Abstract
Polynucleotide phosphorylase (PNPase) is reported to regulate virulence in Salmonella, Yersinia sp. and Campylobacter jejuni, yet its role in Escherichia coli O157:H7 has not been investigated. To gain insights into its roles in E. coli O157:H7 virulence, pnp deletion mutants were generated and the major virulence factors were compared to their parental wild type strains. Deletion of pnp in E. coli O157:H7 dramatically decreased stx2 mRNA expression and Stx2 protein production, and impaired lambdoid prophage activation in E. coli O157:H7. Quantitative PCR further confirmed that the Stx2 phage lytic growth was repressed by pnp deletion. Consistent with reduced Stx2 production and Stx2 phage activation, the transcriptional levels of genes involved in phage lysis and replication were down-regulated. In addition, disruption of pnp in E. coli O157:H7 decreased its adhesion to intestinal epithelial cells as well as cattle colonic explant tissues. On the other hand, PNPase inactivation in E. coli O157:H7 enhanced Tir protein content and the transcription of type three secretion system components, including genes encoding intimin, Tir, and EspB as well as locus of enterocyte and effacement positive regulator, Ler. Collectively, data indicate that PNPase has pleiotropic effects on the virulence of E. coli O157:H7.
Keywords: E. coli O157:H7, PNPase, Shiga toxin 2, prophage, Type three secretion system, intestine, epithelium, adhesion
Introduction
Shiga toxin (Stx) producing Escherichia coli O157:H7 is a major food safety threat that results in significant economic losses, especially in the beef industry. Stx is the major virulence factor in E. coli O157:H7, which causes bloody diarrhea and life threatening hemolytic-uremic syndrome (Mainil and Daube, 2005). The mortality associated with E. coli O157:H7 infection is due to the production and release of Stx, which is composed of a single 32-kDa A subunit and five 7.7-kDa B subunits (Paton and Paton, 1998). Stx binds to receptors on cell surface and is internalized through endocytosis, which inhibits protein synthesis and causes complications associated with E. coli O157:H7 infection (Johannes and Romer, 2010). In addition, E. coli O157:H7 has a chromosomal pathogenicity island, i.e., locus of enterocyte and effacement (LEE; McDaniel et al., 1995), which mediates intimate contact to epithelial cells, further leading to attaching and effacing (AE) lesions (McDaniel et al., 1995). The LEE is composed of five major operons (LEE1-5) that encode type three secretion system (T3SS) apparatus and effector proteins (Deng et al., 2004).
There is a plethora of studies on the regulation of LEE in E. coli O157:H7, which is regulated by multiple proteins such as Ler, H-NS, GrlA, CsrA through direct or indirect interaction (Bhatt et al., 2011). Compared with LEE, the regulation of Stx 2 production in E. coli O157:H7 has only been sparsely studied. Gene stx2 is localized on Stx2 prophage, a lambdoid like bacteriophage, DNA that inserted to E. coli O157:H7 genome (Herold et al., 2004; Allison, 2007; Los et al., 2011). Stx production is generally linked to the expression of induced Stx-phage’s late genes, which results in phage lytic cycle induction and release of Stx (Wagner et al., 2001). The life cycle of prophages is controlled by phage repressor CI proteins in lambdoid phage (Ptashne and Hopkins, 1968). Mutations in the cI gene make a non-cleavable repressor and an uninducible 933W Stx2 prophage (Tyler et al., 2013). RecA can stimulate the self-cleavage of CI further allowing initiation of transcription from the early PL and PR promoters (Little, 2005). The transcription from PL results in expression of N protein, which modified RNA polymerase initiating at PL and PR to form resistant to downstream terminators (Friedman Di, 2006). The transcription from PR results in Q expression, which initiated the transcription from PR′ and the downstream genes expression including stx A and B (Karch et al., 1999). SOS response due to DNA damage and replication arrest can enhance recA expression and further stimulate Stx production (Kimmitt et al., 2000). There is also a RecA independent lambdoid prophage activation pathway for Stx production in E. coli O157:H7 (Imamovic and Muniesa, 2012), which is through regulation of RcsA (Rozanov et al., 1998). However, the mechanism involved in RecA-independent Stx2 933W prophage induction remains unknown (Imamovic and Muniesa, 2012). Stx prophage is also regulated by poly (A) polymerase I (PAP I), and PAP I-deficient cells showed a significant impairment of lysogenization and lytic development by Stx phages (Nowicki et al., 2015).
Polynucleotide phosphorylase (PNPase) has both 3′-5′exoribonuclease activity and 3′ terminal oligonucleotide polymerase activity, which is involved in mRNA degradation and small RNA turnover (Carpousis et al., 1999; Arraiano et al., 2010) and also serves as PAP II (Kushner, 2015). PNPase regulates T3SS expression in Yersinia sp. and Salmonella Typhimurium (Ygberg et al., 2006; Rosenzweig et al., 2007); acts as a global regulator of virulence genes in Salmonella, which repressed invasion and intracellular replication of Salmonella (Clements et al., 2002), but was required for S. Typhimurium gut colonization in swine (Bearson et al., 2013). It enhanced Campylobacter jejuni motility and its chicken gut colonization (Haddad et al., 2012). However, roles of PNPase in the colonization and virulence of E. coli O157:H7 had not been explored, which were examined in the current study.
Materials and Methods
Cell Line, Media, Bacterial Strains, and Plasmids
The human colonic epithelial cell line HT-29 was obtained from the American Type Culture Collection (ATCC® HTB-38TM, Manassas, VA, USA). HT-29 cells were routinely cultured in Dulbecco’s Modified Eagle’s medium (DMEM; Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma), 100 units/ml penicillin G, and 100 μg/ml of streptomycin (Sigma).
The E. coli O157:H7 EDL933, Sakai, and 86-24 strains were obtained from the STEC center at Michigan State University. E. coli O157:H7 was routinely grown in LB broth at 37°C with aeration. E. coli O157:H7 pnp deletion mutant was generated per the published method (Datsenko and Wanner, 2000). Briefly, using the forward primer GGCTTTACCCACATAGAGCTGGGTTAGGGTTGTCATTAGTCGCGAGGATGattccggggatccgtcgacc, and the reverse primer: CCGCCGCAGCGGAYGGCAAATGGCAACCTTACTCGCCCTGTTCAGCAGC tgtaggctggagctgcttcg, Kanamycin resistance cassette (lowercase letter) with pnp homology flanking sequence (uppercase letters) was PCR-amplified from pKD13 and electroporated into E. coli O157:H7 containing the -Red recombinase plasmid. The deletion of pnp was confirmed by PCR amplification using primers external to the disrupted gene (forward primer: TGTCATTAGTCGCGAGGATG; and reverse primer: GCGGAYGGCAAATGGCAACC). To construct the pnp complementary plasmid, the pnp gene was PCR-amplified from E. coli O157:H7 genomic DNA using primers flanking with KpnI and XbaI restriction enzyme sites, respectively (underlined letters): ATGGTACCACGCAAACGACACCGGTTCT and AGTCTAGATTACTCGCCCTGTTCAGCAG, and cloned into pBBR1-MCS3 (Kovach et al., 1995), which was kindly provided by Dr. Mark Gomelsky. The resulting plasmid pBBR1MCS3::pnp and its control vector were electroporated into E. coli O157:H7 EDL933 pnp mutant strains, which resulted in six strains: (1) EDL933; (2) EDL933 Δpnp; (3) EDL933 Δpnp pBBR1; (4) EDL933Δpnp pBBR1::pnp; (5) 86-24 Δpnp; (6) Sakai Δpnp. Given that pnp and its downstream genes, yhbM or nlpI (Lipoprotein NlpI), deaD (ATP-dependent RNA helicase) and mtr (tryptophan permease) are located in one operon, to confirm that pnp deletion did not impact transcription of its downstream genes, we further compared the transcription of yhbM, the nearest downstream gene of pnp, between pnp deletion mutant and wild-type strain using forward primer: CAGTTTGAACAGTGCCGTGG and reverse primer: GATAACACCTCGCTCGCTGA.
Immunoblotting
Supernatant components: overnight E. coli O157:H7 cultures were subcultured at 1:1,000 in LB broth for 14 h at 37°C under shaking at 200 rpm, when bacteria were in stationary phase. Bacterial cultures were centrifuged at 5,000 × g at room temperature for 5 min, and the supernatant was then filtered through 0.22 μm filter (Millipore, Bedford, MA, USA). Trichloroacetic acid was added to a final concentration of 10% to the supernatant and incubated overnight at 4°C to precipitate proteins.
Whole bacteria: overnight E. coli O157:H7 cultures were sub-cultured at 1:1,000 in LB broth for 14 h at 37°C under shaking at 200 rpm, when bacterial culture were used for protein extraction.
Protein extracts were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes and assayed with antibodies specific to Stx2A (Toxin Technology Inc., Sarasota, FL, USA) and Tir (a generous gift from Dr. John Leong, Tufts University). Blotted membranes were visualized using ECLTM Western blotting detection reagents (Amersham Bioscience, Piscataway, NJ, USA).
Phage Spontaneous Induction and Enumeration
Phage enumeration assay was conducted according to our published method (Harris et al., 2012; Yue et al., 2012). Briefly, E. coli O157 cells were activated from frozen glycerol stock and grown in LB broth at 37°C with aeration for 8 h, which were sub-cultured at 1:1,000 in LB broth for 14 h at 37°C under shaking at 200 rpm when cultures were used to assess phage spontaneous induction. Activated bacterial culture was centrifuged at 5,000 × g at 4°C for 5 min. The resulting supernatant was serially diluted in a phage solution containing 10 mM CaCl2 (Sigma) and 5 mM MgSO4 (Sigma). Proper phage titration (200 μl) was mixed with 0.9 ml of the sensitive E. coli strain MG1655 culture and 5 ml of tempered top LB agar (0.7%, W/V) supplemented with 10 mM CaCl2 and 10 mM MgSO4. They were then mixed and poured immediately on top of the bottom LB agar plates (1.5%, W/V) containing 3 μg/ml chloramphenicol (Sigma). Plates were incubated at 37°C and plaque forming units (Pfu) were counted after 36 h incubation. Measurements for each strain had four independent replicates.
Quantitative PCR (qPCR) Analysis of Stx2 Prophage
Shiga toxin2 prophage qPCR was conducted per published methods (Harris et al., 2012; Yue et al., 2012). Briefly, overnight bacterial cultures were centrifuged at 5,000 × g at 4°C for 5 min. The supernatant was then filtered through 0.22 μm filter (Millipore, Bedford, MA, USA) to ensure complete removal of bacteria. The filtrate was centrifuged at 35,000 × g at 4°C for 2 h. The resulting phage pellet was dissolved in sterile ddH2O and treated with DNase I (2 units/μl) at 37°C for 2 h to hydrolyze any remaining contaminated E. coli O157:H7 genomic DNA. The above phage preparation was boiled at 100°C for 10 min to denature DNase I and release phage DNA, and then used as a template for qPCR. PCR analysis of stx2 gene was conducted using stx2A primers and SYBR Green master mix (Bio-Rad, Hercules, CA, USA). Absence of bacterial DNA in phage lysates was confirmed by the absence of tufA DNA as assayed by PCR. Stx2 phages are known to carry only one copy of the stx2 gene, therefore the stx2 gene copy number can be extrapolated to quantify Stx2 prophages.
Adhesion of E. coli O157:H7 to Colonic Epithelial Cells
HT-29 epithelial cells were seeded in 24 well plates and cultured in DMEM media (Sigma) with 10 % FBS (Sigma) until ~90 % confluence. The medium was removed and each well was washed three times with PBS (pH 7.4). The cells in each well were challenged with 107 CFU/well E. coli O157:H7. The HT-29 cells and E. coli O157:H7 were co-cultured at 37°C with 5% CO2 for 2 h. Then the HT-29 cell monolayers were washed three times with PBS, and lysed with 0.2% Triton X-100. Lysates were serially diluted, plated on LB, and bacterial colonies were counted after 18 h incubation (Xicohtencatl-Cortes et al., 2007; Torres et al., 2008).
E. coli O157:H7 Adhesion to Cattle Colonic Explants
Terminal colons (5–10 cm from anus) were aseptically collected from E. coli O157:H7 free beef cattle slaughtered in the University of Wyoming Meat Laboratory and transferred to the Microbiology Lab within 10 min. Specimens were washed five times with 0.9% (W/V) NaCl and the mucosa was dissected from the underlying tissues using sterile dissecting scissors. The trimmed specimens were reduced to 8 mm round pieces using Miltex disposable biopsy punches (Cardinal Health, Cleveland, OH, USA), and transferred to the individual well of 24 well plates and cultured in DMEM medium with 5% FBS for 1 h. E. coli O157:H7 was added to these wells at 107 CFU/well, and cultured at 37°C for 2 h (Xicohtencatl-Cortes et al., 2007). The attached E. coli O157:H7 cells were serially diluted, plated and enumerated.
Quantitative Reverse Transcription PCR (qRT-PCR) Analyses
Total RNA was extracted from E. coli O157:H7 grown in LB broth using RNeasy Protect Bacteria Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcribed using a QuantiTect Reverse Transcription Kit (Qiagen). cDNAs were used as a template for qRT-PCR analysis of selected genes using a CFX96TM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). SYBR Green Master Mix (Bio-Rad, Hercules, CA, USA) was used for all qRT-PCR reactions. Primers for qRT-PCR are listed in Tables 1 and 2. Gene gapA was used as the housekeeping gene. Amplification efficiency was 0.90–0.99.
Table 1.
Primer sets used for quantitative Reverse Transcription PCR (qRT-PCR) for Shiga toxin (Stx) and phage related genes.
| Gene name | Product size | Direction | Sequence |
|---|---|---|---|
| cI | 80 bp | Forward | TCACACGAAGACCAAAGGCA |
| Reverse | TGCCATCGAGATGACCGAAG | ||
| cII | 118 bp | Forward | ACCTGTCAACGCTTACCCAG |
| Reverse | GATGCCATGCCAAAAGCACA | ||
| cIII | 101 bp | Forward | TTCTTTGGGACTCCTGGCTG |
| Reverse | GGCTGCCCTTCCGAATCTTT | ||
| cro | 75 bp | Forward | GAAGTTGCGAAGGCTTGTGG |
| Reverse | AGTCTTAGGGAGGAAGCCGT | ||
| gapA | 103 bp | Forward | CCAGGACATCGTTTCCAAC |
| Reverse | GGTGGTCATCAGACCTTCG | ||
| O | 104 bp | Forward | GATGCTGCAATTCAGAGCGG |
| Reverse | TTTCTGGCTGATGGTGCGAT | ||
| rcsA | 124 bp | Forward | CTCGACGATATCCTTGGCGA |
| Reverse | CCTGACCTGCCATCCACATT | ||
| recA | 70 bp | Forward | CTGGGAGCACAGCAGGTTGTCG |
| Reverse | TGAACACGCGCTGGACCCAATC | ||
| S | 124 bp | Forward | AGTTGCTGGACAGGGTTTCC |
| Reverse | GCCTTACGCCGGTCTTCTTT | ||
| stx1 | 180 bp | Forward | ATAAATCGCCATTCGTTGACTAC |
| Reverse | AGAACGCCCACTGAGATCATC | ||
| stx2A | 133 bp | Forward | CGTCACTCACTGGTTTCATCAT |
| Reverse | TCTGTATCTGCCTGAAGCGTAA |
Table 2.
Primer sets used for qRT-PCR for T3SS related genes.
| Gene name | Product size | Direction | Sequence |
|---|---|---|---|
| csrA | 102 bp | Forward | TCCTTCGGGGCATTTACGCCA |
| Reverse | TCGTCGAGTTGGTGAGACCCTC | ||
| eae | 139 bp | Forward | TCACTTTGAATGGTAAAGGCAGT |
| Reverse | CAAATGGACATAGCATCAGCATA | ||
| espB | 122 bp | Forward | TAAAGGGGCTGGTGAGATTG |
| Reverse | CTGCGACATCAGCAACACTT | ||
| gapA | 103 bp | Forward | CCAGGACATCGTTTCCAAC |
| Reverse | GGTGGTCATCAGACCTTCG | ||
| grlA | 71 bp | Forward | TAGAAAGTCCTGGAACAAC |
| Reverse | AGACTGTCCCACAATACC | ||
| hns | 150 bp | Forward | GCCGGACGCTGAGCACGTTT |
| Reverse | CGCGGCTGCTGCTGAAGTTG | ||
| ler | 71 bp | Forward | CCCGACCAGGTCTGCCCTTCT |
| Reverse | GATGGACTCGCTCGCCGGAAC | ||
| tir | 128 bp | Forward | AACGAAAGAAGCGTTCCAGA |
| Reverse | CTGCTGCTTTAGCCTGCTCT |
Statistical Analysis
Data were analyzed as a randomized design using GLM (General Linear Model of Statistical Analysis System, SAS, 2000). Differences between means were determined using Student’s t-test followed by Duncan’s multiple test when appropriate. P < 0.05 was considered to be statistically significant.
Results
PNPase is Essential for Stx2 Production in E. coli O157:H7 EDL933 Strain
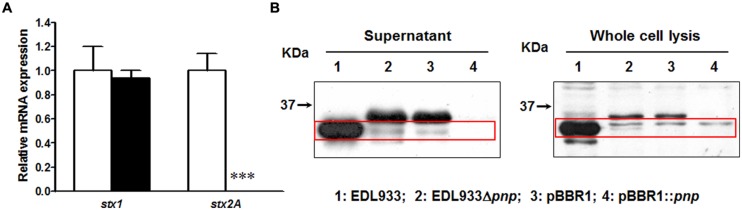
In transcriptional level, deletion of pnp completely eliminated stx2 mRNA expression in E. coli O157:H7 EDL933 strain without affecting stx1 expression (Figure 1A). We further compared the Stx2 production. In wild-type strain, a 32 kDa band Stx2 A subunit was detected in both supernatant fraction and whole cell lysis (Figure 1B). Deletion of pnp resulted in no detection of Stx2A (Figure 1B). This defective in Stx2 protein production was not restored by complementation of pBBR1::pnp (Figure 1B).
FIGURE 1.
Shiga toxin production in E. coli O157:H7 EDL933 strains. (A) Relative mRNA expression; (B) Relative protein content. EDL933: wild-type E. coli O157:H7 strain; EDL933 Δpnp: EDL933 pnp deletion mutant strain; pBBR1: EDL933 Δpnp strain carrying an empty vector pBBR1; pBBR1::pnp: EDL933 Δpnp strain complemented with pBBR1::pnp. □: EDL933; ■: EDL933Δpnp; ***P < 0.001 (Mean ± SEM; n = 4).
PNPase is Indispensable for Stx2 Phage Lytic Growth in E. coli O157:H7
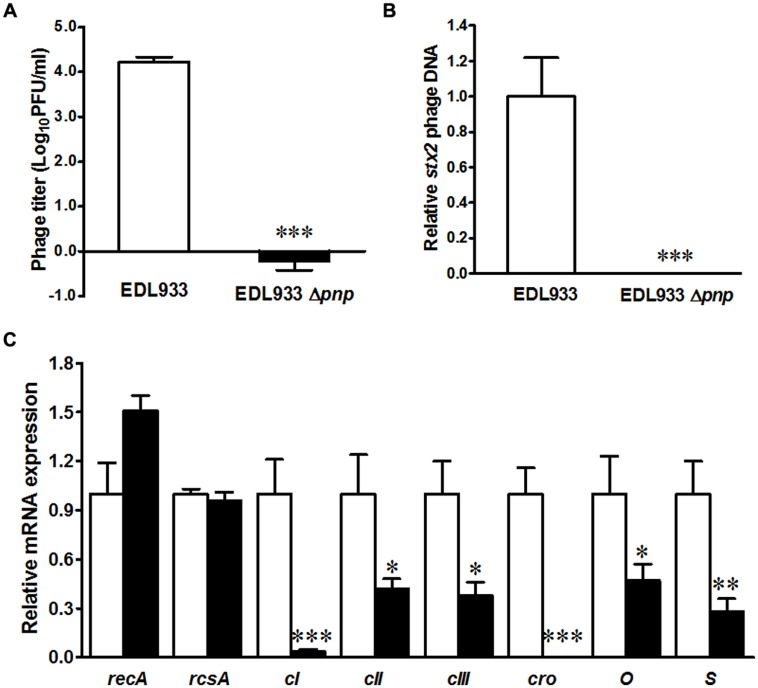
Shiga toxin production is mediated by Stx2 phage activation, thus we further compared phage activation in E. coli O157:H7 EDL933 and its pnp mutant strains. Deletion of pnp completely abolished lambdoid phage progeny production in E. coli O157:H7 (Figure 2A). Quantitative PCR further confirmed that the stx2 phage DNA accumulation was repressed in PNPase defective E. coli O157:H7 strain (Figure 2B). To gain insights into molecular mechanisms of PNPase mediated impairment in Stx phage activation, we further analyzed expression of phage genes regulating prophage activation. Deletion of pnp did not affect recA and rcsA expression (Figure 2C). We could not detect cro mRNA expressions and only detected a very low level of cI expression in Δpnp strain (Figure 2C). In addition, the transcriptional levels of cII and cIII, phage lysis S gene and phage replication gene O were all diminished in Δpnp strain (Figure 2C). Altogether, these data indicated that PNPase in E. coli O157:H7 plays an indispensable role in Stx2 prophage lysis.
FIGURE 2.
Prophage enumeration in E. coli O157:H7 EDL933 wild type (□) and pnp deletion strains (■). (A) Spontaneous lambdoid prophage enumeration; (B) Stx2 phage PCR; (C) Phage lysis related gene expressions. ***P < 0.001, **P < 0.01, *P < 0.05 (Mean ± SEM, n = 4).
Deletion of PNPase in E. coli O157:H7 Impairs Epithelial Adhesion but Enhances T3SS Encoding Transcripts and Proteins
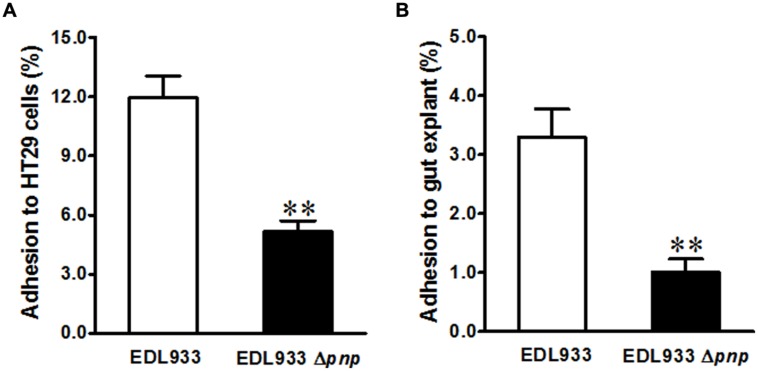
In addition to phage encoding Stx2 production, E. coli O157:H7 has a LEE chromosomal pathogenicity island that encoding T3SS apparatus and effectors, mediating intimate contact to epithelial cells (McDaniel et al., 1995). We further evaluated the significance of pnp deletion in E. coli O157: H7 epithelial adhesion. Deletion of pnp in E. coli O157: H7 EDL933 strain decreased its adhesion to colonic epithelial HT-29 cells by approximately twofold (Figure 3A). Consistently, adhesion to cattle colonic gut explant was reduced about fourfold in EDL933Δpnp (Figure 3B).
FIGURE 3.
Adhesion of E. coli O157:H7 to intestinal epithelium. (A) HT-29 colonic epithelial cell line; (B) Cattle colonic explant tissues. EDL933: wild-type E. coli O157:H7 strain; EDL933 Δpnp: EDL933 pnp deletion mutant strain, **P < 0.01 (Mean ± SEM; n = 8).
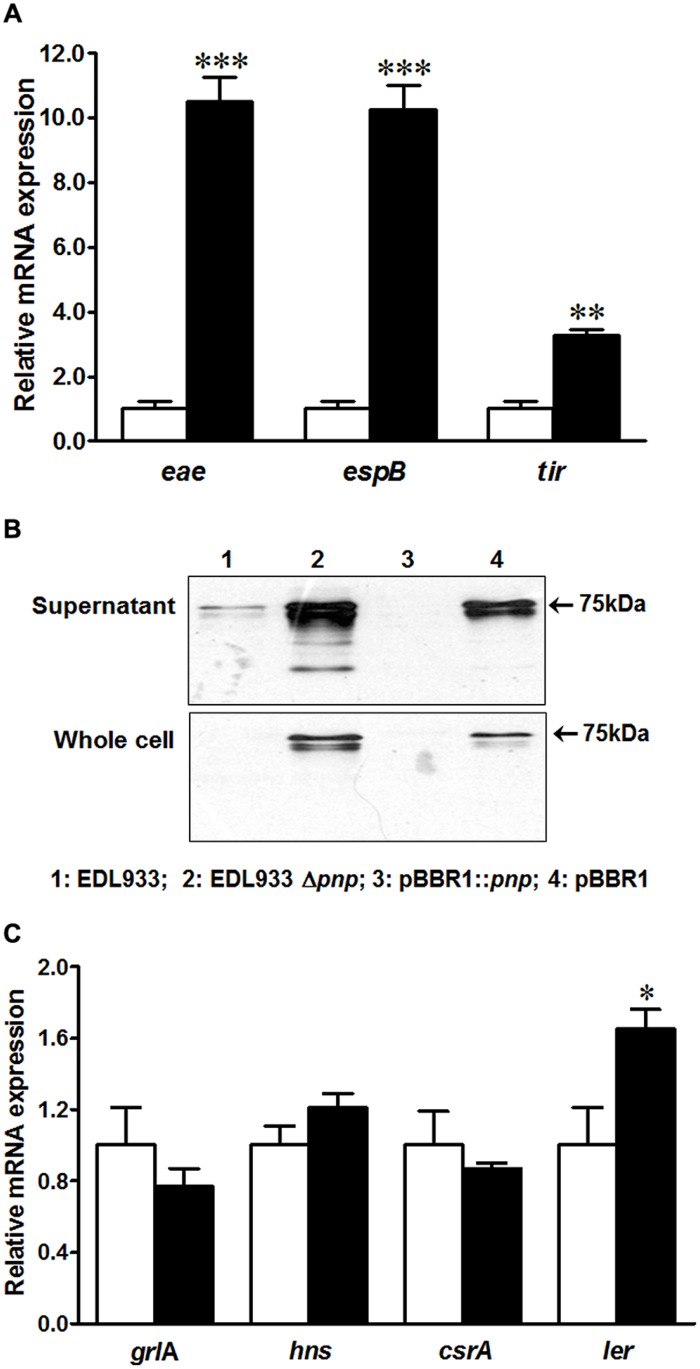
To understand possible factors limiting E. coli O157:H7 epithelial colonization in the absence of PNPase, we further analyzed T3SS apparatus and effectors transcripts and proteins in EDL933Δpnp and its wild type strains. Surprisingly, the transcriptional levels of genes located on LEE region including tir, eae, and espB were enhanced in EDL933Δpnp strain (Figure 4A). Tir protein production was also increased in both supernatant fraction and whole cell lysis of EDL933Δpnp strain compared to the wild-type EDL933 strain (Figure 4B). In line with, the transcriptional level of LEE positive regulators, the ler transcript level, was significantly enhanced in EDL933Δpnp strain (Figure 4C), though other regulators of LEE (hns, csrA, and grlA) did not differ between EDL933Δpnp and its isogenic wild type strain.
FIGURE 4.
Type three secretion system (T3SS) and its regulators in E. coli O157:H7 EDL933 strains. (A) mRNA expressions of T3SS; (B) Tir protein content in E. coli O157:H7; (C) mRNA expression of T3SS regulators. EDL933: wild-type E. coli O157:H7 strain; EDL933 Δpnp: EDL933 pnp deletion mutant strain; pBBR1: EDL933 Δpnp strain carrying an empty vector pBBR1; pBBR1::pnp: EDL933 Δpnp strain complemented with pBBR1::pnp. □: EDL933; ■: EDL933Δpnp; ***P < 0.001, **P < 0.01, *P < 0.05 (Mean ± SEM; n = 4).
Impact of PNPase in Other E. coli O157:H7 Strains
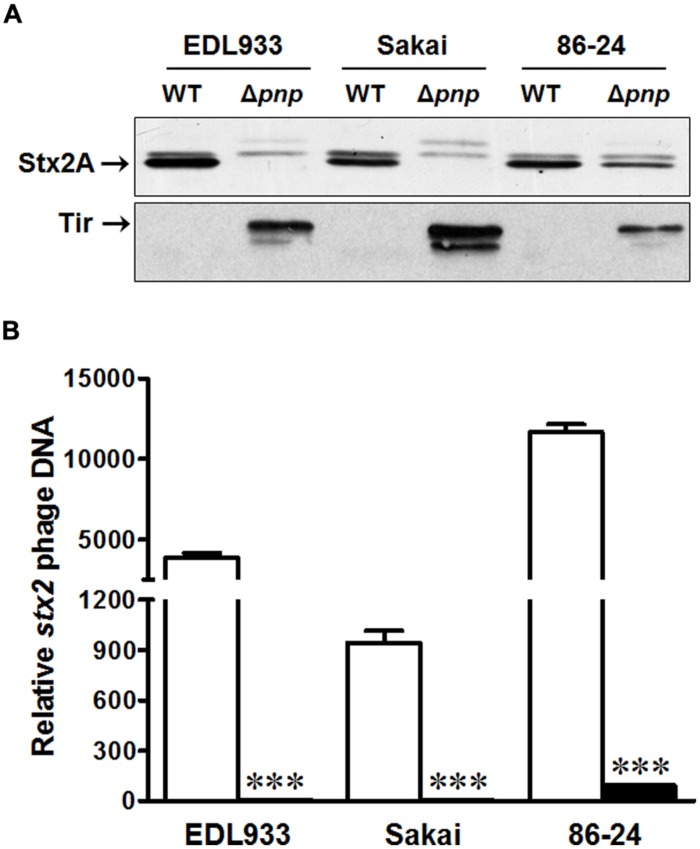
To confirm the results from E. coli O157:H7 strain EDL933, we further examined the Stx2 production and Stx2 phage lytic activation in E. coli O157:H7 strain Sakai and 86-24. Consistent to the finding in EDL933 strain, the pnp deletion decreased Stx2 production (Figure 5A) and impaired Stx2 phage lytic growth (Figure 5B) in Sakai and 86-24. As in strain EDL933, Tir protein content was enhanced in both E. coli O157:H7 strain Sakai and 86-24 (Figure 5A).
FIGURE 5.
Effects of PNPase on the virulence factors of E. coli O157:H7 EDL933, Sakai, and 86-24 strains. (A) Stx 2A and Tir protein contents by immunoblotting; (B) stx2 phage DNA content by qPCR. □: wild-type (WT); ■: pnp deletion mutant (Δpnp). ***P < 0.001 (Mean ± SEM, n = 4).
Discussion
PNPase Regulates Stx2 Production Through Repressing Stx2 Phage Lytic Cycle
Shiga toxin is mainly responsible for the mortality associated with E. coli O157:H7 infection. PNPase is a bifunctional enzyme with an exoribonuclease activity and a poly (A) polymerase activity. Study herein demonstrated that PNPase plays a vital role in Stx production. The deletion of pnp in E. coli O157:H7 completely eliminated stx2 mRNA expression and Stx2 production, which could not be recovered by complementary pnp overexpression. Thus we speculated that the observed effect of PNPase on Stx2 production in EDL933 strain might be due to a secondary mutation in stx2 gene during generation of pnp deletion mutant. Therefore, we did three independent pnp deletion experiments. Different colony from three independent knock out experiments had the same Stx2 profile (data not shown). We further sequenced the promoter region of stx2, which revealed no alteration in the sequence of stx2 promoter region (data not shown). Given that pnp and its downstream genes, yhbM, deaD, and mtr are in one operon, we questioned whether disruption of pnp might change the mRNA levels of pnp downstream genes. Thus, we further analyzed the transcription of the pnp nearest downstream gene, yhbM, and qRT-PCR analysis indicated that it was not affected by pnp deletion (data not shown). These testimonies showed that the decreased Stx2 production was due to pnp deletion. Although the polar effect of pnp deletion would be low in our experiment (Our experiment is in frame deletion), we can’t rule out the possibility that the polar effect of the pnp gene deletion cause the deficiency of Stx production.
Effective production of Stx requires phage lytic status development. Consistent with decreased Stx production, pnp deletion in E. coli O157:H7 repressed Stx2 phage lytic status, indicating that the lytic growth of 933w phage in E. coli O157:H7 was regulated by PNPase. CI protein is required for lysogenic establishment and has self-feedback inhibition activity, which represses prophage replication and lysis (Echols and Green, 1971). cro is the early lytic gene antagonizes CI and regulates phage switch from lysogenic to lytic growth during prophage induction (Svenningsen et al., 2005). Consistent with impaired Stx2 phage lytic growth, transcriptional level of cro were decreased in the EDL933Δpnp strain. Additionally, transcriptional levels of cII and cIII, phage lysis gene, S, and replication genes, O, were also repressed in Δpnp compared to the wild type host. These data demonstrated that the lytic cycle of the Stx2 phage in EDL933Δpnp strain was repressed, which at least partially through influencing transcripts of gene encoding proteins vital for lytic phage growth. In line with our findings, dysfunction of PAP I in E. coli resulted in impaired Stx phage lytic development associated with reduced transcripts of cII, cIII, cro, and O at later time points of induction (Nowicki et al., 2015). Of note, the transcript level of O and cro are altered differently in Δpnp relative to wild-type strain, which might be due to the different post-transcriptional regulation (e.g., different sRNA regulation) and warrants further research.
Similar to what observed in strain EDL933, deletion of pnp significantly decreased Stx2 production in Sakai and 86-24 strains. EDL933 and Sakai strains have both stx1 and stx2 genes, while 86-24 only has stx2 gene. 933W prophage from EDL933 and Sakai Stx2 prophage have different sequence in early gene regulators and replication proteins (Makino et al., 1999), but almost identical sequence for structural genes (Makino et al., 1999). Compared to the genome of 86-24 Stx2 producing phage, there is a 1439 bp insertion replaced a 2091 bp sequence in 933W phage (Kudva et al., 2002). In agreement with diminished Stx2 production, Stx2 phage spontaneous lytic growth was repressed in all tested PNPase deficient strains, indicating that PNPase is important in lytic cycle development of Stx2 phage in E. coli O157:H7 strains.
PNPase Reduced E. coli O157:H7 Intestinal Epithelial Colonization Independent of Expression Levels of T3SS
Deletion of pnp in E. coli O157:H7 decreased its adhesion to epithelial cells and colonic epithelial tissues. In agreement, C. jejun deficient in PNPase has reduced ability to adhere and invade to HT-29 cells, and colonize chick gut (Haddad et al., 2012). PNPase was required for S. Typhimurium gut colonization in swine, PNPase deficiency was associated with reduced fecal S. Typhimurium shedding and intestinal colonization (Bearson et al., 2013). These data collectively indicated PNPase plays a critical role in gut colonization.
Escherichia coli O157:H7 possess LEE pathogenicity island that encode T3SS apparatus and effector proteins, enabling its intimate contact to epithelial cells and further forming AE lesions. In contrast to decreased epithelial adhesion, we revealed that pnp deletion elevated the transcription level of genes located in the LEE region as well as Tir protein content, which is possibly through enhancing ler transcription. These data indicated that PNPase in E. coli O157:H7 might regulate expression and functional activity of T3SS independently, which warrants future research. The reduced T3SS functional activity as indicated by reduced epithelial adhesion might be due to the defectiveness in T3SS apparatus organization or effector secretion. Meanwhile, we speculated that the decreased intestinal adhesion might be partially due to the reduced Stx2 production. Stx regulates the distribution of nucleolin, a host cell protein that is a receptor for intimin in E. coli O157:H7 (Robinson et al., 2006). PNPase deletion decreased Stx2, which might disturb nucleolin distribution and further impair the adherence capacity of E. coli O157:H7.
Our observation was supported by previous studies in Yersinia, where PNPase is required for the proper T3SS function. The pnp mutant strain was defective in rapidly exporting T3SS effector proteins (Rosenzweig et al., 2005), less virulent in mouse, but enhanced T3SS encoding transcripts and proteins compared with its isogenic wild-type Yersinia strain (Rosenzweig et al., 2007). Similarly, in S. Typhimurium, despite of decreased swine intestinal colonization in response to PNPase deficiency (Bearson et al., 2013), T3SS-encoding transcripts and proteins were expressed at higher levels in PNPase inactivated strain (Ygberg et al., 2006). On the other hand, inactivation of PNPase in Salmonella increased invasion and intracellular replication as well as the establishment of persistent infection in mice (Clements et al., 2002). These implicated a complex nature of PNPases in regulating bacterial virulence.
In summary, PNPase has pleiotropic effects on the virulence of E. coli O157:H7. It is not only indispensable for Stx2 phage lytic cycle growth and associated Stx2 production in E. coli O157:H7, but also required for E. coli O157:H7 intestinal epithelial adhesion and regulation of type III secretion system. Alteration in PNPase activity might provide a potential strategy to reduce the virulence of E. coli O157:H7.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Xin Fang and Dr. Mark Gomelsky at Department of Molecular Biology, University of Wyoming for their helpful suggestions. We acknowledge Dr. John Leong at Tufts University and Dr. Mark Gomelsky at University of Wyoming for their generous gifts of Tir antibodies and pBBR1-MCS3 plasmid, respectively. This work was supported by USDA-AFRI 2010-65201-20599 and Washington State University Emerging Research Issues Competitive Grants.
References
- Allison H. E. (2007). Stx-phages: drivers and mediators of the evolution of STEC and STEC-like pathogens. Future Microbiol. 2 165–174. 10.2217/17460913.2.2.165 [DOI] [PubMed] [Google Scholar]
- Arraiano C. M., Andrade J. M., Domingues S., Guinote I. B., Malecki M., Matos R. G., et al. (2010). The critical role of RNA processing and degradation in the control of gene expression. FEMS Microbiol. Rev. 34 883–923. 10.1111/j.1574-6976.2010.00242.x [DOI] [PubMed] [Google Scholar]
- Bearson S. M., Bearson B. L., Lee I. S., Kich J. D. (2013). Polynucleotide phosphorylase (PNPase) is required for Salmonella enterica serovar Typhimurium colonization in swine. Microb. Pathog. 65 63–66. 10.1016/j.micpath.2013.10.001 [DOI] [PubMed] [Google Scholar]
- Bhatt S., Romeo T., Kalman D. (2011). Honing the message: post-transcriptional and post-translational control in attaching and effacing pathogens. Trends Microbiol. 19 217–224. 10.1016/j.tim.2011.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis A. J., Vanzo N. F., Raynal L. C. (1999). mRNA degradation. A tale of poly(A) and multiprotein machines. Trends Genet. 15 24–28. 10.1016/S0168-9525(98)01627-8 [DOI] [PubMed] [Google Scholar]
- Clements M. O., Eriksson S., Thompson A., Lucchini S., Hinton J. C., Normark S., et al. (2002). Polynucleotide phosphorylase is a global regulator of virulence and persistency in Salmonella enterica. Proc. Natl. Acad. Sci. U.S.A. 99 8784–8789. 10.1073/pnas.132047099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko K. A., Wanner B. L. (2000). One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U.S.A. 97 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Puente J. L., Gruenheid S., Li Y., Vallance B. A., Vazquez A., et al. (2004). Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. U.S.A. 101 3597–3602. 10.1073/pnas.0400326101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echols H., Green L. (1971). Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc. Natl. Acad. Sci. U.S.A. 68 2190–2194. 10.1073/pnas.68.9.2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman Di C. D. (2006). “Regulation of lambda gene experssion by transcription termination and antitermination,” in The Bacteriophages, ed. Calendar R. (Oxford: Oxford Press; ), 83–103. [Google Scholar]
- Haddad N., Tresse O., Rivoal K., Chevret D., Nonglaton Q., Burns C. M., et al. (2012). Polynucleotide phosphorylase has an impact on cell biology of Campylobacter jejuni. Front. Cell. Infect. Microbiol. 2:30 10.3389/fcimb.2012.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. M., Yue W. F., Olsen S. A., Hu J., Means W. J., Mccormick R. J., et al. (2012). Salt at concentrations relevant to meat processing enhances Shiga toxin 2 production in Escherichia coli O157:H7. Int. J. Food Microbiol. 159 186–192. 10.1016/j.ijfoodmicro.2012.09.007 [DOI] [PubMed] [Google Scholar]
- Herold S., Karch H., Schmidt H. (2004). Shiga toxin-encoding bacteriophages–genomes in motion. Int. J. Med. Microbiol. 294 115–121. 10.1016/j.ijmm.2004.06.023 [DOI] [PubMed] [Google Scholar]
- Imamovic L., Muniesa M. (2012). Characterizing RecA-independent induction of Shiga toxin2-encoding phages by EDTA treatment. PLoS ONE 7:e32393 10.1371/journal.pone.0032393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L., Romer W. (2010). Shiga toxins–from cell biology to biomedical applications. Nat. Rev. Microbiol. 8 105–116. 10.1038/nrmicro2279 [DOI] [PubMed] [Google Scholar]
- Karch H., Schmidt H., Janetzki-Mittmann C., Scheef J., Kroger M. (1999). Shiga toxins even when different are encoded at identical positions in the genomes of related temperate bacteriophages. Mol. Gen. Genet. 262 600–607. 10.1007/s004380051122 [DOI] [PubMed] [Google Scholar]
- Kimmitt P. T., Harwood C. R., Barer M. R. (2000). Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg. Infect. Dis. 6 458–465. 10.3201/eid0605.000503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach M. E., Elzer P. H., Hill D. S., Robertson G. T., Farris M. A., Roop R. M., II, et al. (1995). Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166 175–176. 10.1016/0378-1119(95)00584-1 [DOI] [PubMed] [Google Scholar]
- Kudva I. T., Evans P. S., Perna N. T., Barrett T. J., Ausubel F. M., Blattner F. R., et al. (2002). Strains of Escherichia coli O157:H7 differ primarily by insertions or deletions, not single-nucleotide polymorphisms. J. Bacteriol. 184 1873–1879. 10.1128/JB.184.7.1873-1879.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R. (2015). Polyadenylation in E. coli: a 20 year odyssey. RNA 21 673–674. 10.1261/rna.049700.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. (2005). “Lysogeny, prophage induction, and lysogenic conversion,” in Phages: Their Role in Bacterial Pathogenesis and Biotechnology, eds Friedman D. I., Waldor M. K., Adhya S. L. (Washington, DC: ASM Press; ), 37–54. [Google Scholar]
- Los J. M., Los M., Wegrzyn G. (2011). Bacteriophages carrying Shiga toxin genes: genomic variations, detection and potential treatment of pathogenic bacteria. Future Microbiol. 6 909–924. 10.2217/fmb.11.70 [DOI] [PubMed] [Google Scholar]
- Mainil J. G., Daube G. (2005). Verotoxigenic Escherichia coli from animals, humans and foods: who’s who? J. Appl. Microbiol. 98 1332–1344. 10.1111/j.1365-2672.2005.02653.x [DOI] [PubMed] [Google Scholar]
- Makino K., Yokoyama K., Kubota Y., Yutsudo C. H., Kimura S., Kurokawa K., et al. (1999). Complete nucleotide sequence of the prophage VT2-Sakai carrying the verotoxin 2 genes of the enterohemorrhagic Escherichia coli O157:H7 derived from the Sakai outbreak. Genes Genet. Syst. 74 227–239. 10.1266/ggs.74.227 [DOI] [PubMed] [Google Scholar]
- McDaniel T. K., Jarvis K. G., Donnenberg M. S., Kaper J. B. (1995). A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. U.S.A. 92 1664–1668. 10.1073/pnas.92.5.1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki D., Bloch S., Nejman-Falenczyk B., Szalewska-Palasz A., Wegrzyn A., Wegrzyn G. (2015). Defects in RNA polyadenylation impair both lysogenization by and lytic development of Shiga toxin-converting bacteriophages. J. Gen. Virol. 10.1099/vir.0.000102 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Paton J. C., Paton A. W. (1998). Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin. Microbiol. Rev. 11 450–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Hopkins N. (1968). The operators controlled by the lambda phage repressor. Proc. Natl. Acad. Sci. U.S.A. 60 1282–1287. 10.1073/pnas.60.4.1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C. M., Sinclair J. F., Smith M. J., O’Brien A. D. (2006). Shiga toxin of enterohemorrhagic Escherichia coli type O157 : H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. U.S.A. 103 9667–9672. 10.1073/pnas.0602359103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig J. A., Chromy B., Echeverry A., Yang J., Adkins B., Plano G. V., et al. (2007). Polynucleotide phosphorylase independently controls virulence factor expression levels and export in Yersinia spp. FEMS Microbiol. Lett. 270 255–264. 10.1111/j.1574-6968.2007.00689.x [DOI] [PubMed] [Google Scholar]
- Rosenzweig J. A., Weltman G., Plano G. V., Schesser K. (2005). Modulation of Yersinia type three secretion system by the S1 domain of polynucleotide phosphorylase. J. Biol. Chem. 280 156–163. 10.1074/jbc.M405662200 [DOI] [PubMed] [Google Scholar]
- Rozanov D. V., D’Ari R., Sineoky S. P. (1998). RecA-independent pathways of lambdoid prophage induction in Escherichia coli. J. Bacteriol. 180 6306–6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsen S. L., Costantino N., Court D. L., Adhya S. (2005). On the role of Cro in lambda prophage induction. Proc. Natl. Acad. Sci. U.S.A. 102 4465–4469. 10.1073/pnas.0409839102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A. G., Slater T. M., Patel S. D., Popov V. L., Arenas-Hernandez M. M. (2008). Contribution of the Ler- and H-NS-regulated long polar fimbriae of Escherichia coli O157:H7 during binding to tissue-cultured cells. Infect. Immun. 76 5062–5071. 10.1128/IAI.00654-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. S., Beeri K., Reynolds J. L., Alteri C. J., Skinner K. G., Friedman J. H., et al. (2013). Prophage induction is enhanced and required for renal disease and lethality in an EHEC mouse model. PLoS Pathog. 9:e1003236 10.1371/journal.ppat.1003236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. L., Neely M. N., Zhang X., Acheson D. W., Waldor M. K., Friedman D. I. (2001). Role for a phage promoter in Shiga toxin 2 expression from a pathogenic Escherichia coli strain. J. Bacteriol. 183 2081–2085. 10.1128/JB.183.6.2081-2085.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xicohtencatl-Cortes J., Monteiro-Neto V., Ledesma M. A., Jordan D. M., Francetic O., Kaper J. B., et al. (2007). Intestinal adherence associated with type IV pili of enterohemorrhagic Escherichia coli O157:H7. J. Clin. Invest. 117 3519–3529. 10.1172/JCI30727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ygberg S. E., Clements M. O., Rytkonen A., Thompson A., Holden D. W., Hinton J. C., et al. (2006). Polynucleotide phosphorylase negatively controls spv virulence gene expression in Salmonella enterica. Infect. Immun. 74 1243–1254. 10.1128/IAI.74.2.1243-1254.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue W. F., Du M., Zhu M. J. (2012). High temperature in combination with UV irradiation enhances horizontal transfer of stx2 gene from E. coli O157:H7 to non-pathogenic E. coli. PLoS ONE 7:e31308 10.1371/journal.pone.0031308 [DOI] [PMC free article] [PubMed] [Google Scholar]