Abstract
Attention deficit/hyperactivity disorder (ADHD) is one of the most common child psychiatric disorders, and is often treated with stimulant medication. Nonpharmacological treatments include dietary supplementation with omega-3 fatty acids, although their effectiveness remains to be shown conclusively. In this study, we investigated the effects of dietary omega-3 fatty acid supplementation on ADHD symptoms and cognitive control in young boys with and without ADHD. A total of 40 boys with ADHD, aged 8–14 years, and 39 matched, typically developing controls participated in a 16-week double-blind randomized placebo-controlled trial. Participants consumed 10 g of margarine daily, enriched with either 650 mg of eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) each or placebo. Baseline and follow-up assessments addressed ADHD symptoms, fMRI of cognitive control, urine homovanillic acid, and cheek cell phospholipid sampling. EPA/DHA supplementation improved parent-rated attention in both children with ADHD and typically developing children. Phospholipid DHA level at follow-up was higher for children receiving EPA/DHA supplements than placebo. There was no effect of EPA/DHA supplementation on cognitive control or on fMRI measures of brain activity. This study shows that dietary supplementation with omega-3 fatty acids reduces symptoms of ADHD, both for individuals with ADHD and typically developing children. This effect does not appear to be mediated by cognitive control systems in the brain, as no effect of supplementation was found here. Nonetheless, this study offers support that omega-3 supplementation may be an effective augmentation for pharmacological treatments of ADHD (NCT01554462: The Effects of EPA/DHA Supplementation on Cognitive Control in Children with ADHD; http://clinicaltrials.gov/show/NCT01554462).
INTRODUCTION
Attention deficit/hyperactivity disorder (ADHD) is one of the most common child psychiatric disorders and is characterized by symptoms of inattention, hyperactivity, and impulsivity (American Psychiatric Association, 1994). Because of their efficacy, pharmacological treatments using stimulant medications, such as methylphenidate, have typically been the primary treatment for ADHD. However, in recent years there has been a growing interest in nonpharmacological treatments to provide an alternative for parents and clinicians looking for alternative or additive treatments.
One popular nonpharmacological treatment for ADHD is dietary supplementation using long-chain omega-3 polyunsaturated fatty acids (LC-PUFAs). However, there has been a fair amount of debate on the efficacy of this treatment. Two early studies reported a significant reduction of ADHD symptoms in healthy children after supplementation with the omega-3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) (Richardson and Montgomery, 2005; Sinn and Bryan, 2007). However, a later attempt to replicate these findings in a group of children diagnosed with ADHD failed to find such effects (Johnson et al, 2009). In fact, even though reduced plasma omega-3 PUFA levels have been reported in children and adolescents with ADHD (Antalis et al, 2006; Burgess et al, 2000; Chen et al, 2004; Colter et al, 2008; Spahis et al, 2008), to date most intervention studies investigating the efficacy of omega-3 PUFAs to reduce ADHD symptoms have yielded conflicting or negative results (Bélanger et al, 2009; Dean et al, 2014; Gustafsson et al, 2010; Hirayama et al, 2004; Raz et al, 2009; Richardson and Puri, 2002; Stevens et al, 2003; Vaisman et al, 2008; Voigt et al, 2001).
Recently however, two independent meta-analyses provided new evidence for possible beneficial effects of omega-3 PUFA supplementation on symptoms of ADHD with small but positive effect sizes between 0.14 (Sonuga-Barke et al, 2013) and 0.31 (Bloch and Qawasmi, 2011). Furthermore, an open-label, exploratory study by Barragán et al (2014) showed clinical benefits of treatment with omega-3 PUFA supplements in addition to methylphenidate (MPH) over treatment with MPH alone in children with ADHD.
The neurobiological mechanism underlying an effect of omega-3 supplementation is far from clear. Omega-3 PUFAs are thought to play an important role in cell membrane elasticity and myelination and may thus affect neural signal transduction (Bazinet and Layé, 2014). Animal studies indicated that omega-3 PUFA deficiency, and specifically DHA deficiency, results in decreased neuron size in rats that were raised on a DHA-deficient diet (Ahmad et al, 2002). Furthermore, both dopaminergic and serotonergic neurotransmission have been shown to be reduced in rats on an omega-3 PUFA-deficient diet (Chalon, 2006; Zimmer et al, 2000, 2002), with marked effects in the frontal cortex. Specifically, lower frontal cortical omega-3 PUFA status correlated with hyperactive and impulsive behavior in rats (Vancassel et al, 2007). Interestingly, a vast body of human literature has implied dopamine dysfunction and impairments in cognitive control in ADHD (Durston and Konrad, 2007; Kirley et al, 2002; Swanson et al, 2000). Taken all this together, this may suggest a potential target for omega-3 supplementation in the treatment of this disorder.
Therefore, we set out to investigate the effects of omega-3 PUFA dietary supplementation on ADHD symptoms in young boys with and without ADHD in a randomized, placebo-controlled trial. We included a typically developing reference group to investigate the specificity of treatment to subjects with ADHD. Our hypotheses were that dietary supplementation with omega-3 PUFAs would: improve symptoms of ADHD, increase phospholipid PUFA status as assessed by cheek cell phospholipid composition, and increase the rate of dopamine turnover as assessed by homovanillic acid (HVA) excretion in urine. Furthermore, we expected that dietary supplementation with omega-3 PUFAs would improve cognitive control in ADHD and that it would increase activation in the associated prefrontal and striatal areas, as assessed with fMRI.
MATERIALS AND METHODS
Participants
The study was approved by the Ethics Committee of the University Medical Centre Utrecht, The Netherlands, and took into account the ethical principles for medical research involving human subjects as stated in the declaration of Helsinki (amendment of Washington, 2002). Written and oral information was provided, after which written informed consent was obtained from all parents. All children provided written assent.
A total of 40 boys between 8 and 14 years of age with a DSM-IV diagnosis of ADHD were recruited through the Department of Psychiatry at the University Medical Center in Utrecht, and through advertising. The clinical diagnosis was confirmed by a trained researcher using the Diagnostic Interview Schedule for Children–Parent Version (DISC-P). We chose to only include boys in this study as we wanted to minimize the number of potential confounds (such as gender) on brain activity, and ADHD is more prevalent in boys than girls. The children with ADHD were either medication naive or using psychostimulant medication (methylphenidate only). No other forms of psychoactive medication were accepted in this study (Table 1). Children with ADHD who were on stimulant medication were instructed not to take their medication for 24 h before the fMRI scan. However, children were allowed to use their medication throughout the intervention period (16 weeks). Medication continued to be managed by the outside provider (eg, general practitioner, pediatrician, or psychiatrist). Any changes in medication status were recorded on a monthly basis by the research team.
Table 1. Demographic Data and Compliance.
| ADHD, N=40 | RG, N=39 | P-value | ||
|---|---|---|---|---|
| General | ||||
| Age | Mean±SD (range) | 10.3±2.0 (8.0–15.0) | 10.9±2.0 (8.2–15.1) | 0.163 |
| Total IQ | Mean±SD (range) | 104.3±16.2 (76–144) | 113.6±17.4 (75–145) | 0.017 |
| Parental Education in years | Mean±SD | 13.5±2.0 | 13.9±2.5 | 0.422 |
| Hand preference | N Right/left/ambidextrous | 39/0/1 | 37/0/2 | 0.610 |
| Medication (MPH) | N | 38/40 | — | |
| Intervention | ||||
| Body mass index | Mean±SD (range) | 16.8±2.8 (12.8–26.3) | 17.7±2.1 (14.0–22.5) | 0.122 |
| Percentage compliance | Mean±SD | 92.2±6.9 | 91.4±6.5 | 0.573 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; IQ, intelligence quotient; MPH, methylphenidate; RG, reference group of typically developing children.
A total of 39 typically developing boys matched to the patients for age, hand preference, and body mass index (BMI; at inclusion) were recruited as a reference group (hereafter also referred to as RG) through advertising at primary and secondary schools in the wider Utrecht area, and from the pool of volunteers participating in studies by our lab.
All subjects were screened by telephone interview to check for major neurological or psychiatric disorders, as well as psychiatric diagnoses in subjects and their first-degree relatives. Parents of the typically developing subjects participated in a DISC-P interview with trained researchers to confirm the absence of any psychiatric conditions and their first-degree relatives. None of the typically developing subjects were using any form of psychoactive medication.
IQ was assessed for all participants using a four subtest short form of the Wechsler Intelligence Scale for Children (WISC-III; Kort et al, 2005). As is often reported, children with ADHD had a slightly lower total IQ score than typically developing children (Table 1). As this is typical of the ADHD phenotype, IQ was not entered as a covariate in any of the designs to prevent partialling out variance that is potentially relevant to the disorder (Dennis et al, 2009; de Zeeuw et al, 2012). Hand preference was assessed using the Edinburgh Handedness Inventory (Oldfield, 1971). There were no significant differences in hand preference, nor were there any differences in age, parental education (measured in years), and BMI (Table 1).
Design
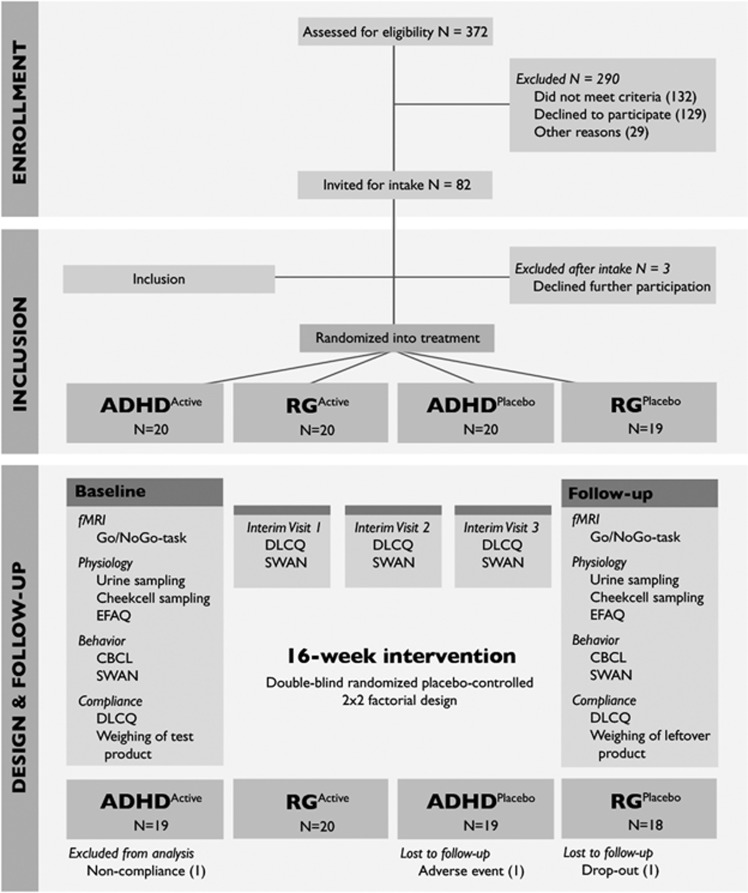
The 16-week intervention followed a double-blind randomized placebo-controlled design, where investigators, parents, and participants were all blind to the treatment conditions (Figure 1). The 2 × 2 factorial design included four groups: children with ADHD receiving either placebo or omega-3 fortified margarine (ADHDPlacebo and ADHDActive, respectively) and children from the reference group receiving the same treatment (RGPlacebo and RGActive). All participants were randomly assigned to one of the treatment conditions by a member of the Unilever Center for Nutritional Intervention Trials.
Figure 1.
Trial design. Schematic overview of the design of this double-blind randomized placebo-controlled trial, including all measures that were collected and the number of participants that were included at baseline. After 1, 2, and 3 months, interim visits took place during which compliance and behavior were measured. ADHD, attention deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; DLCQ, Diet and Lifestyle Change Questionnaire; EFAQ, Essential Fatty Acids Questionnaire; SWAN, Strengths and Weaknesses of ADHD symptoms and Normal behavior scale; RG, reference group of typically developing children.
Intervention
This trial was registered at clinicaltrials.gov under number NCT01554462. All participants were instructed to consume a daily dose of 10 g of either normal or omega-3 fortified margarine. The active product was full fat (80%) margarine, containing 650 mg DHA and 650 mg EPA per 10 g serving. The dose of the active ingredients DHA and EPA in the intervention product was under the US Generally Recognized As Safe (GRAS) level (FDA, 2004). The placebo product was a similar margarine with the same sensory properties, but with monounsaturated fatty acids (refined plant oils) instead of EPA and DHA; the total amount of saturated fatty acids and omega-6 fatty acid were matched in the placebo and active product (see Supplementary Materials 1 for more details).
Compliance was assessed by weighing the leftover products that parents returned to the investigators on a monthly basis. Furthermore, participants and parents kept a calendar, on which daily margarine consumption was recorded. Participants were asked to maintain their usual diet throughout the intervention period, with the following constraints: they were not allowed to use other supplements containing omega-3 or foods fortified with EPA and DHA during the intervention, nor were they to consume fatty fish more than once a week. Compliance to these requirements was measured on a monthly basis using the Diet and Lifestyle Change Questionnaire (DLCQ).
Physiological and Behavioral Measures
During the baseline and follow-up visits, cheek cell samples were collected using cotton swabs for analysis of phospholipid fatty acid levels. This method was chosen over blood sampling in children, as it is not invasive. Analyses of fatty acids were performed by gas chromatography after phospholipid isolation by thin-layer chromatography as described previously (Koletzko et al, 1999). Furthermore, urine samples were collected to measure the HVA to creatinine ratio, as a proxy for dopamine turnover. The Essential Fatty Acids Questionnaire (EFAQ) was collected to assess symptoms of FA deficiency (see Supplement Materials 1 for methodological details).
Finally, the parent-rated Child Behavior Checklist (CBCL) and Strengths and Weaknesses of ADHD symptoms and Normal behavior scale (SWAN) were collected for all participants, both of which measure the severity of ADHD symptoms (see Figure 1). The CBCL (collected twice) was used as the primary outcome measure, whereas the SWAN (collected five times) was used to measure change over time. In addition to the parent-rated questionnaires, the Teacher Report Form (TRF) was sent out to classroom teachers or mentors of the participants at baseline and follow-up. However, the response rate for this instrument was very low (54 of 79 returned at baseline, 31 of 76 at follow-up), and we had to exclude these data from further analysis.
fMRI Acquisition and Analysis
The fMRI study included a traditional Go-NoGo paradigm that has been described in previous work (Durston et al, 2003, 2006, 2009). Standard preprocessing was performed in SPM8 (Wellcome Department of Cognitive Neurology, www.fil.ion.ucl.ac.uk). MRI scans were excluded if head motion exceeded 3 mm or the size of one voxel at baseline and/or follow-up. Hence, 13 children with ADHD and 6 typically developing children were excluded from the SPM fMRI analysis. For a full description of the first- and second-level analysis of the fMRI study, please see Supplementary Material 2.
Statistics
All statistical analyses on the demographic, behavioral, and physiological measures were performed with the SPSS statistical package 20.0 (SPSS, Chicago, IL). Group differences on the demographic variables were analyzed using χ2, independent samples t-tests, or Mann–Whitney U-tests, as appropriate.
For the behavioral and physiological measures, independent samples t-tests or Mann–Whitney U-tests were used to analyze group differences at baseline between the diagnostic groups, and between the intervention groups. To investigate treatment effects, all variables of interest were fed into an ANCOVA (analysis of covariance) model with the baseline measurement entered as a covariate (Vickers, 2005). Intention-to-treat (ITT) analyses, including all subjects randomized into the trial, were performed using a linear mixed effects (LME) model with restricted maximum likelihood (REML) estimation where diagnostic status and treatment condition were included as fixed factors. Mean adjusted difference (MAD) and 95% confidence intervals (CIs) were also calculated.
Initially, age included as a covariate in the analyses. However, it did not correlate with any of the measures of interest, and as such it was excluded from the final models. Significant findings were further analyzed using paired or independent samples t-tests. Pearson's correlations were performed to analyze the relationship between behavioral and physiological measures. Finally, a post hoc power analysis was conducted to determine the retrospective power of the study.
RESULTS
After inclusion, 77 children successfully completed the study and participated in both fMRI sessions. One RGplacebo participant dropped out after 3 months for personal reasons. One RGactive participant dropped out after developing a skin rash that later turned out to be unrelated to the test product. After dropping out of the study, the parents of this second participant were notified about the contents of the test product by an independent member of Unilever Center for Nutritional Intervention Trial. The researchers remained blind throughout the whole study period. Compliance was considered acceptable if two-thirds of the test product was consumed during the intervention period, with no periods of nonconsumption longer than 7 days (eg, because of illness). One participant in the ADHDplacebo group did not meet these criteria, and was excluded from the analyses. There were no differences in compliance between the four treatment groups (χ2= 2.733, p=0.435, Table 1).
Behavioral Results
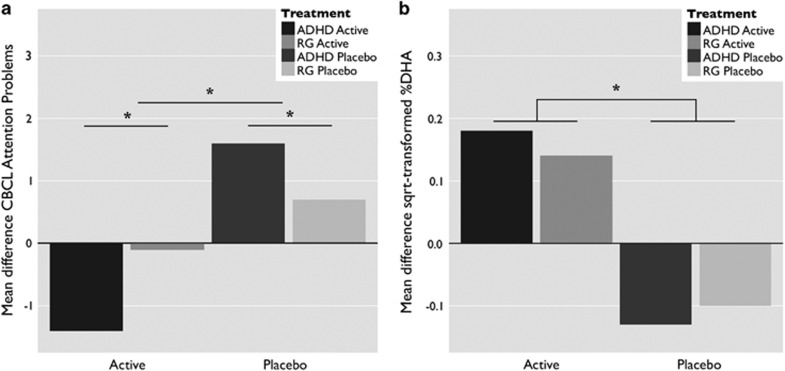
At baseline, subjects with ADHD scored higher than the reference group on the CBCL subscales Attention Problems (p<0.001, see Table 2), Rule Breaking Behavior (p<0.001), and Aggressive Behavior (p<0.001). CBCL scores did not correlate with age in either diagnostic group. Completer analyses by means of ANCOVA showed a main effect of diagnostic status (F(1, 67)=6.92, p=0.011) at follow-up, where children with ADHD scored higher on CBCL attention problems. ANCOVA further showed a main effect of treatment condition, where after supplementation with omega-3 PUFAs, scores on CBCL attention problems were reduced in comparison with supplementation with placebo (Figure 2a: F(1, 67)=14.99, p<0.001). Although there was no interaction between diagnostic status and treatment condition, it should be noted that the typically developing group receiving active treatment did not show a significant reduction in attention problems. The ITT analysis using the LME model yielded similar results for CBCL attention problems showing a significant effect of diagnostic status (MAD=6.37, 95% CI 8.31–4.42), t(90)=6.51, p<0.001) and an interaction effect between treatment condition and time (MAD=−1.83, 95% CI (−2.91 to −0.76), t(67)=3.42, p=0.001). There were no significant effects of treatment on the CBCL Rule Breaking and Aggressive Behavior subscales, or on the SWAN questionnaire.
Table 2. Behavioral and Physiological Measures per Treatment Group.
| Baseline |
Follow-up |
|||||||
|---|---|---|---|---|---|---|---|---|
| RG |
ADHD |
RG |
ADHD |
|||||
| Placebo | Active | Placebo | Active | Placebo | Active | Placebo | Active | |
| Behavior, mean (SD) | ||||||||
| CBCL ADHDa | 1.9 (2.1) | 2.1 (2.3) | 9.0 (3.1) | 8.8 (2.1) | 2.5 (2.3) | 1.8 (1.6) | 10.1 (2.2) | 7.6 (3.5) |
| CBCL Attention Problems | 2.7 (2.8) | 2.5 (3.6) | 8.9 (3.5) | 9.1 (2.5) | 3.4 (2.8)b | 2.4 (2.6)b | 10.5 (3.3)b | 7.7 (3.0)b |
| CBCL Rule Breaking | 1.4 (2.2) | 0.7 (0.9) | 3.8 (2.3) | 3.1 (2.8) | 1.0 (1.5) | 1.2 (1.8) | 4.1 (2.4) | 2.4 (1.9) |
| CBCL Aggressive Behavior | 2.2 (3.7) | 2.2 (2.7) | 11.0 (5.8) | 9.7 (7.2) | 1.8 (3.1) | 2.7 (3.2) | 11.8 (5.4) | 7.8 (3.5) |
| Physiology, mean (SD) | ||||||||
| %DHA (C22:6 n-3)c | 0.58 (0.23) | 0.54 (0.24) | 0.67 (0.19) | 0.49 (0.13) | 0.48 (0.19)d | 0.68 (0.24)d | 0.54 (0.15)d | 0.67 (0.27)d |
| Urine samples HVAe | 1.4 (0.2) | 1.4 (0.3) | 1.5 (0.3) | 1.5 (0.4) | 1.3 (0.2) | 1.3 (0.3) | 1.4 (0.3) | 1.5 (0.3) |
| EFAQf | 1.3 (2.3) | 0.9 (1.8) | 1.8 (1.7) | 1.8 (2.1) | 1.3 (2.3) | 0.5 (0.9) | 1.6 (2.0) | 1.8 (1.7) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; DHA, docosahexaenoic acid; EFAQ, Essential Fatty Acids Questionnaire; HVA, homovanillic acid; RG, reference group of typically developing children.
RGplacebo: 1 baseline, 2 follow-up; RGactive: 2 follow-up; ADHDactive: 1 baseline, 6 follow-up.
Main effect of diagnosis and treatment group at follow-up (ANCOVA and LME: p≤0.001).
RGplacebo: 1 baseline, 2 follow-up; RGactive: 1 baseline, 2 follow-up; ADHDplacebo: 3 baseline, 1 follow-up; ADHDactive: 1 baseline, 2 follow-up, 1 both (missing data).
Main effect of treatment group at follow-up (ANCOVA and LME: p<0.001).
RGplacebo: 2 follow-up; RGactive: 1 baseline, 2 follow-up; ADHDactive: 1 baseline, 2 follow-up (missing data).
RGplacebo: 2 follow-up; RGactive: 1 follow-up; ADHDactive: 1 baseline, 1 follow-up (missing data).
Figure 2.
Main effect of omega-3 PUFA supplementation. (a) The mean difference between baseline and follow-up CBCL attention problems in both diagnostic groups, with main effects of diagnosis and the intervention. (b) The mean difference between baseline and follow-up square-root transformed %DHA levels as collected from cheek cell samples, with similar main effects. The asterisks denote significance at p<0.01. ADHD, attention deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; DHA, docosahexaenoic acid; RG, reference group of typically developing children.
Three children in the ADHDActive group and four children in the ADHDPlacebo group reported that the dosage of their stimulant medication had been increased during the intervention period. The treatment effect on the Attention Problems subscale was still found in a reanalysis of the CBCL data excluding these participants (ANCOVA: F(1, 61)=14.22, p<0.001, LME: MAD=−1.91, 95% CI (−3.10 to −0.77), t(60)=3.34, p=0.001).
Physiological Results
Scores on the EFAQ (Table 2) were normalized by square root transformation. At baseline, there was a difference between children with ADHD and children in the reference group on the EFAQ (t(75)=2.72, p=0.008), where children with ADHD scored higher on symptoms of FA deficiency. However, only four children met cutoff criteria for FA deficiency (3 children with ADHD and 1 RG). There was no significant effect of supplementation with omega-3 PUFAs on the EFAQ, neither for subjects with ADHD nor the RG.
Analysis of the square-root transformed cheek cell phospholipids showed no differences between the four groups at baseline (F(3, 69)=1.92, p=0.135). At follow-up, there was a main effect of treatment (F(1, 63)=13.03, p=0.001), where %DHA in phospholipids was higher after supplementation with omega-3 PUFAs in comparison with supplementation with the placebo product (see Figure 2b and Table 2). The ITT analysis accounted for missing data at either baseline (6 samples) or follow-up (8 samples) and similarly showed an interaction between treatment condition and time (MAD=0.34, 95% CI (0.14–0.53), t(72)=3.47, p=0.001). For some samples, the amount of cheek cell phospholipids was too low to assess DHA levels reliably, leading to floor values of 0 for a number of samples (24 at baseline and 21 at follow-up). Reanalysis without these samples showed the same effect (ANCOVA: F(1, 29)=8.00, p=0.009), LME: MAD=0.28, 95% CI (0.12–0.44), t(51)=3.48, p=0.001). However, these results should be interpreted with caution, as cheek cell fatty acid data were below detection threshold for some subjects and missing for others.
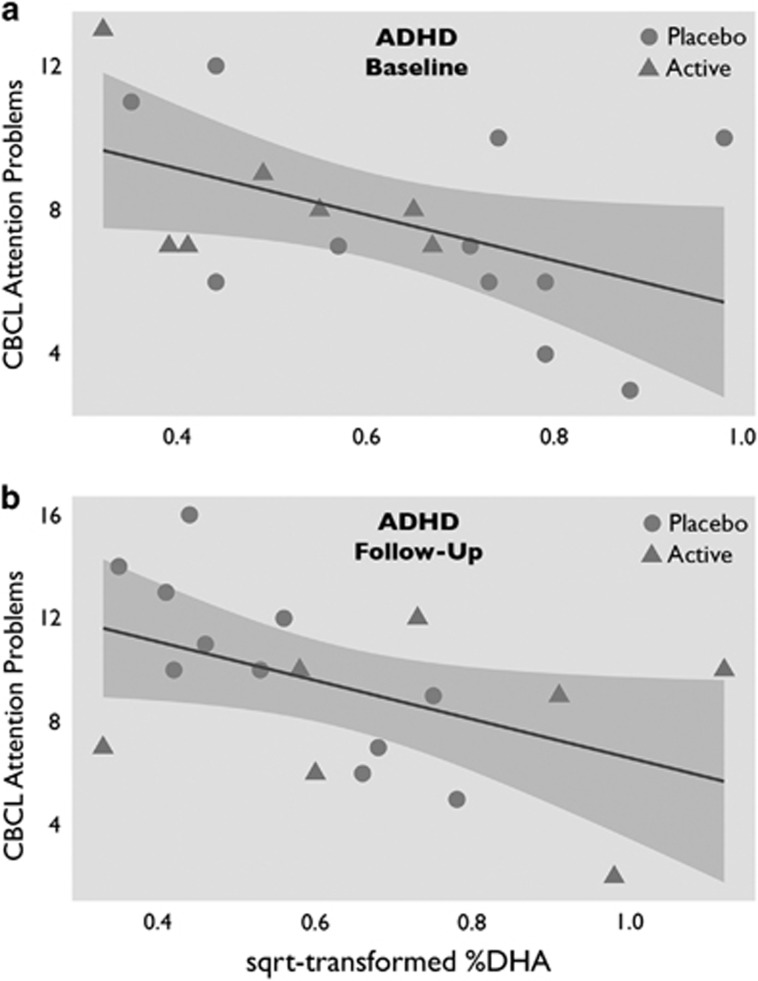
Follow-up Pearson's correlation analyses showed that at baseline CBCL attention problems correlated negatively with omega-3 LC-PUFA status in the group of children with ADHD as a whole (r=−0.47, p=0.048; see Figure 3a), but not in children in the reference group (r=0.28, p=0.166). The correlation in the ADHD group persisted at follow-up (r=−0.48, p=0.042; see Figure 3b).
Figure 3.
The relation between omega-3 fatty acids and attention problems in ADHD, at baseline and at follow-up. (a) Correlation at baseline between square-root transformed %DHA and CBCL attention problems in children with ADHD (r=−0.47). (b) The same correlation at follow-up (r=−0.48). There was no correlation between %DHA and CBCL attention problems in typically developing children. ADHD, attention deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; DHA, docosahexaenoic acid.
The data on the HVA to creatinine ratio were normalized using a natural logarithm. At baseline, the difference in the HVA ratio (μmol/mmol creatinine) approached significance (t(72)=1.92, p=0.059), with subjects with ADHD having slightly higher levels of this ratio than the reference group. No effects of the intervention were found on these measures, including in analyses with age or BMI included as covariates in the design.
fMRI Task
Results typical of this task were found (Durston et al, 2003, 2006); see Supplementary Materials 2 and 3 for a detailed description of the fMRI results. There was no effect of dietary omega-3 supplementation on task performance or brain activation during the cognitive control task.
DISCUSSION
Omega-3 PUFA dietary supplementation improved symptoms of inattention in boys with and without ADHD in a double-blind randomized controlled trial. This effect did not appear to be mediated by dopaminergic cognitive control networks, as measures of dopamine turnover and neural activity during cognitive control were unaffected by the intervention.
At baseline, boys with ADHD had higher symptoms of inattention than typically developing boys. Furthermore, there was an effect of treatment on parent-rated symptoms of ADHD, regardless of diagnosis. This effect was driven by the measures of inattention at follow-up: subjects who had received omega-3 PUFAs had lower scores on the CBCL attention problems subscale than subjects on placebo. This ties in with earlier studies that have suggested that omega-3 PUFA supplementation improves symptoms of inattention specifically, and not symptoms of ADHD more generally (Gustafsson et al, 2010; Richardson and Montgomery, 2005; Sinn and Bryan, 2007). In line with recent meta-analyses (Bloch and Qawasmi, 2011; Sonuga-Barke et al, 2013), our results suggest that supplementation with omega-3 PUFAs may be beneficial in ADHD.
Moreover, our results indicate that typically developing children also benefit, showing the importance of omega-3 PUFA intake during development in general (Eilander et al, 2007; Schuchardt et al, 2010). Previous studies using sustained attention tasks have shown that dietary supplementation of omega-3 PUFAs leads to increases in brain activation in prefrontal areas and improved task performance in both children with ADHD and typically developing children (Bauer et al, 2014; Mcnamara et al, 2010; McNamara et al, 2013; Vaisman et al, 2008). We used a cognitive control task and found no changes in brain activity or task performance after supplementation. Furthermore, dopamine turnover did not appear to be affected by the supplementation, as HVA levels in urine were not affected. Therefore, it appears that the neural mechanism underlying improvements in attention did not involve dopaminergic cognitive control networks.
The dietary intervention affected omega-3 PUFA levels in cheek cell phospholipids, as the level of DHA was significantly higher at follow-up for subjects who had been treated with the active product than for those who had received placebo. This observation was supported by the returned margarine tubs and diaries, and showed that the intervention was effective. Furthermore, in subjects with ADHD, higher levels of DHA were associated with lower attention problems, both at baseline and follow-up. The detected increase in DHA levels was more modest than in earlier studies where DHA was measured in plasma or erythrocytes (Muthayya et al, 2009; Osendarp et al, 2007). Cheek cell samples are more prone to contamination than blood samples and this may have contributed to this difference. An alternative explanation may be that the intervention was less effective than expected.
Most studies to date have suggested that omega-3 supplementation is effective through higher levels of EPA as opposed to DHA (Bloch and Qawasmi, 2011; Gustafsson et al, 2010). The biological mechanism through which DHA acts on human brain function is not clear, even though this fatty acid is abundantly present in the brain's phospholipid membranes (Bazinet and Layé, 2014; Horrocks and Farooqui, 2004). As biosynthesis of DHA from its precursors α-linolenic acid and EPA is very low, a notable increase in DHA plasma level can only be accomplished through direct DHA intake (Plourde and Cunnane, 2007). Many of the omega-3 supplements used in previous studies, with often negative results, contained a relatively low DHA to EPA ratio, possibly obscuring direct effects of DHA. However, a recent H-MRS study by McNamara et al (2013) showed that higher erythrocyte DHA levels were related to a better performance on a sustained attention task and ACC metabolic function in typically developing children. The results from previous work combined with the current study suggest that the combined supplementation with EPA and DHA accounts for the treatment effect in the present study and may thus yield the greatest improvements in behavior.
Although evidence of a moderate effect of dietary omega-3 PUFA supplementation is converging, it is unlikely that it will ever achieve the same behavioral improvements as stimulant medication in ADHD, with effect sizes ranging from 0.54 to 0.78 (Schachter et al, 2001). However, it is noteworthy that in the present study the majority of children with ADHD received the omega-3 PUFA supplements in addition to their regular medication. The behavioral improvement persisted even when changes in medication were taken into account in the analyses. This suggests that omega-3 PUFAs may be useful as an augmentation to standard pharmacological therapies. Indeed, recent reports showed that children receiving a combination of MPH and omega-3 PUFAs needed lower doses of MPH compared with children only receiving MPH to achieve the same clinical benefits (Barragán et al, 2014; MTA Cooperative Group, 1999).
Obvious strengths of our study include the randomized placebo-controlled double-blind design and the inclusion of a typically developing reference group to assess whether effects were specific to ADHD. However, there are also some limitations that should be taken into consideration. The first limitation is that in some subjects the quality of the cheek cell samples did not permit a reliable detection of DHA. However, this did not affect our statistical analyses negatively, as the effect of the dietary intervention reached statistical significance even without these values included. Phospholipid values did not show the expected extent of separation between placebo and intervention groups. However, these measures did differ between the active product and placebo intervention groups, and correlated with behavioral outcomes. A second limitation is that a small number of participants with ADHD had changes made to their medication during the intervention. However, reanalysis without these participants showed that the effect of the intervention still held without these subjects. Finally, sample sizes in the fMRI study were smaller than in the main study as a result of subject motion. However, the remaining sample was well matched and yielded only a tiny effect size of the intervention on MR signal. We conducted a post hoc power analysis to investigate what sample size would be necessary to show an effect of the intervention on brain activity, if this was indeed a real effect. The results of this power analysis indicated that only a huge sample size (N=718) would have shown a significant effect of treatment and suggests therefore that the nonsignificant differences in this study are most likely attributable to noise. Taken together, the absence of a treatment effect on the brain activity during a cognitive control task, on performance of the task, and on dopamine turnover suggest that the effect of the intervention on ADHD symptoms is unlikely to be mediated by dopamine systems.
In conclusion, this study provides new evidence that dietary supplementation using omega-3 PUFAs may be an effective augmentation of pharmacological treatments of ADHD. This effect does not appear to be mediated by dopaminergic cognitive control networks, but may involve other systems implicated in ADHD, such as attention networks.
FUNDING AND DISCLOSURE
Marco Hoeksma and Ans Eilander are employees of Unilever. The other authors declare no conflict of interest.
Acknowledgments
We thank all the participants and their parents for their commitment to this study. We also thank two anonymous reviewers for their constructive input and Dr Harry Hiemstra for his valuable advice. This study was financially supported by Unilever Research & Development, Vlaardingen, The Netherlands. Unilever Research & Development was involved in the conception and design of the study. They provided financial support for research staff to run the study and provided the intervention product.
Author Contributions
Sarah Durston and the staff at the NICHE lab at the Department of Psychiatry of the UMC Utrecht were responsible for data collection and analysis, and for drafting and deciding upon the final content of the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Ahmad A, Moriguchi T, Salem N. Decrease in neuron size in docosahexaenoic acid-deficient brain. Pediatr Neurol. 2002;26:210–218. doi: 10.1016/s0887-8994(01)00383-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing: Washington DC; 1994. [Google Scholar]
- Antalis CJ, Stevens LJ, Campbell M, Pazdro R, Ericson K, Burgess JR. Omega-3 fatty acid status in attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2006;75:299–308. doi: 10.1016/j.plefa.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Barragán E, Breuer D, Döpfner M.2014Efficacy and safety of omega-3/6 fatty acids, methylphenidate, and a combined treatment in children with ADHD J Atten Disord(in press). [DOI] [PubMed]
- Bauer I, Hughes M, Rowsell R, Cockerell R, Pipingas A, Crewther S, et al. Omega-3 supplementation improves cognition and modifies brain activation in young adults. Hum Psychopharmacol. 2014;29:133–144. doi: 10.1002/hup.2379. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat Rev Neurosci. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- Bélanger SA, Vanasse M, Spahis S, Sylvestre M-P, Lippé S, L'heureux F, et al. Omega-3 fatty acid treatment of children with attention-deficit hyperactivity disorder: a randomized, double-blind, placebo-controlled study. Paediatr Child Health. 2009;14:89–98. doi: 10.1093/pch/14.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50:991–1000. doi: 10.1016/j.jaac.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JR, Stevens L, Zhang W, Peck L. Long-chain polyunsaturated fatty acids in children with attention-deficit hyperactivity disorder. Am J Clin Nutr. 2000;71:327S–330SS. doi: 10.1093/ajcn/71.1.327S. [DOI] [PubMed] [Google Scholar]
- Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Chen J-R, Hsu S-F, Hsu C-D, Hwang L-H, Yang S-C. Dietary patterns and blood fatty acid composition in children with attention-deficit hyperactivity disorder in Taiwan. J Nutr Biochem. 2004;15:467–472. doi: 10.1016/j.jnutbio.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Colter AL, Cutler C, Meckling KA. Fatty acid status and behavioural symptoms of attention deficit hyperactivity disorder in adolescents: a case-control study. Nutr J. 2008;7:8. doi: 10.1186/1475-2891-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean AJ, Bor W, Adam K, Bowling FG, Bellgrove MA. A randomized, controlled, crossover trial of fish oil treatment for impulsive aggression in children and adolescents with disruptive behavior disorders. J Child Adolesc Psychopharmacol. 2014;24:140–148. doi: 10.1089/cap.2013.0093. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes Ma, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Konrad K. Integrating genetic, psychopharmacological and neuroimaging studies: a converging methods approach to understanding the neurobiology of ADHD. Dev Rev. 2007;27:374–395. [Google Scholar]
- Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Durston S, Nederveen H, van Dijk S, van Belle J, de Zeeuw P, Langen M, et al. Magnetic resonance simulation is effective in reducing anxiety related to magnetic resonance scanning in children. J Am Acad Child Adolesc Psychiatry. 2009;48:206–207. doi: 10.1097/CHI.0b013e3181930673. [DOI] [PubMed] [Google Scholar]
- Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti I-M, Yang Y, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Eilander A, Hundscheid DC, Osendarp SJ, Transler C, Zock PL. Effects of n-3 long chain polyunsaturated fatty acid supplementation on visual and cognitive development throughout childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids. 2007;76:189–203. doi: 10.1016/j.plefa.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Gustafsson Pa, Birberg-Thornberg U, Duchén K, Landgren M, Malmberg K, Pelling H, et al. EPA supplementation improves teacher-rated behaviour and oppositional symptoms in children with ADHD. Acta Paediatr. 2010;99:1540–1549. doi: 10.1111/j.1651-2227.2010.01871.x. [DOI] [PubMed] [Google Scholar]
- Hirayama S, Hamazaki T, Terasawa K. Effect of docosahexaenoic acid-containing food administration on symptoms of attention-deficit/hyperactivity disorder - a placebo-controlled double-blind study. Eur J Clin Nutr. 2004;58:467–473. doi: 10.1038/sj.ejcn.1601830. [DOI] [PubMed] [Google Scholar]
- Horrocks La, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Johnson M, Ostlund S, Fransson G, Kadesjö B, Gillberg C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: a randomized placebo-controlled trial in children and adolescents. J Atten Disord. 2009;12:394–401. doi: 10.1177/1087054708316261. [DOI] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, et al. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology. 2002;27:607–619. doi: 10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Koletzko B, Knoppke B, Schenck U, von, Demmelmair H, Damli A. Noninvasive assessment of essential fatty acid status in preterm infants by buccal mucosal cell phospholipid analysis. J Pediatr Gastroenterol Nutr. 1999;29:467–474. doi: 10.1097/00005176-199910000-00018. [DOI] [PubMed] [Google Scholar]
- Kort W, Schittekatte M, Dekker PH, Verhaeghe P, Compaan EL, Bosmans M. Wechsler Intelligence Scale for Children - Third Edition, Dutch Version. Psychological Corporation: London; 2005. [Google Scholar]
- Mcnamara RK, Able J, Jandacek R, Rider T, Tso P, Eliassen JC, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr. 2010;91:1060–1067. doi: 10.3945/ajcn.2009.28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Tso P, Weber W, Chu W-J, Strakowski SM, et al. Low docosahexaenoic acid status is associated with reduced indices in cortical integrity in the anterior cingulate of healthy male children: a 1H MRS Study. Nutr Neurosci. 2013;16:183–190. doi: 10.1179/1476830512Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA Cooperative Group A 14-month randomized clinical trial of treatment strategies for attention-deficit/ hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Muthayya S, Eilander A, Transler C, Thomas T, Van Der Knaap HCM, Srinivasan K, et al. Effect of fortification with multiple micronutrients and n 2 3 fatty acids on growth and cognitive performance in Indian schoolchildren: the CHAMPION (Children ' s Health and Mental Performance Influenced by Optimal Nutrition) Study 1–3. Am J Clin Nutr. 2009;89:1766–1775. doi: 10.3945/ajcn.2008.26993. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osendarp SJM, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaine M, et al. Effect of a 12-mo micronutrient intervention on learning and memory in well-nourished and marginally nourished school-aged children: 2 parallel, randomized, placebo-controlled studies in Australia and. Am J Clin Nutr. 2007;86:1082–1093. doi: 10.1093/ajcn/86.4.1082. [DOI] [PubMed] [Google Scholar]
- Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab. 2007;32:619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- Raz R, Carasso RL, Yehuda S. The influence of short-chain essential fatty acids on children with attention-deficit/hyperactivity disorder: a double-blind placebo-controlled study. J Child Adolesc Psychopharmacol. 2009;19:167–177. doi: 10.1089/cap.2008.070. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Montgomery P. The Oxford-Durham study: a randomized, controlled trial of dietary supplementation with fatty acids in children with developmental coordination disorder. Pediatrics. 2005;115:1360–1366. doi: 10.1542/peds.2004-2164. [DOI] [PubMed] [Google Scholar]
- Richardson AJ, Puri BK. A randomized double-blind, placebo-controlled study of the effects of supplementation with highly unsaturated fatty acids on ADHD-related symptoms in children with specific learning difficulties. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:233–239. doi: 10.1016/s0278-5846(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Schachter HM, Pham B, King J, Langford S, Moher D. How efficacious and safe is short- acting methylphenidate for the treatment of attention-deficit disorder in children and adolescents? A meta-analysis. CMAJ. 2001;165:1475–1488. [PMC free article] [PubMed] [Google Scholar]
- Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur J Pediatr. 2010;169:149–164. doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- Sinn N, Bryan J. Effect of supplementation with polyunsaturated fatty acids and micronutrients on learning and behavior problems associated with child ADHD. J Dev Behav Pediatr. 2007;28:82–91. doi: 10.1097/01.DBP.0000267558.88457.a5. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Brandeis D, Cortese S, Daley D, Ferrin M, Holtmann M, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–289. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- Spahis S, Vanasse M, Bélanger Sa, Ghadirian P, Grenier E, Levy E. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot Essent Fatty Acids. 2008;79:47–53. doi: 10.1016/j.plefa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Stevens L, Zhang W, Peck L, Kuczek T, Grevstad N, Mahon A, et al. EFA supplementation in children with inattention, hyperactivity, and other disruptive behaviors. Lipids. 2003;38:1007–1021. doi: 10.1007/s11745-006-1155-0. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Flodman P, Kennedy J, Spence Ma, Moyzis R, Schuck S, et al. Dopamine genes and ADHD. Neurosci Biobehav Rev. 2000;24:21–25. doi: 10.1016/s0149-7634(99)00062-7. [DOI] [PubMed] [Google Scholar]
- Vaisman N, Kaysar N, Zaruk-adasha Y, Pelled D, Brichon G, Zwingelstein G, et al. Correlation between changes in blood fatty acid composition and visual sustained attention performance in children with inattention. Am J Clin Nutr. 2008;87:1170–1180. doi: 10.1093/ajcn/87.5.1170. [DOI] [PubMed] [Google Scholar]
- Vancassel S, Blondeau C, Lallemand S, Cador M, Linard A, Lavialle M, et al. Hyperactivity in the rat is associated with spontaneous low level of n-3 polyunsaturated fatty acids in the frontal cortex. Behav Brain Res. 2007;180:119–126. doi: 10.1016/j.bbr.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Vickers AJ. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med Res Methodol. 2005;5:35. doi: 10.1186/1471-2288-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RG, Llorente AM, Jensen CL, Fraley JK, Berretta MC, Heird WC. A randomized, double-blind, placebo-controlled trial of docosahexaenoic acid supplementation in children with attention-deficit/hyperactivity disorder. J Pediatr. 2001;139:189–196. doi: 10.1067/mpd.2001.116050. [DOI] [PubMed] [Google Scholar]
- de Zeeuw P, Schnack HG, van Belle J, Weusten J, van Dijk S, Langen M, et al. Differential brain development with low and high IQ in attention-deficit/hyperactivity disorder. PLoS One. 2012;7:e35770. doi: 10.1371/journal.pone.0035770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer L, Delion-Vancassel S, Durand G, Guilloteau D, Bodard S, Besnard JC, et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. J Lipid Res. 2000;41:32–40. [PubMed] [Google Scholar]
- Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr. 2002;75:662–667. doi: 10.1093/ajcn/75.4.662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.