Abstract
Hypothesis. Cancer patients frequently suffer from poor sleep quality. Bryophyllum pinnatum is a herbal medication used in anthroposophic medicine, which has been shown to be associated with improvements in sleep quality during pregnancy with only few and minor or moderate side-effects reported. In this study, the sleep quality of cancer patients during treatment with B pinnatum was investigated. Study Design. In this prospective, observational study, cancer patients suffering from sleep problems were treated with B pinnatum (350 mg tablets, corresponding to 50% of leaf pressed juice [Weleda AG, Arlesheim, Switzerland], dosage at physician’s consideration, but most frequently 2 tablets with evening meal and 2 before going to bed). Methods. Sleep quality (Pittsburgh Sleep Quality Index [PSQI]), daily sleepiness (Epworth Sleeping Scale [ESS]), and fatigue (Fatigue Severity Scale [FSS]) were assessed at the beginning of the treatment and after 3 weeks. Possible adverse drug reactions perceived by the patients during the treatment were recorded. From the 28 recruited patients, 20 completed both questionnaires and were considered in the present analysis. Data are expressed as mean ± standard deviation. Results. Patients were 61 ± 10.4 years old and the majority were female (17 out of 20). During treatment with B pinnatum, the PSQI decreased from 12.2 ± 3.62 to 9.1 ± 3.61 (P < .01), and ESS changed from 8.4 ± 3.18 to 7.1 ± 3.98 (P < .05). There was no change in FSS. The treatment was well tolerated by the majority of patients, with only 6 patients reporting discomfort that might have been caused by B pinnatum (fatigue n = 3, dry throat n = 1, agitation n = 1, difficult digestion n = 1). No serious adverse drug reactions were detected. Conclusion. B pinnatum may be a suitable treatment for sleep problems of cancer patients. Controlled, randomized clinical trials of the use of B pinnatum in sleep disorders are urgently needed.
Keywords: cancer, Bryophyllum pinnatum, Kalanchoe pinnata, sleep, sleep quality, sleep problems
Introduction
Cancer patients suffer frequently from poor sleep quality, which is often associated with worries, coping with illness, pain, and treatment side effects.1-4 Among the various cancer types, breast cancer and lung cancer seem to lead most often to sleep disturbance, insomnia, and fatigue.1,4 Whereas some sleep problems are directly associated with the disease, others derive from recent or current anticancer therapies. While or after undergoing cancer therapies, the most common symptoms cancer patients experience include excessive fatigue, leg restlessness, insomnia, and increased sleepiness.1 Suffering from sleep problems affects both physical and mental components of quality of life. The observed association between insomnia and sedative/hypnotic use4 suggests that the standard medications are not effective enough. This and the fact that cancer patients often take several medications, some of which are associated with numerous and strong side-effects, makes the search for new, effective, and well-tolerated medications for sleeping problems worthwhile.
Bryophyllum pinnatum (Lamarck) Oken [syn.: Kalanchoe pinnata (Lamarck) Persoon, syn.: Bryophyllum calycinum Salisbury] is a plant of the family Crassulaceae. It is originally from Madagascar but can now be found across tropical Africa, tropical America, India, China, and Australia.5 The plant is known under various common names, such as life plant, air plant, love plant, Cathedral bells, and Goethe plant. Leaf extracts from B pinnatum have been used in traditional medicine of the regions where it grows and are suggested to have the following properties: sedative, central nervous system depressant, anticancer, analgesic, antipyretic, muscle relaxant, gastroprotective, anti-inflammatory, antiseptic, anti-allergic, anti-anaphylactic, immunomodulating, and wound healing.5-10 Until recently, evidence for the sedative effects was exclusively preclinical, with different fractions shown to potentiate pentobarbitone-induced sleeping time in in vivo models, that is, to possess a depressant action on the central nervous system.11,12 The anticancer potential of B pinnatum has been demonstrated by a study showing that crude leaf extracts inhibit in vitro growth of human cervical cancer cells and induce apoptosis.10 Its safety was recently supported by a study showing that at relatively high concentrations, both aqueous and ethanolic B pinnatum leaf extracts can inhibit N-diethylnitrosamine-induced hepatic injury in rats.13
In the 1920s, in Europe, B pinnatum started to be used in anthroposophic medicine as a treatment for “hysteria.”14 A half century later, B pinnatum was introduced in obstetrics at the anthroposophic Herdecke Community Hospital (Germany) for the treatment of premature labor.15 Subsequently, several experimental and clinical studies corroborated its tocolytic effects and revealed the corresponding mechanism of action.16-21 More recently, B pinnatum was shown to have potential in the treatment of overactive bladder.22,23 A characterization of the B pinnatum prescribing pattern among a network of anthroposophic physicians revealed sleep disorders to be one of the most frequent diagnoses for which it is prescribed.24 A first observational study on pregnant women confirmed that improvements in sleep quality can be observed during treatment with B pinnatum.25 This patient group was chosen because pregnant women have poorer sleep quality than nonpregnant women,26 while treatment with sedatives is particularly problematic during pregnancy due to possible undesirable effects for mother and embryo/fetus. Irrespective of the indication (preterm contractions,18 overactive bladder,23 and sleep problems during pregnancy25), all clinical studies documented a good tolerability of B pinnatum.
This favorable tolerability, the previous observational study on pregnant women, and the particular vulnerabilities of cancer patients prompted us to conduct the present observational, prospective study on the treatment of cancer-related sleeping problems with B pinnatum. The suspected anticancer properties of B pinnatum preparations were not addressed in the present study; they are however reassuring inasmuch as a possible disadvantageous impact of the extracts on tumor progression can be ruled out.
Materials and Methods
Study Design
This prospective, observational study was authorized by the ethical committee of canton of Zurich as well as by the Swiss Agency for Therapeutic Products and was performed according to good clinical practice. Written informed consent was obtained from all study participants. Patients of the Paracelsus-Hospital Richterswil were included in the study if (a) they were outpatients; (b) their age was between 18 and 80 years; (c) they had an oncological disease; (d) their Eastern Cooperative Oncology Group (ECOG) index was 0, 1, or 2 (ie, patients could be up more than 50% of waking hours); (e) they had been prescribed B pinnatum 50% (chewable tablets 350 mg, dosage depending on physician’s prescription) for at least 21 consecutive days for sleep disorders or changes in sleep behavior perceived by the patients as being associated with the cancer disease; and (f) they had basic knowledge of German. Excluded from this study were cancer patients who did not fulfill the inclusion criteria or (a) had received treatment for their sleep problems before the onset of the cancer disease; (b) had an alcohol dependence and/or consumed intoxicants; (c) or presented with acute impairment of consciousness, acute psychosis, or dementia; or (d) were under acute life-threatening conditions. At the beginning of the study, an additional exclusion criterion was treatment with soporifics (affecting 11 patients); after an authorized amendment to the study protocol, this criterion was replaced by a change in the dosage of medication likely to affect sleep quality, either during the 3 weeks before the study started or during the study period.
By analogy with the previous observational study on pregnant women, the study had been designed to include 50 patients.25 Due to the slow recruitment rate, however, the study was stopped when 40% of the patients had finished the study. An analysis of the main outcomes was performed and the data are presented here.
Study Medication
Participants were treated with B pinnatum (chewable tablets 350 mg, 50% leaf pressed juice on lactose, corresponding to 175 mg of fresh plant per tablet) based on a clinical indication. The dosage depended on the physician’s prescription (3-8 tablets per day). The tablets were manufactured by Weleda AG, Arlesheim, Switzerland, with B pinnatum plants provided by Weleda, Brazil, and are registered at the Swiss Agency for Therapeutic Products (without indication). A voucher specimen of B pinnatum plants provided by Weleda, Brazil (No. ZSS 29717) has been deposited at the Zurich Succulent Plant Collection, Switzerland. Previous analytical work on the pressed juice of fresh or fresh frozen B pinnatum leaves, which are routinely used to manufacture B pinnatum chewable tablets, revealed the presence of flavonoid glycosides as major constituents, and several bufadienolides as minor components.27
Measurements
A detailed questionnaire was completed by the participants on day 1 (before treatment with B pinnatum) and on day 22 (at the end of 21 days of treatment). At each occasion, it addressed the 2 previous weeks and contained the Pittsburgh Sleep Quality Index (PSQI) to assess sleep quality,28,29 the Epworth Sleeping Scale (ESS),30 and the Fatigue Severity Scale (FSS).31 In addition, patients were asked to specify possible discomforts that they believed to be caused by B pinnatum.
The PSQI index was developed as a self-rated questionnaire to characterize sleep quality, and the corresponding values can vary between 0 and 21, with higher values representing worse sleep quality, whereby a cutoff of >5 has considerable diagnostic sensitivity (89.6%) and specificity (86.5%) in distinguishing good and poor sleepers.28 The PSQI index comprises the following components: “sleep quality” (Comp1), “sleep latency” (Comp2), “sleep duration” (Comp3), “habitual sleep efficiency” (Comp4), “sleep disturbance” (Comp5), “use of sleeping medication” (Comp6), and “daytime dysfunction” (Comp7).
Statistics
The statistical analysis was performed in SPSS for Windows, Version 19; differences between the scores before and at the end of treatment were compared with the t test for paired samples if the data had a normal distribution (ie, Z-score for both skewness and kurtosis ≤1.96), otherwise using the Wilcoxon signed rank test. Differences were considered to be statistically significant if P ≤ .05. Data are expressed as mean ± standard deviation (SD). For each question, the total number of answers (n) is shown, if different from 20.
Results
From February 2013 until March 2014, 28 patients were recruited, of whom 21 finished the study. The reasons for study dropout were the following: therapy stopped (n = 4; in 2 cases due to lack of compliance; in the others, because curative therapies were seen as more important), therapy had been carried out but the questionnaires had not been completed (n = 2, due to lack of time and lost questionnaire), and unknown (n = 1). Of the 21 participants who finished the study, one was excluded because more than 67% of the questions of the second questionnaire had not been answered. Hence, data from 20 patients could be evaluated. All 20 evaluated patients filled in the questionnaire at the beginning of the treatment and 3 weeks later.
The main demographic characteristics and disease characteristics of these patients are depicted in Table 1. The mean patient age was 61 years. The majority lived with a partner, were of female gender, were postmenopausal, exhibited a normal body mass index, had additional diseases (other than cancer), and were taking other medications. All patients complained of sleep disorders or changes in sleep behavior (one of the inclusion criteria), which were mostly long-lasting (2 years on average, median 1 year). The severity of their sleep problems translated into relatively high PSQI values at study beginning (12 ± 3.6 in a 0 to 21 scale; Figure 1; see Discussion) that in all but one case (5; ie, borderline) were above the cutoff value used to define poor sleepers (>5). Concerning the malignant disease, most patients were suffering from breast cancer, were in remission, and had a favorable ECOG performance status. The cancer disease had been diagnosed on average 5 ± 6.2 years previously (median 2 years). Most patients had undergone several cancer-related therapies in the past (more than 1 month before the study); in the month prior to the study as well as during the study, mistletoe therapy was the most frequently mentioned therapy.
Table 1.
Patient Characteristics and Reported Discomforts Possibly Caused by Bryophyllum pinnatuma.
| N (or mean ± SD) | |
|---|---|
| Age (years) | 61 ± 10.4 |
| Female patients | 17 |
| Household structure | 14 |
| Lives alone | 6 |
| Lives with partner | 10 |
| Lives with partner and children | 3 |
| Lives with a daughter | 1 |
| BMI (n = 17) | 20 ± 4.8 |
| Hormonal situation (women only) | |
| Premenopausal | 2 |
| Perimenopausal | 1 |
| Postmenopausal | 14 |
| NK | 3 |
| Duration of sleep problems (n = 17, in years) | 2 ± 2.1b |
| Cancer type | |
| Breast | 12 |
| Prostate | 2 |
| Colon | 2 |
| Lung | 1 |
| Uterus sarcoma | 1 |
| Oral | 1 |
| Hairy cell leukemia | 1 |
| Cancer disease activity | |
| Complete remission | 10 |
| Partial remission | 2 |
| Small change | 3 |
| Progression | 2 |
| ECOG-Karnofsky index | |
| 0 | 16 |
| 1 | 4 |
| Time since cancer diagnosis (years) | 5 ± 6.2 |
| Anticancer therapies (before last month) | |
| Surgery | 14 |
| Chemotherapy | 7 |
| Radiation therapy | 3 |
| Hormonal therapy | 7 |
| Mistletoe therapy | 13 |
| Hyperthermia | 5 |
| Anticancer therapies (last month) | |
| Surgery | 1 |
| Chemotherapy | 3 |
| Radiation therapy | 3 |
| Hormonal therapy | 3 |
| Mistletoe therapy | 11 |
| Hyperthermia | 5 |
| Anticancer therapies (during the study) | |
| Surgery | 1 |
| Chemotherapy | 3 |
| Radiation therapy | 1 |
| Hormonal therapy | 6 |
| Mistletoe therapy | 16 |
| Hyperthermia | 4 |
| Additional diseases | 13 |
| Hypertonia | 6 |
| Psychological diseases | 4 |
| Allergies | 5 |
| Others | 4 |
| Current additional medications | 15 |
| High blood pressure medications | 6 |
| Cholesterol-lowering drugs | 3 |
| Soporifics | 3 |
| Analgesics | 2 |
| Others | 11 |
| Discomforts possibly caused by B pinnatum |
6 |
| Fatigue | 3 |
| Dry throat | 1 |
| Agitation | 1 |
| Difficult digestion | 1 |
Abbreviations: NK, not known; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group index.
N = 20; except when indicated.
One patient answered “several weeks,” 2 patients wrote “several years”; for the calculations, “several” was replaced by “2”; median was 1 year.
Figure 1.
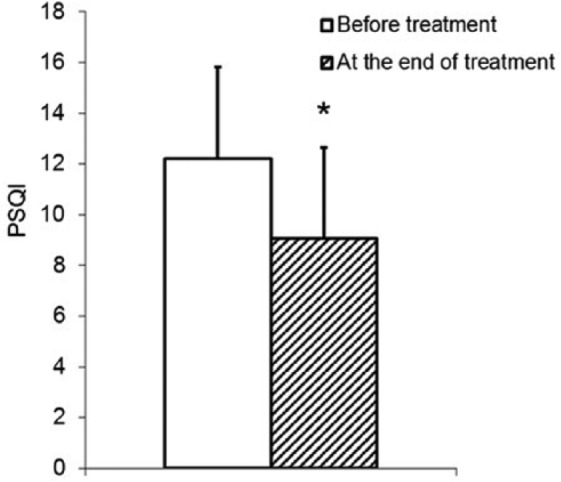
Improvement in sleep quality of cancer patients as assessed by the Pittsburgh Sleep Quality Index (PSGI; P = .002) during treatment with B pinnatum for 3 weeks.
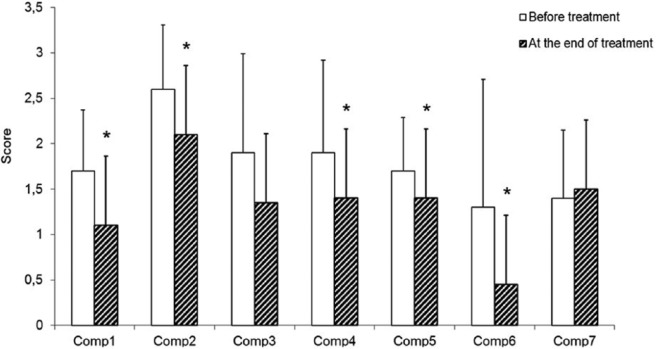
During treatment with B pinnatum, sleep quality of the participating cancer patients improved markedly, inasmuch as a statistically significant reduction of PSQI was observed (from 12.2 ± 3.62 down to 9.1 ± 3.61, P = .002; Figure 1). It should be kept in mind that the higher the values of the global PSQI (and of the corresponding components), the worse the sleep quality. The number of patients below the cutoff for poor sleepers (ie, PSQI >5) increased from 1 at study beginning (PSQI = 5) to 3 (PSQI values of 4, 3, and 3). When the different PSQI components were compared at the beginning and at the end of the treatment (Figure 2), improvements of subjective sleep quality (1.7 ± 0.67 vs 1.1 ± 0.79, P = .008), sleep latency (2.6 ± 0.71 vs 2.1 ± 0.75, P = .024), habitual sleep efficiency (1.9 ± 1.02 vs 1.4 ± 1.04, P = .045), sleep disturbance (1.7 ± 0.59 vs 1.4 ± 0.59, P = .014), and a reduction in the use of sleeping medication (1.3 ± 1.41 vs 0.45 ± 1.10, P = .035) were detected. A tendency for an improvement was observed in the case of sleep duration (1.9 ± 1.09 vs 1.35 ± 1.19, P = .160). No significant alteration was detected in daytime dysfunction (1.4 ± 0.75 vs 1.5 ± 0.76, P = .739).
Figure 2.
Most components of the Pittsburgh Sleep Quality Index improved during treatment of cancer patients with B pinnatum for 3 weeks.
ESS analysis revealed a statistically significant decrease in mean daily sleepiness (Figure 3; P = .048). On the other hand, no alteration in fatigue could be observed during the study period (36.6 ± 11.75 at the treatment start vs 36.2 ± 12.56 at the end of treatment, P = .802).
Figure 3.
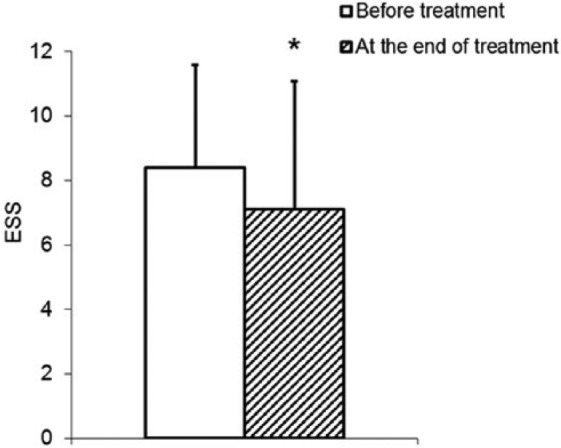
Reduction of daily sleepiness (Epworth Sleeping Scale [ESS], P = .048) of cancer patients during treatment with B pinnatum for 3 weeks.
Although the treating physicians were free to decide on the medication dosage, in most cases (n = 17), the patients had been instructed to take 2 B pinnatum tablets, twice daily (with the evening meal and when going to bed). A few discomforts, all of minor severity, were reported by patients to be possibly caused by the study medication (see Table 1). They consisted of fatigue, dry throat, agitation, and difficult digestion. No severe adverse reactions were reported by any patient at any time. One patient in the perimenopausal period reported personally on her own initiative a clear-cut improvement in hot flashes.
Discussion
Some of the symptoms triggered by a cancer disease and initial treatment, for example, sleep disturbances and fatigue, are long-lasting, with cancer survivors experiencing them even more than 10 years thereafter.2 In the present study, patients had become aware of their cancer disease on average 5 years (median 2 years) before entering the study, and their sleep disturbances were mostly long-lasting (2 years on average, median 1 year) and strong. Indeed, the average baseline PSQI of 12 is relatively high in comparison with values from other studies (often 8-932-35 is reported among breast cancer patients, or 8 for elderly patients36), rather corresponding to the value initially found among depressive patients.28 In the present study, the majority of patients were not undergoing invasive anticancer therapies—surgery, chemotherapy, and radiotherapy—during or in the month before the study, that is, the short-term effects of such therapies were unlikely to be affecting day-to-day well-being or activity.
During treatment with B pinnatum, a statistically significant (P = .002) improvement of sleep quality as assessed by the PSQI was observed. This averaged index decreased from 12 to 9 during treatment, which is likely to be clinically relevant, even though most patients did not attain values characteristic of good sleepers at the end of the study. Whether a 3-point improvement renders the sleep problems of cancer patients such as those of the present study bearable or whether additional treatments should be followed requires further investigation. However, a 3-point improvement compares well with differences of 1 to 1.5 points after 4 weeks of (electro)acupuncture for breast cancer patients34,37 and is only slightly inferior to the decrease in 4 points during acupuncture that was observed among elderly patients.36 A previous study on the effect of mindfulness-based stress reduction on subjective and objective sleep parameters in women with breast cancer revealed only a tendency for a reduction of approximately 0.6 in PSQI, which was however inferior to the improvement detected by objective sleep parameters.33
In the present study, all PSQI components but one—daytime dysfunction—were higher at study beginning than after 3 weeks of treatment, suggesting a manifold improvement. The present observations corroborate the results obtained in the former prospective clinical study about the effect of B pinnatum on sleep quality during pregnancy.25 Here, significant improvements in subjective sleep quality and a reduction in sleep disturbances were detected. Taken together, the 2 studies indicate that B pinnatum is effective for multiple types of sleep problems. The improvements in PSQI observed here are highly statistically significant even though the number of patients was smaller than initially planned and in comparison with the previous study (20 vs 50). This suggests that the improvements were more clear-cut than initially expected.
Cancer patients suffer not only from sleep problems at night but also from daily sleepiness and fatigue.1 The statistically significant ESS reduction toward a value similar to the average obtained with healthy subjects (6 ± 2.230) that was observed here suggests that B pinnatum might be helpful in this respect as well. Comparable results were obtained in a previous study with pregnant women.25 Also as in this previous study, no improvement in fatigue was detected with the FSS, an observation that is corroborated by the lack of change in the PSQI component daytime dysfunction. These results must be interpreted in view of the study population, which at treatment beginning exhibited initial FSS scores only slightly higher than those typical for healthy subjects, that is, up to 36.31 Furthermore, the lack of change in average fatigue and daytime dysfunction suggests that the 3 cases of patients mentioning fatigue as a discomfort that might have been caused by B pinnatum should be interpreted with caution. Indeed, only in one of these cases were concomitant increases in FSS and in daytime dysfunction during the study period detected (data not shown). The discomforts mentioned might reflect patients’ own subjective perceptions (particularly comprehensible in the course of malignant diseases), which might be associated with the (expected) sedative and/or muscle relaxing effect of the study medication.11,12
The main limitation of this study is the lack of a control group and patient randomization, which prevents the demonstration of a causal relationship between intake of B pinnatum and improvement of sleep quality. However, improvements were observed in most but not all measured parameters, suggesting that the patients were answering the questions truthfully and not merely trying to fulfill the researchers’ expectations. Since in most cases patients had been aware of their cancer disease for a considerable period and the sleep problems mentioned were long-lasting, one can assume that patients’ situation was relatively stable and spontaneous improvements were unlikely.
Herbal medications, such as those prepared from B pinnatum, are often multitarget medicines with a pleiotropic effect profile.38 Accordingly, B pinnatum preparations are frequently prescribed as a treatment for organic and nonorganic sleep disorders, but also for several other diagnoses.24 To a certain extent, some of these—depressions, anxiety disorders, reactions to severe stress, and adjustment disorders, symptoms, and signs involving emotional state as well as menopausal and other perimenopausal disorders—might have been pertinent to the outcomes of the present study. In other words, possible improvements in some of these (co)diagnoses might have contributed to the observed improvements in sleep quality and daily sleepiness. Whereas this multitude of possible indications might shed some doubts on which might be the exact condition that was most improved during treatment with B pinnatum, it can be clinically advantageous. The example of the patient who took the initiative of reporting an improvement in hot flashes illustrates this pragmatic perspective.
Conclusion
The present work shows that the sleep quality of cancer patients significantly improved under treatment with B pinnatum and that this medication was well tolerated in this clinical context. The significant and additionally observed favorable effect of B pinnatum on daytime sleepiness may be associated with the improvement in sleep quality and turned out to be independent of fatigue, which did not change during the study. No serious adverse event occurred during treatment.
Acknowledgments
We express our gratitude to the participating patients and thank the outpatient clinic Paracelsus-Hospital Richterswil for the opportunity to perform this study. We thank Claudia Lambrigger-Steiner, who contributed to the elaboration of the study documents and Heather Murray for language corrections. Weleda AG (Arlesheim, Switzerland) provided the Bryophyllum pinnatum tablet medication free of charge to the study participants. Financial support by the Johannes Kreyenbühl Foundation and by Weleda AG is gratefully acknowledged.
Footnotes
Authors’ Note: In addition to A. P. Simões-Wüst, PhD, K. Fürer, PhD, and U. von Mandach, PhD, the Bryophyllum Collaborative Group includes C. Betschart, MD, R. Brenneisen, PhD, M. Hamburger, PhD, M. Mennet, PhD, O. Potterat, PhD, and M. Schnelle, MD.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ana Paula Simões-Wüst and Ursula von Mandach received over the last 5 years occasional research funds from the Weleda AG, the producer of Bryophyllum pinnatum preparations.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Financial support by the Johannes Kreyenbühl Foundation and by Weleda AG.
References
- 1. Parish JM. Sleep-related problems in common medical conditions. Chest. 2009;135:563-572. [DOI] [PubMed] [Google Scholar]
- 2. Harrington CB, Hansen JA, Moskowitz M, Todd BL, Feuerstein M. It’s not over when it’s over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatry Med. 2010;40:163-181. [DOI] [PubMed] [Google Scholar]
- 3. Sateia MJ, Lang BJ. Sleep and cancer: recent developments. Curr Oncol Rep. 2008;10:309-318. [DOI] [PubMed] [Google Scholar]
- 4. Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309-1321. [DOI] [PubMed] [Google Scholar]
- 5. Kamboj A, Saluja A. Bryophyllum pinnatum (Lam.) Kurz.: phytochemical and pharmacological profile: a review. Pharmacognosy Rev. 2009;3:364-374. [Google Scholar]
- 6. El Abdellaoui S, Destandau E, Toribio A, et al. Bioactive molecules in Kalanchoe pinnata leaves: extraction, purification, and identification. Anal Bioanal Chem. 2010;398:1329-1338. [DOI] [PubMed] [Google Scholar]
- 7. Nayak BS, Marshall JR, Isitor G. Wound healing potential of ethanolic extract of Kalanchoe pinnata Lam. Leaf—a preliminary study. Indian J Exp Biol. 2010;48:572-576. [PubMed] [Google Scholar]
- 8. Kamgang R, Mboumi RY, Fondjo AF, Tagne MA, N’Dille GP, Yonkeu JN. Antihyperglycaemic potential of the water-ethanol extract of Kalanchoe crenata (Crassulaceae). J Nat Med. 2008;62:34-40. [DOI] [PubMed] [Google Scholar]
- 9. Ojewole JA. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J Ethnopharmacol. 2005;99:13-19. [DOI] [PubMed] [Google Scholar]
- 10. Mahata S, Maru S, Shukla S, et al. Anticancer property of Bryophyllum pinnata (Lam.) Oken. leaf on human cervical cancer cells. BMC Complement Altern Med. 2012;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pal S, Sen T, Chaudhuri AK. Neuropsychopharmacological profile of the methanolic fraction of Bryophyllum pinnatum leaf extract. J Pharm Pharmacol. 1999;51:313-318. [DOI] [PubMed] [Google Scholar]
- 12. Yemitan OK, Salahdeen HM. Neurosedative and muscle relaxant activities of aqueous extract of Bryophyllum pinnatum. Fitoterapia. 2005;76:187-193. [DOI] [PubMed] [Google Scholar]
- 13. Afzal M, Kazmi I, Anwar F. Antineoplastic potential of Bryophyllum pinnatum Lam. on chemically induced hepatocarcinogenesis in rats. Pharmacognosy Res. 2013;5:247-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daems W. Kurzgefsste Byophyllum-Chronologie. Korrespondenzblätter für Ärzte, Arlesheim. 1982;105. [Google Scholar]
- 15. Hassauer W, Schreiber K, Von der Decken D. Ein neuer Weg in der tokolytischen Therapie. Erfahrungsheikunde. 1985;34:683-687. [Google Scholar]
- 16. Daub E. Vorzeitige Wehentätigkeit. Ihre Behandlung mit pflanzlichen Substanzen, eine klinische Studie. Stuttgart, Germany: Urachhaus; 1989. [Google Scholar]
- 17. Vilaghy I. Decreasing the rate of premature delivery with phytotherapy—results from general practice. Ther Umsch. 2002;59:696-701. [DOI] [PubMed] [Google Scholar]
- 18. Plangger N, Rist L, Zimmermann R, von Mandach U. Intravenous tocolysis with Bryophyllum pinnatum is better tolerated than beta-agonist application. Eur J Obstet Gynecol Reprod Biol. 2006;124:168-172. [DOI] [PubMed] [Google Scholar]
- 19. Gwehenberger B, Rist L, Huch R, von Mandach U. Effect of Bryophyllum pinnatum versus fenoterol on uterine contractility. Eur J Obstet Gynecol Reprod Biol. 2004;113:164-171. [DOI] [PubMed] [Google Scholar]
- 20. Wächter R, Brenneisen R, Hamburger M, et al. Leaf press juice from Bryophyllum pinnatum (Lamarck) Oken induces myometrial relaxation. Phytomedicine. 2011;19:74-82. [DOI] [PubMed] [Google Scholar]
- 21. Simões-Wüst AP, Grãos M, Duarte CB, et al. Juice of Bryophyllum pinnatum (Lam.) inhibits oxytocin-induced increase of the intracellular calcium concentration in human myometrial cells. Phytomedicine. 2010;17:980-986. [DOI] [PubMed] [Google Scholar]
- 22. Schuler V, Suter K, Fürer K, et al. Bryophyllum pinnatum inhibits detrusor contractility in porcine bladder strips—a pharmacological study towards a new treatment option of overactive bladder. Phytomedicine. 2012;19:947-951. [DOI] [PubMed] [Google Scholar]
- 23. Betschart C, von Mandach U, Seifert B, et al. Randomized, double-blind placebo-controlled trial with Bryophyllum pinnatum versus placebo for the treatment of overactive bladder in postmenopausal women. Phytomedicine. 2012;20:351-358. [DOI] [PubMed] [Google Scholar]
- 24. Simões-Wüst AP, Jeschke E, Mennet M, Schnelle M, Matthes H, von Mandach U. Prescribing pattern of Bryophyllum preparations among a network of anthroposophic physicians. Forsch Komplementmed. 2012;19:293-301. [DOI] [PubMed] [Google Scholar]
- 25. Lambrigger-Steiner C, Simões-Wüst AP, Kuck A, Fürer K, Hamburger M, von Mandach U. Sleep quality in pregnancy during treatment with Bryophyllum pinnatum: an observational study. Phytomedicine. 2013;21:753-757. [DOI] [PubMed] [Google Scholar]
- 26. Brunner DP, Munch M, Biedermann K, Huch R, Huch A, Borbely AA. Changes in sleep and sleep electroencephalogram during pregnancy. Sleep. 1994;17:576-582. [DOI] [PubMed] [Google Scholar]
- 27. Fürer K, Raith M, Brenneisen R, et al. Two new flavonol glycosides and a metabolite profile of Bryophyllum pinnatum, a phytotherapeutic used in obstetrics and gynaecology. Planta Med. 2013;79:1565-1571. [DOI] [PubMed] [Google Scholar]
- 28. Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 29. Monk TH, Reynolds CF, Kupfer DJ, et al. The Pittsburgh Sleep Diary. J Sleep Res. 1994;3:111-120. [PubMed] [Google Scholar]
- 30. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540-545. [DOI] [PubMed] [Google Scholar]
- 31. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121-1123. [DOI] [PubMed] [Google Scholar]
- 32. Khoramirad A, Mousavi M, Dadkhahtehrani T, Pourmarzi D. Relationship between sleep quality and spiritual well-being/religious activities in Muslim women with breast cancer [published online December 9, 2014]. J Relig Health. doi: 10.1007/s10943-014-9978-0. [DOI] [PubMed] [Google Scholar]
- 33. Lengacher CA, Reich RR, Paterson CL, et al. The effects of mindfulness-based stress reduction on objective and subjective sleep parameters in women with breast cancer: a randomized controlled trial [published online June 18, 2014]. Psycho-oncology. doi: 10.1002/pon.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bao T, Cai L, Snyder C, et al. Patient-reported outcomes in women with breast cancer enrolled in a dual-center, double-blind, randomized controlled trial assessing the effect of acupuncture in reducing aromatase inhibitor-induced musculoskeletal symptoms. Cancer. 2014;120:381-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ancoli-Israel S, Liu L, Rissling M, et al. Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study. Support Care Cancer. 2014;22:2535-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zuppa C, Prado CH, Wieck A, Zaparte A, Barbosa A, Bauer ME. Acupuncture for sleep quality, BDNF levels and immunosenescence: a randomized controlled study. Neurosci Lett. 2014;587C:35-40. [DOI] [PubMed] [Google Scholar]
- 37. Mao JJ, Farrar JT, Bruner D, et al. Electroacupuncture for fatigue, sleep, and psychological distress in breast cancer patients with aromatase inhibitor-related arthralgia: a randomized trial. Cancer. 2014;120:3744-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schwabl H, Vennos C, Saller R. Tibetan formulas as pleiotropic signatures—application of network medicines in multimorbidity. Forsch Komplementmed. 2013;20 (suppl 2):35-40. [DOI] [PubMed] [Google Scholar]