Abstract
Genetic polymorphisms in DNA repair genes may induce individual variations in DNA repair capacity, which may in turn contribute to the risk of cancer developing. Homologous recombination repair (HRR) plays a critical role in maintaining chromosomal integrity and protecting against carcinogenic factors. The aim of the present study was to evaluate the relationship between prostate cancer risk and the presence of single nucleotide polymorphisms (SNPs) in the genes involved in HRR, that is, RAD51 (rs1801320 and rs1801321), RAD51B (rs10483813 and rs3784099), XRCC2 (rs3218536), and XRCC3 (rs861539). Polymorphisms were analyzed by PCR-RFLP and Real-Time PCR in 101 patients with prostate adenocarcinoma and 216 age- and sex-matched controls. A significant relationship was detected between the RAD51 gene rs1801320 polymorphism and increased prostate cancer risk. Our results indicate that the RAD51 gene rs1801320 polymorphism may contribute to prostate cancer susceptibility in Poland.
1. Introduction
Prostate cancer (PC) is the second most commonly diagnosed malignant disease in men and the sixth leading cause of cancer-related death among men worldwide, with an estimated 10948 (14.3%) registered new cases and 4199 (8%) deaths in 2012 in Poland [1]. Although prostate cancer is one of the most common cancers in men, the genetic defects underlying its pathogenesis remain poorly understood. While being over 65 years and the presence of a family history are the strongest risk factors for prostate cancer, ethnicity has also been shown to be a risk factor, with the lowest incidence rates of prostate cancer being observed in Asian men, particularly in India, China, and Japan. Higher incidence rates are seen in black men. The risk of developing prostate cancer is thought to be 1.3–2.0 times higher in African-American than Caucasian men [2].
Genetic defects in DNA repair and DNA damage response genes often lead to an increase of cancer incidence. The most deleterious form of DNA damage is the DNA double-strand break (DSB), which can be formed by free radicals derived from the metabolism, ionizing radiation, or DNA cross-linking agents or which can naturally occur during DNA replication. Unrepaired DSBs lead to mutations, rearrangements, and/or loss of chromosomes, causing genome instability and the development of tumors or cell death. In order to maintain genetic stability, DNA double-strand breaks must be repaired by homologous recombination or nonhomologous end-joining. The key protein in homologous recombination is RAD51, which displaces replication protein A (RPA) and forms a helical nucleofilament on the exposed single-stranded DNA flanking the DSB. This nucleofilament performs a homology search for repair template and then catalyses DNA strand invasion [3, 4].
RAD51 is a homologue of the RecA protein and contains 339 amino acids. The RAD51 gene is located at human chromosome 15q15.1 and is highly polymorphic. Five RAD51 paralogs, that is, RAD51B, RAD51C, RAD51D, XRCC2, and XRCC3, have been identified in the human genome. They are key components of homologous recombination, and their loss can result in the development of genetic instability. The RAD51 paralogs form two major complexes, RAD51B-RAD51C-RAD51D-XRCC2 (BCDX2) and RAD51C-XRCC3 (CX3), as well as two subcomplexes, RAD51B-RAD51C (BC) and RAD51D-XRCC2 (DX2). The CX3 complex is known to catalyze strand exchange in vitro. Similarly, the BC subcomplex of the BCDX2 complex has been reported to have in vitro RAD51 mediator activity and the DX2 subcomplex has been reported to have strand exchange activity. However, the specific activities of the RAD51 paralog complexes and subcomplexes have not been defined in vivo [5–7].
The genetic variations of RAD51 and its paralogs may contribute to the development of cancer, as has been shown in the case of breast, ovarian, endometrial, colorectal, and head and neck cancer and acute leukemia [8–11].
The aim of the work was to evaluate the significance of common genetic variation in four genes involved in DNA double-strand break repair via homologous recombination, that is, RAD51, RAD51B, XRCC2, and XRCC3, in prostate cancer susceptibility, tagging six most widely studied single nucleotide polymorphisms (SNPs) in these genes. The association between the presence of polymorphisms rs1801320 and rs1801321 of RAD51, rs10483813 and rs3784099 of RAD51B, rs3218536 of XRCC2, and rs861539 of XRCC3 and the risk of prostate cancer was examined.
2. Material and Methods
2.1. Study Subjects
The study included 101 men with prostate adenocarcinoma and 216 sex- and age-matched persons who have not been diagnosed with cancer to serve as a control group. Peripheral blood from the patients with prostate adenocarcinoma was collected at the 2nd Department of Urology, Medical University of Lodz, Poland, between October 2009 and December 2011. Peripheral blood from the control group was obtained from the Maria Sklodowska-Curie Memorial Hospital, Zgierz, Poland. Blood samples were collected on EDTA and frozen. The clinical characteristics of the prostate cancer patients and controls are presented in Table 1. None of the patients underwent any anticancer treatment. The samples were obtained in accordance with guidelines concerning ethical and legal requirements. Informed consent was obtained from patients, and the studies were approved by the Independent Ethical Committees of the Medical University of Lodz, Poland (RNN/59/09/KE), and the University of Lodz, Poland (KBBN-UŁ/II/25/2012).
Table 1.
Clinical characteristic of studied material.
| Prostate cancer patients | Control group | |
|---|---|---|
| Age (year) | ||
| Range | 49–86 | 43–84 |
| Mean ± SD | 71 ± 9 | 63 ± 9 |
| Median | 71 | 63 |
|
| ||
| PSAT (ng/mL) | ||
| Range | 4.01–1489 | 0.004–3.94 |
| Mean ± SD | 59.96 ± 182.67 | 1.09 ± 0.88 |
| Median | 10.57 | 0.90 |
2.2. DNA Isolation
DNA was isolated from the blood samples from the prostate adenocarcinoma patients and controls using an AxyPrep Blood Genomic DNA Miniprep Kit (Axygen, USA) and the phenol-chloroform method. DNA purity and quantity were estimated by UV-spectroscopy (Eppendorf BioPhotometer TM Plus, Eppendorf, Germany). DNA purity was determined by the 260/280 nm absorbance ratio, with a value between 1.8 and 2.0 being acceptable.
2.3. Genotyping
Single nucleotide polymorphisms (SNPs) of the RAD51 (rs1801320 and rs1801321), RAD51B (rs10483813 and rs3784099), XRCC2 (rs3218536), and XRCC3 (rs861539) genes were analyzed (Table 2).
Table 2.
SNPs analyzed.
| Gene | Polymorphism | Other names | SNP position | Chromosome | Global MAF | Method |
|---|---|---|---|---|---|---|
| RAD51 | rs1801320 |
c. -98G>C G135C |
UTR-5, Exon | 15:40695330 | 13.04% | PCR-RFLP |
| rs1801321 | c. -61G>T, G172T | UTR-5, Exon | 15:40695367 | 26.63% | Real-Time PCR | |
|
| ||||||
| RAD51B | rs10483813 | c. 1037-29918T>A | Intron | 14:68564567 | 12.49% | Real-Time PCR |
| rs3784099 | c. 757-8674G>A | Intron | 14:68283210 | 37.79% | Real-Time PCR | |
|
| ||||||
| XRCC2 | rs3218536 | c. 563G>A, p. Arg188His | Missense | 7:152648922 | 4.27% | PCR-RFLP |
|
| ||||||
| XRCC3 | rs861539 | c. 722C>T, p. Thr241Met | Intron, Missense | 14:103699416 | 25.07% | PCR-RFLP |
The genotyping of rs1801320, rs3218536, and rs861539 polymorphisms was determined by PCR-RFLP. The primers and PCR conditions for the polymorphic sites of these genes are shown in Table 2. The PCR was run in 10 μL reactions containing 10 ng of genomic DNA, 0.2 mM of each primer, 2.5 mM MgCl2, 1 mM deoxyribonucleotide triphosphates (dNTPs), 3 U HOT FIREPol DNA polymerase, and 1x Solis BioDyne buffer B1. The primers were synthesized by Sigma-Aldrich (USA), and PCR reagents were obtained from Solis BioDyne (Estonia) and Applied Biosystems (USA). Thermal cycling was performed as follows: initial activation at 95°C for 12 min, followed by 30 amplification cycles consisting of denaturation at 95°C for 30 s, annealing at 64°C (rs3218536, rs861539) or 65°C (rs1801320) for 30 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min.
To genotype the rs1801320, rs3218536, and rs861539 polymorphisms, 10 μL of each PCR product was digested with either 2 U of MvaI (BstNI) (Thermo Scientific, USA), 1 U of SexAI (New England Biolabs Inc., USA), or 0.5 units of NlaIII (New England Biolabs Inc., USA), respectively, for 16 h at 37°C. The genotypes were determined by running the digested products in 3% agarose gel with ethidium bromide (1 μL/mL) for UV visualization. The products for each genotype of the tested genes are shown in Table 3. Examples of the obtained restriction patterns are presented in Figure 1.
Table 3.
Details of PCR-RFLP of the studied SNPs.
| SNP | Primer sequences | Annealing temp. (°C) | Product size (bp) | Enzyme | Genotype | Fragment sizes (bp) |
|---|---|---|---|---|---|---|
| rs1801320 | (F) 5′TGGGAACTGCAACTCATCTGG3′ (R) 5′GCGCTCCTCTCTCCAGCAG3′ |
GG | 71, 86 | |||
| 65 | 157 | MvaI | GC | 71, 86, 157 | ||
| CC | 157 | |||||
|
| ||||||
| rs3218536 | (F) 5′CGTCAATGGAGGAGAAAGTGTG3′ (R) 5′TCGAGAGGCATGAGAAGGTT3′ |
GG | 166 | |||
| 64 | 166 | SexA1 | GA | 79, 87, 166 | ||
| AA | 79, 87 | |||||
|
| ||||||
| rs861539 | (F) 5′TAAGAAGGTCCCCGTACTCC3′ (R) 5′CTGCGCATCAACCAGGTGAG3′ |
CC | 34, 161 | |||
| 64 | 195 | NlaIII | CT | 56, 105, 161 | ||
| TT | 56, 105 | |||||
Figure 1.
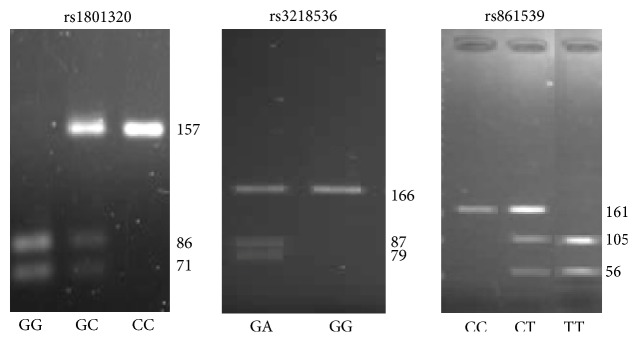
Genotyping of rs1801320, rs3218536, and rs861539 polymorphisms by PCR-RFLP.
The rs1801321, rs10483813, and rs3784099 polymorphisms were genotyped by Real-Time PCR (Mastercycler ep realplex, Eppendorf USA) using TaqMan SNP Genotyping Assays (Applied Biosystems). The PCR amplification was performed according to the manufacturer's recommendations in 10 μL reactions containing 10 ng of genomic DNA, 0.25 μL SNP Genotyping Assay Mix (40x) (rs1801321 Assay ID: C_7482700_10; rs10483813 Assay ID: C_2564845_10; rs3784099 Assay ID: C_27481679_10), and 5 μL TaqMan Universal PCR Master Mix (2x). The amplification conditions consisted of initial AmpliTaq Gold 1 activation at 95°C for 10 min, followed by 40 amplification cycles consisting of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min.
2.4. Statistical Analysis
The chi-square test was used to analyze the Hardy-Weinberg frequencies for the RAD51, RAD51B, XRCC2, and XRCC3 genotypes in the control and patients populations. The differences in genotype and allele frequency between prostate cancer patients and controls were also evaluated with the chi-square test. The odds ratio (OR) and corresponding 95% confidence intervals (CI) were determined through unconditional multiple logistic regression. Statistical analysis was carried out using Statistica version 9.0 (StatSoft, Poland).
3. Results
The study comprised 101 prostate cancer cases and 216 sex- and age-matched controls. The genotypes of six genetic variants of the RAD51, RAD51B, XRCC2, and XRCC3 genes were determined. The genotypes and allele frequencies for each polymorphism of the studied genes are summarized in Table 4. The observed genotype frequencies were found to be consistent with the Hardy-Weinberg equilibrium at all studied polymorphic loci, except for the RAD51 polymorphism. No statistically significant differences were found between prostate cancer patients and cancer-free controls with regard to the distribution of genotypes and alleles, apart from the rs1801320 polymorphism of the RAD51 gene.
Table 4.
Distribution of genotypes and allele frequency in the RAD51, RAD51B, XRCC2, and XRCC3 loci among prostate cancer patients.
| Gene | Genotype/allele | Prostate cancer patients (n = 101) |
Control group (n = 216) |
P |
|---|---|---|---|---|
|
RAD51
rs1801320 |
GG | 66 | 172 | 0.017 |
| GC | 27 | 37 | ||
| CC | 8 | 7 | ||
| G | 159 | 381 | 0.002 | |
| C | 43 | 51 | ||
| rs1801321 | GG | 39 | 67 | 0.392 |
| TG | 4 | 8 | ||
| TT | 58 | 141 | ||
| G | 82 | 421 | 0.058 | |
| T | 120 | 290 | ||
|
| ||||
|
RAD51B
rs10483813 |
TT | 56 | 134 | 0.350 |
| TA | 40 | 68 | ||
| AA | 5 | 14 | ||
| T | 152 | 336 | 0.481 | |
| A | 50 | 96 | ||
| rs3784099 | GG | 49 | 122 | 0.225 |
| GA | 41 | 80 | ||
| AA | 11 | 14 | ||
| G | 139 | 324 | 0.102 | |
| A | 63 | 108 | ||
|
| ||||
|
XRCC2
rs3218536 |
GG | 90 | 196 | 0.648 |
| GA | 11 | 20 | ||
| AA | 0 | 0 | ||
| G | 191 | 412 | 0.800 | |
| A | 11 | 20 | ||
|
| ||||
|
XRCC3
rs861539 |
CC | 54 | 119 | 0.776 |
| CT | 34 | 75 | ||
| TT | 13 | 22 | ||
| C | 142 | 313 | 0.574 | |
| T | 60 | 119 | ||
The genotype frequencies for each polymorphism, in both the prostate cancer group and the control group, were analyzed using a logistic regression model (Table 5). Among the six polymorphisms examined, the rs1801320 RAD51 gene polymorphism was found to be significantly associated with prostate cancer susceptibility. The rs1801320 RAD51 gene polymorphism was verified by sequencing analysis with BigDye Terminator Cycle Sequencing Ready Reaction Kits version 1.1 (see Supplementary Material available online at http://dx.doi.org/10.1155/2015/828646). Heterozygous (OR = 1.90) and homozygous (OR = 2.98) C genotype variants and C allele (OR = 2.02, P < 0.01) appeared to increase prostate cancer risk.
Table 5.
Genotype distribution and prostate cancer risk for the RAD51, RAD51B, XRCC2, and XRCC3 polymorphisms in prostate cancer patients and control group.
| Genotype/allele | Prostate cancer patients (n = 101) |
Control group (n = 216) |
OR [95% Cl] | P value |
|---|---|---|---|---|
| rs1801320 | ||||
| GG | 66 (65.3) | 172 (79.5) | 1 [Ref.] | |
| GC | 27 (26.7) | 37 (17.3) | 1.90 [1.07–3.37] | 0.03 |
| CC | 8 (8.0) | 7 (3.2) | 2.98 [1.04–8.54] | 0.04 |
| G | 159 (78.7) | 381 (88.2) | 1 [Ref.] | |
| C | 43 (21.3) | 51 (11.8) | 2.02 [1.29–3.16] | <0.01 |
|
| ||||
| rs1801321 | ||||
| GG | 39 (38.6) | 67 (31.0) | 1 [Ref.] | |
| TG | 4 (4.0) | 8 (3.7) | 0.86 [0.24–3.04] | 1 |
| TT | 58 (57.4) | 141 (65.3) | 0.71 [0.43–1.16] | 0.17 |
| G | 82 (40.6) | 142 (32.9) | 1 [Ref.] | |
| T | 120 (59.4) | 290 (67.1) | 0.72 [0.51–1.01] | 0.06 |
|
| ||||
| rs10483813 | ||||
| TT | 56 (55.4) | 134 (62.0) | 1 [Ref.] | |
| TA | 40 (39.6) | 68 (31.5) | 1.41 [0.85–2.32] | 0.18 |
| AA | 5 (5.0) | 14 (6.5) | 0.85 [0.29–2.49] | 0.78 |
| T | 152 (75.2) | 336 (77.8) | 1 [Ref.] | |
| A | 50 (24.8) | 96 (22.2) | 1.15 [0.78–1.70] | 0.48 |
|
| ||||
| rs3784099 | ||||
| GG | 49 (48.5) | 122 (56.5) | 1 [Ref.] | |
| GA | 41 (40.6) | 80 (37.0) | 1.28 [0.77–2.11] | 0.34 |
| AA | 11 (10.9) | 14 (6.5) | 1.96 [0.83–4.61] | 0.12 |
| G | 139 (68.8) | 324 (75.0) | 1 [Ref.] | |
| A | 63 (31.2) | 108 (25.0) | 1.36 [0.94–1.97] | 0.10 |
|
| ||||
| rs3218536 | ||||
| GG | 90 (89) | 196 (90.7) | 1 [Ref.] | |
| GA | 11 (11.0) | 20 (9.3) | 1.20 [0.55–2.60] | 0.65 |
| AA | 0 (0.0) | 0 (0.0) | — | |
| G | 191 (94.6) | 412 (95.4) | 1 [Ref.] | |
| A | 11 (5.4) | 20 (4.6) | 1.19 [0.56–2.53] | 0.65 |
|
| ||||
| rs861539 | ||||
| CC | 54 (53.5) | 119 (55.1) | 1 [Ref.] | |
| CT | 34 (33.7) | 75 (34.7) | 1.00 [0.60–1.68] | 1.00 |
| TT | 13 (12.8) | 22 (10.2) | 1.30 [0.61–2.78] | 0.49 |
| C | 142 (70.3) | 313 (72.5) | 1 [Ref.] | |
| T | 60 (29.7) | 119 (27.5) | 1.11 [0.77–1.61] | 0.57 |
The association between the C allele of RAD51 gene rs1801320 polymorphism and the age and PSAT serum level of the cancer patients was analyzed. No statistically significant relationship was found between the presence of the C allele for rs1801320 and either age or PSAT serum level (Table 6).
Table 6.
Relationship between C allele for the RAD51 gene rs1801320 polymorphism and patient's age and PSAT level.
| rs1801320 | Age | OR [95% Cl] | P value | PSAT | OR [95% Cl] | P value | ||
|---|---|---|---|---|---|---|---|---|
| ≤71 | >71 | <4–10 | >10 | |||||
| G | 86 | 16 | 1 [Ref.] | 0.05 | 77 | 21 | 1 [Ref.] | 0.88 |
| C | 73 | 27 | 1.99 [0.99–3.97] | 82 | 22 | 0.98 [0.50–1.93] | ||
4. Discussion
Despite the high incidence of prostate cancer, the exact cause of its development is not known. It is assumed that, as in the case of other cancers, prostate cancer occurs as a result of the interaction between environmental factors and genetic predisposition. The standard biomarker associated with prostate pathology is prostate specific antigen (PSA). PSA is a kallikrein-like serine protease secreted by the epithelial cells of the prostate and encoded by an androgen-responsive gene (19q 13.3–13.4). The major role of PSA is the liquefaction of human semen by its proteolytic activity. However, because of the low specificity of PSA, unnecessary biopsies and mistaken diagnoses frequently occur. As prostate cancer has a variety of features, prognosis following diagnosis is greatly variable. Hence, there is a need for new biomarkers to improve clinical management of prostate cancer [12].
DNA is replicated with extremely high fidelity in normal cells, with a mutation rate of 10−10 per base pair per cell division. DNA damage typically occurs through exposure to genotoxic chemicals, ultraviolet and ionizing radiation, failures in normal cellular DNA processing and replication events (stalled replication forks), and spontaneous DNA-damaging events. These processes contribute to oxidation, alkylation, cross-linking, and dimerization and cause strand breaks in DNA, whose repair is essential for maintaining the integrity of the genome and preventing cancer. The DNA double-strand break is the most lethal form of DNA damage, as it can lead to significant DNA damage by multiple genomic changes, including translocation, deletion, and amplification, resulting in heritable cellular genomic instability that can lead to malignancy [12, 13].
The repair of a double-strand DNA break is performed by homologous recombination. This process is promoted by recombinase RAD51, which catalyzes the key reactions that typify HRR, that is, homology search and DNA strand invasion. Several RAD family members, including its paralogs RAD51B, XRCC2, and XRCC3, assist RAD51 in this process [14–16].
There is a growing body of evidence, which suggests that polymorphic variants of genes involved in DNA repair could modulate DNA repair capacity and thus have a great impact on genomic stability and cancer prevention. Human RAD51 and its paralogs RAD51B, XRCC2, and XRCC3 are highly polymorphic. The RAD51 polymorphisms c. -98G>C (rs1801320; 135G>C) and c. -61G>T (rs1801321; 172G>T) are two of the most common polymorphisms and are located at 5′UTR. Although the functional consequence of the c. -98G>C polymorphism is unknown, it is speculated that because it alters a CpG island pattern in the promoter region, it may regulate the expression of RAD51 and its mRNA levels. The c. -61G>T is located in a binding site for the transcription factor P300/CBP. Current models suggest that the binding of P300/CBP cofactors to transcription factor activation domains positions histone acetyltransferases near specific nucleosomes in the promoter regions of the target gene. In contrast to the c. -61T allele, the c. -61G allele does not form a binding site for cis-transcriptional elements for P300/CBP. Thus, the presence of the T allele results in a greater effect on RAD51 gene expression [17–19].
It was found that the RAD51 polymorphism rs1801320 is associated with an elevated risk of breast [20–22] and triple-negative breast cancer [23] and ovarian [24, 25], endometrial [26–28], colorectal [9, 29, 30], gastric [31], and head and neck cancer [32, 33] and myelodysplastic syndrome [34], as well as keratoconus and Fuchs endothelial corneal dystrophy [35]. The significance of the RAD51 polymorphism rs1801320 has been best characterized in patients with breast cancer, especially in carriers of BRCA2 gene mutations. Antoniou et al. [17] report that the risk of developing breast cancer increases in the case of a polymorphic variant c. -98G>C and the presence of mutations in the BRCA2 gene. No correlations of this polymorphism were found in carriers of BRCA1 gene mutations. These results are confirmed by numerous studies [36–39]. However, on the contrary, Wang et al. [40] observe that the RAD51 gene rs1801320 polymorphism reduces the risk of developing ovarian cancer in carriers of the BRCA2 mutations. In addition, Ribeiro Junior et al. [41] indicate that the rs1801320 polymorphism of RAD51 gene is associated with a decreased risk of developing myelodysplastic syndrome. No correlation was found between the presence of the RAD51 rs1801321 polymorphism and the risk of developing endometrial and ovarian cancer [28, 42]. However, the variant c. -61G>T in the RAD51 gene seems to affect the development of breast and triple-negative breast cancer, acute myeloid leukemia, and head and neck cancer [32, 43–46].
The RAD51B gene encodes one of five RAD51 paralogs, which play an important role in DNA repair through homologous recombination. RAD51B-deficient chicken B lymphocyte DT40 cells have been observed to impair homologous recombination and are sensitive to cross-linking agents. It has been suggested that RAD51B promotes the assembly of the RAD51 nucleoprotein filaments during HRR. Inactivation of RAD51B by translocation between chromosomes 12 and 14 is a frequent finding in uterine leiomyoma, supporting a role for the inactivation of RAD51B in tumorigenesis [47]. In the case of the RAD51B gene, a correlation has been demonstrated between the presence of the rs3784099 polymorphism and the risk of breast cancer [48]. The relationship between the RAD51B gene rs10483813 polymorphic variants and risk of triple-negative breast cancer and age-related macular degeneration has also been indicated [49, 50].
The XRCC2 and XRCC3 genes are recognized as essential parts of the homologous recombination repair pathway. They are required for correct chromosome segregation and apoptotic response to DSB [51, 52]. A meta-analysis of data from available literature revealed no direct relationship between polymorphic variants rs3218536 of the XRCC2 gene and the risk of breast cancer [53]. However, the XRCC2 Arg188His polymorphism (c. 563G>A; rs3218536) is suggested to modify the risk of breast cancer, including triple-negative breast cancer [20, 54, 55]. A correlation was found between the prevalence of the XRCC2 gene rs3218536 polymorphism and morbidity of triple-negative breast cancer in the female population in Poland [55]. The XRCC2 gene rs3218536 polymorphism has been found to possibly increase the risk of developing cervical cancer and breast cancer in women in Pakistan [11, 56]. In contrast, no direct relation was observed between the occurrence of rs3218536 variant of the XRCC2 gene and the incidence of lung cancer or sporadic colorectal cancer [57, 58]. Similarly, no correlation has been found with hereditary colon cancer, known as Lynch syndrome [59].
The Thr241Met substitution is the most thoroughly analyzed polymorphism in the XRCC3 gene (c. 722C>T; rs861539). Although the functional relevance of the XRCC3 Thr241Met variation is unknown, some studies have reported that this polymorphism is associated with increased risk of breast cancer [20]. A meta-analysis has revealed that the XRCC3 gene rs861539 polymorphism also increases the risk of developing hepatocellular carcinoma, as well as head and neck, bladder, and breast cancer [11, 32, 33, 60–62]. However, this polymorphism has also been shown to have a weak association with the risk of breast cancer as well as lung cancer [63, 64] and no connection with the development of ovarian, cervical, or colorectal cancer or renal cell carcinoma [9, 65–67]. No direct relationship has been found between the XRCC3 gene rs861539 polymorphism and the risk of leukemia in the general population. However, studies performed in Asian populations have implicated it in the risk of incidence of leukemia [68].
The results of the present study show that the RAD51 gene rs1801320 polymorphism doubles the risk of prostate cancer in the studied population. Statistically significant differences in allele distribution were identified between the control group and prostate cancer patients (P < 0.05). Dhillon et al. [69] do not report any such correlation in an Australian population. Hence, the RAD51 gene rs1801320 polymorphism may act as an independent biomarker of prostate cancer risk in Polish population.
No associations were found between the polymorphic variants of the RAD51B, XRCC2, and XRCC3 genes evaluated in the present study and the susceptibility of prostate cancer. However, considering that the occurrence of cancer is the result of complex interactions between genetic changes and environmental factors, polymorphic variants of the RAD51B, XRCC2, and XRCC3 genes other than those studied may yet have an impact on the development of prostate cancer.
Supplementary Material
Associated with risk of prostate cancer the rs1801320 polymorphism of RAD51 gene was verified by sequencing analysis. PCR products of each genotype were sequenced according to the manufacturer's protocol using BigDye Terminator Cycle Sequencing Ready Reaction Kits version 1.1 in ABI PRISM 377™ DNA Sequencer (Applied Biosystems). Supplementary figure has shown results of sequencing of GG, GC, CC genotypes for the rs1801320 polymorphism in RAD51 gene.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Wojciechowska U., Didkowska J., Zatoński W. Cancer in Poland in 2012. Warsaw, Poland: Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Polish National Cancer Registry; 2014. [Google Scholar]
- 2.National Collaborating Centre for Cancer. Prostate Cancer: Diagnosis and Treatment. London, UK: National Institute for Health and Care Excellence (NICE); 2014. [Google Scholar]
- 3.Kitagishi Y., Kobayashi M., Matsuda S. Defective DNA repair systems and the development of breast and prostate cancer. International Journal of Oncology. 2013;42(1):29–34. doi: 10.3892/ijo.2012.1696. [DOI] [PubMed] [Google Scholar]
- 4.Serra H., Da Ines O., Degroote F., Gallego M. E., White C. I. Roles of XRCC2, RAD51B and RAD51D in RAD51-independent SSA recombination. PLoS Genetics. 2013;9(11) doi: 10.1371/journal.pgen.1003971.e1003971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun J., Buechelmaier E. S., Powell S. N. Rad51 paralog complexes BCDX2 and CX3 act at different stages in the BRCA1-BRCA2-dependent homologous recombination pathway. Molecular and Cellular Biology. 2013;33(2):387–395. doi: 10.1128/mcb.00465-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulíková S., Chmelařová M., Petera J., Palička V., Paulík A. Hypermethylation of RAD51L3 and XRCC2 genes to predict late toxicity in chemoradiotherapy-treated cervical cancer patients. Folia Biologica. 2013;59(6):240–245. [PubMed] [Google Scholar]
- 7.Shi S., Qin L., Tian M., et al. The effect of RAD51 135 G>C and XRCC2 G>A (rs3218536) polymorphisms on ovarian cancer risk among Caucasians: a meta-analysis. Tumor Biology. 2014;35(6):5797–5804. doi: 10.1007/s13277-014-1769-4. [DOI] [PubMed] [Google Scholar]
- 8.Cheng D., Shi H., Zhang K., Yi L., Zhen G. RAD51 gene 135G/C polymorphism and the risk of four types of common cancers: a meta-analysis. Diagnostic Pathology. 2014;9, article 18 doi: 10.1186/1746-1596-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupa R., Sliwinski T., Wisniewska-Jarosinska M., et al. Polymorphisms in RAD51, XRCC2 and XRCC3 genes of the homologous recombination repair in colorectal cancer—a case control study. Molecular Biology Reports. 2011;38(4):2849–2854. doi: 10.1007/s11033-010-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao L., Qian X. F., Yang G. H., Zhao L. D. Relationship between RAD51-G135C and XRCC3-C241T single nucleotide polymorphisms and onset of acute myeloid leukemia. Journal of Experimental Hematology. 2015;23(3):605–611. doi: 10.7534/j.issn.1009-2137.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi Z., Mahjabeen I., Baig R. M., Kayani M. A. Correlation between selected XRCC2, XRCC3 and RAD51 gene polymorphisms and primary breast cancer in women in Pakistan. Asian Pacific Journal of Cancer Prevention. 2014;15(23):10225–10229. doi: 10.7314/apjcp.2014.15.23.10225. [DOI] [PubMed] [Google Scholar]
- 12.Esfahani M., Ataei N., Panjehpour M. Biomarkers for evaluation of prostate cancer prognosis. Asian Pacific Journal of Cancer Prevention. 2015;16(7):2601–2611. doi: 10.7314/apjcp.2015.16.7.2601. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson L. R., Chen H H., Collins A. R., et al. Genomic instability in human cancer: molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Seminars in Cancer Biology. 2015 doi: 10.1016/j.semcancer.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krejci L., Altmannova V., Spirek M., Zhao X. Homologous recombination and its regulation. Nucleic Acids Research. 2012;40(13):5795–5818. doi: 10.1093/nar/gks270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.San Filippo J., Sung P., Klein H. Mechanism of eukaryotic homologous recombination. Annual Review of Biochemistry. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- 16.Walsh C. S. Two decades beyond BRCA1/2: homologous recombination, hereditary cancer risk and a target for ovarian cancer therapy. Gynecologic Oncology. 2015;137(2):343–350. doi: 10.1016/j.ygyno.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Antoniou A. C., Sinilnikova O. M., Simard J., et al. RAD51 135G→C modifies breast cancer risk among BRCA2 mutation carriers: results from a combined analysis of 19 studies. The American Journal of Human Genetics. 2007;81(6):1186–1200. doi: 10.1086/522611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasselbach L., Haase S., Fischer D., Kolberg H.-C., Stürzbecher H.-W. Characterisation of the promoter region of the human DNA-repair gene Rad51. European Journal of Gynaecological Oncology. 2005;26(6):589–598. [PubMed] [Google Scholar]
- 19.Chekravarti D., LaMorte V. J., Nelson M. C., et al. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383(6595):99–103. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 20.Romanowicz-Makowska H., Smolarz B., Zadrozny M., et al. The association between polymorphisms of the RAD51-G135C, XRCC2-Arg188His and XRCC3-Thr241Met genes and clinico-pathologic features in breast cancer in Poland. European Journal of Gynaecological Oncology. 2012;33(2):145–150. [PubMed] [Google Scholar]
- 21.Sun H., Bai J., Chen F., et al. RAD51 G135C polymorphism is associated with breast cancer susceptibility: a meta-analysis involving 22,399 subjects. Breast Cancer Research and Treatment. 2011;125(1):157–161. doi: 10.1007/s10549-010-0922-z. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B.-B., Wang D.-G., Xuan C., Sun G.-L., Deng K.-F. Genetic 135G/C polymorphism of RAD51 gene and risk of cancer: a meta-analysis of 28,956 cases and 28,372 controls. Familial Cancer. 2014;13(4):515–526. doi: 10.1007/s10689-014-9729-0. [DOI] [PubMed] [Google Scholar]
- 23.Smolarz B., Zadrozny M., Duda-Szymańska J., et al. RAD51 genotype and triple-negative breast cancer (TNBC) risk in Polish women. Polish Journal of Pathology. 2013;64(1):39–43. doi: 10.5114/PJP.2013.34602. [DOI] [PubMed] [Google Scholar]
- 24.Romanowicz-Makowska H., Smolarz B., Samulak D., et al. A single nucleotide polymorphism in the 5′ untranslated region of RAD51 and ovarian cancer risk in Polish women. European Journal of Gynaecological Oncology. 2012;33(4):406–410. [PubMed] [Google Scholar]
- 25.Smolarz B., Makowska M., Samulak D., et al. Association between polymorphisms of the DNA repair gene RAD51 and ovarian cancer. Polish Journal of Pathology. 2013;64(4):290–295. doi: 10.5114/pjp.2013.39338. [DOI] [PubMed] [Google Scholar]
- 26.Krupa R., Sobczuk A., Popławski T., Wozniak K., Blasiak J. DNA damage and repair in endometrial cancer in correlation with the hOGG1 and RAD51 genes polymorphism. Molecular Biology Reports. 2011;38(2):1163–1170. doi: 10.1007/s11033-010-0214-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michalska M. M., Samulak D., Romanowicz H., Smolarz B. Association of polymorphisms in the 5′ untranslated region of RAD51 gene with risk of endometrial cancer in the Polish population. Archives of Gynecology and Obstetrics. 2014;290(5):985–991. doi: 10.1007/s00404-014-3305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smolarz B., Samulak D., Michalska M., et al. 135G>C and 172G>T polymorphism in the 5′ untranslated region of RAD51 and sporadic endometrial cancer risk in Polish women. Polish Journal of Pathology. 2011;62(3):157–162. [PubMed] [Google Scholar]
- 29.Nissar S., Baba S. M., Akhtar T., Rasool R., Shah Z. A., Sameer A. S. RAD51 G135C gene polymorphism and risk of colorectal cancer in Kashmir. European Journal of Cancer Prevention. 2014;23(4):264–268. doi: 10.1097/cej.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 30.Romanowicz-Makowska H., Samulak D., Michalska M., et al. RAD51 gene polymorphisms and sporadic colorectal cancer risk in Poland. Polish Journal of Pathology. 2012;63(3):193–198. doi: 10.5114/pjp.2012.31505. [DOI] [PubMed] [Google Scholar]
- 31.Popławski T., Arabski M., Kozirowska D., et al. DNA damage and repair in gastric cancer—a correlation with the hOGG1 and RAD51 genes polymorphisms. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2006;601(1-2):83–91. doi: 10.1016/j.mrfmmm.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Kayani M. A., Khan S., Baig R. M., Mahjabeen I. Association of RAD51 135 G/C, 172 G/T and XRCC3 Thr241Met gene polymorphisms with increased risk of head and neck cancer. Asian Pacific Journal of Cancer Prevention. 2014;15(23):10457–10462. doi: 10.7314/apjcp.2014.15.23.10457. [DOI] [PubMed] [Google Scholar]
- 33.Śliwiński T., Walczak A., Przybyłowska K., et al. Polymorphisms of the XRCC3 C722T and the RAD51 G135C genes and risk of a head and neck cancer in a Polish population. Experimental and Molecular Pathology. 2010;89(3):358–366. doi: 10.1016/j.yexmp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 34.He Y.-Z., Hu X., Chi X.-S., et al. Association between RAD51 gene polymorphism (-135G/C) and susceptibility of myelodysplastic syndrome and acute leukemia: evidence based on a meta-analysis. Tumor Biology. 2014;35(1):615–621. doi: 10.1007/s13277-013-1085-4. [DOI] [PubMed] [Google Scholar]
- 35.Synowiec E., Wojcik K. A., Izdebska J., et al. Polymorphisms of the homologous recombination gene RAD51 in keratoconus and fuchs endothelial corneal dystrophy. Disease Markers. 2013;35(5):353–362. doi: 10.1155/2013/851817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadouri L., Kote-Jarai Z., Hubert A., et al. A single-nucleotide polymorphism in the RAD51 gene modifies breast cancer risk in BRCA2 carriers, but not in BRCA1 carriers or noncarriers. British Journal of Cancer. 2004;90(10):2002–2005. doi: 10.1038/sj.bjc.6601837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy-Lahad E., Lahad A., Eisenberg S., et al. A single nucleotide polymorphism in the RAD51 gene modifies cancer risk in BRCA2 but not BRCA1 carriers. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(6):3232–3236. doi: 10.1073/pnas.051624098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Synowiec E., Stefańska J., Morawiec Z., Blasiak J., Wozniak K. Association between DNA damage, DNA repair genes variability and clinical characteristics in breast cancer patients. Mutation Research. 2008;648(1-2):65–72. doi: 10.1016/j.mrfmmm.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., Li J.-L., He X.-F., et al. Association between the RAD51 135 G>C polymorphism and risk of cancer: a meta-analysis of 19,068 cases and 22,630 controls. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0075153.e75153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W. W., Spurdle A. B., Kolachana P., et al. A single nucleotide polymorphism in the 5′ untranslated region of RAD51 and risk of cancer among BRCA1/2 mutation carriers. Cancer Epidemiology, Biomarkers & Prevention. 2001;10(9):955–960. [PubMed] [Google Scholar]
- 41.Ribeiro Junior H. L., de Oliveira R. T., Maia A. R., et al. Polymorphisms of DNA repair genes are related to the pathogenesis of myelodysplastic syndrome. Hematological Oncology. 2014 doi: 10.1002/hon.2175. [DOI] [PubMed] [Google Scholar]
- 42.Auranen A., Song H., Waterfall C., et al. Polymorphisms in DNA repair genes and epithelial ovarian cancer risk. International Journal of Cancer. 2005;117(4):611–618. doi: 10.1002/ijc.21047. [DOI] [PubMed] [Google Scholar]
- 43.Kuschel B., Auranen A., McBride S., et al. Variants in DNA double-strand break repair genes and breast cancer susceptibility. Human Molecular Genetics. 2002;11(12):1399–1407. doi: 10.1093/hmg/11.12.1399. [DOI] [PubMed] [Google Scholar]
- 44.Lee K.-M., Choi J.-Y., Kang C., et al. Genetic polymorphisms of selected DNA repair genes, estrogen and progesterone receptor status, and breast cancer risk. Clinical Cancer Research. 2005;11(12):4620–4626. doi: 10.1158/1078-0432.ccr-04-2534. [DOI] [PubMed] [Google Scholar]
- 45.Michalska M. M., Samulak D., Romanowicz H., Smolarz B. Single nucleotide polymorphisms (SNPs) of RAD51-G172T and XRCC2-41657C/T homologous recombination repair genes and the risk of triple- negative breast cancer in Polish women. Pathology & Oncology Research. 2015 doi: 10.1007/s12253-015-9922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rollinson S., Smith A. G., Allan J. M., et al. RAD51 homologous recombination repair gene haplotypes and risk of acute myeloid leukaemia. Leukemia Research. 2007;31(2):169–174. doi: 10.1016/j.leukres.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Wadt K. A. W., Aoude L. G., Golmard L., et al. Germline RAD51B truncating mutation in a family with cutaneous melanoma. Familial Cancer. 2015;14(2):337–340. doi: 10.1007/s10689-015-9781-4. [DOI] [PubMed] [Google Scholar]
- 48.Shu X. O., Long J., Lu W., et al. Novel genetic markers of breast cancer survival identified by a genome-wide association study. Cancer Research. 2012;72(5):1182–1189. doi: 10.1158/0008-5472.can-11-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Figueroa J. D., Garcia-Closas M., Humphreys M., et al. Associations of common variants at 1p11.2 and 14q24.1 (RAD51l1) with breast cancer risk and heterogeneity by tumor subtype: findings from the Breast Cancer Association Consortium. Human Molecular Genetics. 2011;20(23):4693–4706. doi: 10.1093/hmg/ddr368.ddr368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou J., Wang D., Zhang J., et al. RAD51 gene is associated with advanced age-related macular degeneration in Chinese population. Clinical Biochemistry. 2013;46(16-17):1689–1693. doi: 10.1016/j.clinbiochem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Bashir N., Sana S., Mahjabeen I., Kayani M. A. Association of reduced XRCC2 expression with lymph node metastasis in breast cancer tissues. Familial Cancer. 2014;13(4):611–617. doi: 10.1007/s10689-014-9745-0. [DOI] [PubMed] [Google Scholar]
- 52.Song Y. Z., Han F. J., Liu M., et al. Association between single nucleotide polymorphisms in XRCC3 and radiation-induced adverse effects on normal tissue: a meta-analysis. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0130388.e0130388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu K.-D., Chen A.-X., Qiu L.-X., Fan L., Yang C., Shao Z.-M. XRCC2 Arg188His polymorphism is not directly associated with breast cancer risk: evidence from 37,369 subjects. Breast Cancer Research and Treatment. 2010;123(1):219–225. doi: 10.1007/s10549-010-0753-y. [DOI] [PubMed] [Google Scholar]
- 54.Silva S. N., Tomar M., Paulo C., et al. Breast cancer risk and common single nucleotide polymorphisms in homologous recombination DNA repair pathway genes XRCC2, XRCC3, NBS1 and RAD51. Cancer Epidemiology. 2010;34(1):85–92. doi: 10.1016/j.canep.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 55.Smolarz B., Makowska M., Samulak D., et al. Association between single nucleotide polymorphisms (SNPs) of XRCC2 and XRCC3 homologous recombination repair genes and triple-negative breast cancer in Polish women. Clinical and Experimental Medicine. 2015;15(2):151–157. doi: 10.1007/s10238-014-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pérez L. O., Crivaro A., Barbisan G., Poleri L., Golijow C. D. XRCC2 R188H (rs3218536), XRCC3 T241M (rs861539) and R243H (rs77381814) single nucleotide polymorphisms in cervical cancer risk. Pathology & Oncology Research. 2013;19(3):553–558. doi: 10.1007/s12253-013-9616-2. [DOI] [PubMed] [Google Scholar]
- 57.Curtin K., Lin W.-Y., George R., et al. Genetic variants in XRCC2: new insights into colorectal cancer tumorigenesis. Cancer Epidemiology Biomarkers and Prevention. 2009;18(9):2476–2484. doi: 10.1158/1055-9965.epi-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hung R. J., Christiani D. C., Risch A., et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiology, Biomarkers and Prevention. 2008;17(11):3081–3089. doi: 10.1158/1055-9965.epi-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reeves S. G., Meldrum C., Groombridge C., et al. DNA repair gene polymorphisms and risk of early onset colorectal cancer in Lynch syndrome. Cancer Epidemiology. 2012;36(2):183–189. doi: 10.1016/j.canep.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Costa S., Pinto D., Pereira D., et al. DNA repair polymorphisms might contribute differentially on familial and sporadic breast cancer susceptibility: a study on a Portuguese population. Breast Cancer Research and Treatment. 2007;103(2):209–217. doi: 10.1007/s10549-006-9364-z. [DOI] [PubMed] [Google Scholar]
- 61.Luo H.-C., Zhang H.-B., Xin X.-J., Huang W.-X. Haplotype-based case-control study of DNA repair gene XRCC3 and hepatocellular carcinoma risk in a Chinese population. Tumor Biology. 2014;35(4):3415–3419. doi: 10.1007/s13277-013-1451-2. [DOI] [PubMed] [Google Scholar]
- 62.Ma Q., Zhao Y., Wang S., et al. Genetic polymorphisms of XRCC3 Thr241Met (C18067T, rs861539) and bladder cancer risk: a meta-analysis of 18 research studies. Tumor Biology. 2014;35(2):1473–1480. doi: 10.1007/s13277-013-1203-3. [DOI] [PubMed] [Google Scholar]
- 63.Ding P., Yang Y., Cheng L., et al. The relationship between seven common polymorphisms from five DNA repair genes and the risk for breast cancer in northern Chinese women. PLoS ONE. 2014;9(3) doi: 10.1371/journal.pone.0092083.e92083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hung R. J., Christiani D. C., Risch A., et al. International lung cancer consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiology Biomarkers and Prevention. 2008;17(11):3081–3089. doi: 10.1158/1055-9965.epi-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Margulis V., Lin J., Yang H., Wang W., Wood C. G., Wu X. Genetic susceptibility to renal cell carcinoma: the role of DNA double-strand break repair pathway. Cancer Epidemiology Biomarkers and Prevention. 2008;17(9):2366–2373. doi: 10.1158/1055-9965.epi-08-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pearce C. L., Near A. M., van den Berg D. J., et al. Validating genetic risk associations for ovarian cancer through the international Ovarian Cancer Association Consortium. British Journal of Cancer. 2009;100(2):412–420. doi: 10.1038/sj.bjc.6604820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pérez L. O., Crivaro A., Barbisan G., Poleri L., Golijow C. D. XRCC2 R188H (rs3218536), XRCC3 T241M (rs861539) and R243H (rs77381814) single nucleotide polymorphisms in cervical cancer risk. Pathology and Oncology Research. 2013;19(3):553–558. doi: 10.1007/s12253-013-9616-2. [DOI] [PubMed] [Google Scholar]
- 68.Yan Y., Liang H., Li T., et al. Association of XRCC3 Thr241Met polymorphism and leukemia risk: evidence from a meta-analysis. Leukemia and Lymphoma. 2014;55(9):2130–2134. doi: 10.3109/10428194.2013.853303. [DOI] [PubMed] [Google Scholar]
- 69.Dhillon V. S., Phil M., Yeoh E., Fenech M. DNA repair gene polymorphisms and prostate cancer risk in South Australia—results of a pilot study. Urologic Oncology: Seminars and Original Investigations. 2011;29(6):641–646. doi: 10.1016/j.urolonc.2009.08.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associated with risk of prostate cancer the rs1801320 polymorphism of RAD51 gene was verified by sequencing analysis. PCR products of each genotype were sequenced according to the manufacturer's protocol using BigDye Terminator Cycle Sequencing Ready Reaction Kits version 1.1 in ABI PRISM 377™ DNA Sequencer (Applied Biosystems). Supplementary figure has shown results of sequencing of GG, GC, CC genotypes for the rs1801320 polymorphism in RAD51 gene.


