowing function was perceived with gradual perturbation, consequently inducing no systematic error-reduction response. The significant decreases in hyoid and laryngeal superior peaks in the gradual-unmasked group during the perturbation period likely only occurred as a response to the effect of the gradually increasing stimulation intensity. In the gradual-masked group, it is notable that resistance to the perturbation was eventually induced, but it occurred during the last two perturbation trials (9th and 10th swallows) after the peaks had reached their lowest level (comparable to the initial perturbation effects in the abrupt paradigms). By the 10th perturbed swallow in the gradual-masked condition, the participants were able to reestablish hyo-laryngeal peaks at similar levels to the early perturbation effects. This may suggest there could be a threshold for triggering hyo-laryngeal range of motion error-based learning when anterior neck surface electrical stimulation is used as a perturbation.
Abrupt perturbation.
As expected, the abrupt-unmasked condition appears to have induced error-based learning during the period of the perturbation; however, the abrupt-masked paradigm did not. This is in contrast to Humbert et al. (11), which found that a masked abrupt paradigm leads to gradually overcoming the effect of the perturbation. Two important differences might be responsible for the opposing outcomes. First, the perturbation period in Humbert et al. (11) was twice a long, possibly allowing more time for hyo-laryngeal magnitude to approximate baseline levels with less variability among participants. Second, in the current study, we chose to compare the first two perturbed swallows to the last two perturbed swallows. The abrupt-masked condition appears to have a faster rate of error-reduction than the abrupt-unmasked condition shown in the first two swallows (abrupt-masked: 0.16 then 0.28; abrupt-unmasked: 0.20 then 0.20).
Presence of Aftereffects
Aftereffects were only observed in the abrupt-masked paradigm. This has also been shown in Humbert et al. (10) where abrupt paradigms without masking may have impacted feed-forward processing by cueing the system about the absence of the perturbation leading to immediate changes in error-reduction behaviors (10). Immediate aftereffects were only evident in hyoid superior magnitude, but delayed aftereffects occurred in all measures (hyoid and laryngeal superior magnitude and hyoid anterior magnitude). Unlike the current study, Humbert et al. (11) reported immediate aftereffects in laryngeal superior magnitude, likely because the perturbation period was longer (18 perturbation swallows) than the current study (only 10 perturbation swallows). The absence of immediate laryngeal aftereffects may have also occurred because the perturbation may have opposed hyoid elevation more than laryngeal elevation, possibly because there are more hyoid depressors (thyrohyoids, sternohyoids, and omohyoids) than laryngeal depressors (sternothyroids) in the anterior neck. Thus laryngeal peak elevation aftereffects may only be induced in response to a greater perturbation effect (number and intensity of trials) than was applied in the current study. Aftereffects for hyoid movement in the anterior direction have not previously been examined. We suspect that no immediate aftereffects were found in hyoid anterior movement because the horizontal trajectory was not directly perturbed. In other words, the perturbation caused significant inferior hyoid movement rather than posterior movement at rest. This likely occurred because we did not target the muscles that move the hyoid posteriorly (posterior belly of the digastrics, stylohyoid) with our electrode placement. During the postperturbation period, it is notable that none of the measures returned to baseline levels in the abrupt-masked condition after 10 trials, possibly because deadaptation of hyo-laryngeal range of motion requires many more trials to occur. This offers potentially good possibilities for longer term outcomes in rehabilitation programs where aftereffects are considered to be beneficial.
Surface Electrical Stimulation as an Experimental Perturbation
Unlike most motor adaptation studies of the limb that mechanically perturb movement trajectories, our perturbation involves an unavoidable and salient sensory experience that could be exciting critical brainstem and cortical regions involved in processing swallowing behavior. Indeed, we have previously reported cortical activity and connectivity in the primary motor cortex and insula when swallowing with concurrent surface electrical stimulation to the submental region (13). It is unknown whether a mechanical hyo-laryngeal perturbation might induce the same adaptation outcomes as surface electrical stimulation. Still, given the widespread use of surface electrical stimulation to treat dysphagia, the outcomes of this study could have relevant clinical implications. Future studies could bypass the possible effects of the sensory stimulation by using intramuscular (percutaneous) electrical stimulation to the hyoid and laryngeal depressors to examine motor adaptation.
Limitations
There are several potential limitations in this study that are important to note. Firstly, in this study we did not analyze rates of retention, despite the importance of retention in motor learning and adaptation research. This study focused primarily on the effects of error size on adaptation rates through varied perturbation force fields. Secondly, maximum electrical stimulation thresholds were based on self-report, providing the possibility that this introduced some degree of variability. Additionally, although there are theoretical explanations to consider, the possibility of differential effects of masking (low level surface electrical stimulation) on hyo-laryngeal function is not clear. Another potential limitation of this study is that it did not consider the effect of the perturbation on swallowing fatigue, a possibility that should be further investigated. Finally, it cannot be assumed, based on these data, that the results will generalize to patients or all etiologies of swallowing dysfunction (e.g., gradual age related changes vs. abrupt changes following sudden neurologic insult). Regardless, to increase understanding of dysfunctional swallowing behavior and ways to correct impaired swallowing, it is crucial to understanding the same concepts in healthy people.
Conclusions
Adaptation research arguably can provide valuable insight into the mechanisms of swallowing as it has in other realms of rehabilitation research. Despite the consideration that research has yet to comprehensively define variation in adaptation, it has nonetheless been demonstrated that adaptation and skill learning are possibilities suggesting potential for the central nervous system and brain to mediate learning and improving function for patients (10, 11, 23). Adaptation research in swallowing is not extensive, but other areas of research may yield some insight into application of the principles of motor learning to swallowing research. An important question to ask is whether reflexive swallowing can be reorganized. That is, can a highly automated process such as swallowing be trained to reduce errors quickly and efficiently? We believe that studies of adaptation are an important step in that direction.
GRANTS
This work was supported by Office of Extramural Research, National Institute of Deafness and Other Communications Disorders Grant 1R01-DC-01428501A1 (to I. A. Humbert) and American Heart Association Grant 14BGIA20380348 (to I. A. Humbert).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.A., P.M., and I.A.H. performed experiments; C.A., P.M., I.T.-K., S.S., and I.A.H. analyzed data; C.A., P.M., S.S., A.V., and I.A.H. interpreted results of experiments; C.A., S.S., and I.A.H. drafted manuscript; C.A., P.M., I.T.-K., S.S., A.V., and I.A.H. edited and revised manuscript; C.A., P.M., I.T.-K., S.S., A.V., and I.A.H. approved final version of manuscript; I.A.H. conception and design of research; I.A.H. prepared Figs.
REFERENCES
- 1.Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21: 628–633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol 103: 2275–2284, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol 114: 2226–2244, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Fink BR. Folding mechanism of the human larynx. Acta Otolaryngol 78: 124–128, 1974. [DOI] [PubMed] [Google Scholar]
- 6.Fink BR, Demarest RJ. Laryngeal Biomechanics. New York: Raven, 1978. [Google Scholar]
- 7.Fink BR, Martin RW, Rohrmann CA. Biomechanics of the human epiglottis. Acta Otolaryngol 87: 554–559, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29: 9115–9122, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galea JM, Vazquez A, Pasricha N, de Xivry JJ, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: 1761–1770, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Humbert IA, Christopherson H, Lokhande A. Surface electrical stimulation perturbation context determines the presence of error-reduction in swallowing hyo-laryngeal kinematics. Am J Speech Lang Pathol 24: 72–80, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbert IA, Christopherson H, Lokhande A, German R, Gonzalez-Fernandez M, Celnik P. Human hyolaryngeal movements show adaptive motor learning during swallowing. Dysphagia 28: 139–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Humbert IA, German RZ. New directions for understanding neural control in swallowing: the potential and promise of motor learning. Dysphagia 20: 1–10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Humbert IA, Joel S. Tactile, gustatory, and visual biofeedback stimuli modulate neural substrates of deglutition. Neuroimage 59: 1485–1490, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert IA, Poletto CJ, Saxon KG, Kearney PR, Crujido L, Wright-Harp W, Payne J, Jeffries N, Sonies BC, Ludlow CL. The effect of surface electrical stimulation on hyolaryngeal movement in normal individuals at rest and during swallowing. J Appl Physiol 101: 1657–1663, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert IA, Robbins J. Normal swallowing and functional magnetic resonance imaging: a systematic review. Dysphagia 22: 266–275, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayaram G, Galea JM, Bastian AJ, Celnik P. Human locomotor adaptive learning is proportional to depression of cerebellar excitability. Cereb Cortex 21: 1901–1909, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jean A. Brainstem organization of the swallowing network. Brain Behav Evol 25: 109–116, 1984. [DOI] [PubMed] [Google Scholar]
- 17a.Kargerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Kendall KA, McKenzie S, Leonard RJ, Goncalves MI, Walker A. Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia 15: 74–83, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Klassen J, Tong C, Flanagan JR. Learning and recall of incremental kinematic and dynamic sensorimotor transformations. Exp Brain Res 164: 250–259, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Krakauer JW, Mazzoni P. Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21: 636–644, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Ludlow CL, Humbert I, Saxon K, Poletto C, Sonies B, Crujido L. Effects of surface electrical stimulation both at rest and during swallowing in chronic pharyngeal dysphagia. Dysphagia 22: 1–10, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macrae P, Anderson C, Humbert I. Mechanisms of airway protection during chin-down swallowing. J Speech Lang Hear Res 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macrae P, Humbert IA. Exploiting experience-dependent plasticity in dysphagia rehabilitation: current evidence and future directions. Curr Phys Med Rehabil Rep 1: 231–241, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molfenter SM, Steele CM. Physiological variability in the deglutition literature: hyoid and laryngeal kinematics. Dysphagia 26: 67–74, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molfenter SM, Steele CM. Temporal variability in the deglutition literature. Dysphagia 27: 162–177, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molfenter SM, Steele CM. Use of an anatomical scalar to control for sex-based size differences in measures of hyoid excursion during swallowing. J Speech Lang Hear Res 57: 768–778, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molfenter SM, Steele CM. Variation in temporal measures of swallowing: sex and volume effects. Dysphagia 28: 226–233, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Park T, Kim Y, Ko DH, McCullough G. Initiation and duration of laryngeal closure during the pharyngeal swallow in poststroke patients. Dysphagia 25: 177–182, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33: 89–108, 2010. [DOI] [PubMed] [Google Scholar]
- 31.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25: 323–333, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu-Wilson M, Tian J, Shadmehr R, Zee DS. TMS perturbs saccade trajectories and unmasks an internal feedback controller for saccades. J Neurosci 31: 11537–11546, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeiler SR, Krakauer JW. The interaction between training and plasticity in the poststroke brain. Curr Opin Neurol 26: 609–616, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]