Abstract
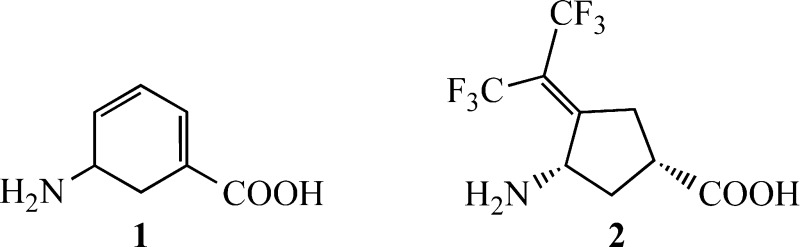
Hepatocellular carcinoma is the second leading cause of cancer death worldwide. DNA microarray analysis identified the ornithine aminotransferase (OAT) gene as a prominent gene overexpressed in hepatocellular carcinoma (HCC) from Psammomys obesus. In vitro studies demonstrated inactivation of OAT by gabaculine (1), a neurotoxic natural product, which suppressed in vitro proliferation of two HCC cell lines. Alpha-fetoprotein (AFP) secretion, a biomarker for HCC, was suppressed by gabaculine in both cell lines, but not significantly. Because of the active site similarity between GABA aminotransferase (GABA-AT) and OAT, a library of 24 GABA-AT inhibitors was screened to identify a more selective inhibitor of OAT. (1S,3S)-3-Amino-4-(hexafluoropropan-2-ylidene)cyclopentane-1-carboxylic acid (2) was found to be an inactivator of OAT that only weakly inhibits GABA-AT, l-aspartate aminotransferase, and l-alanine aminotransferase. In vitro administration of 2 significantly suppressed AFP secretion in both Hep3B and HepG2 HCC cells; in vivo, 2 significantly suppressed AFP serum levels and tumor growth in HCC-harboring mice, even at 0.1 mg/kg. Overexpression of the OAT gene in HCC and the ability to block the growth of HCC by OAT inhibitors support the role of OAT as a potential therapeutic target to inhibit HCC growth. This is the first demonstration of suppression of HCC by an OAT inactivator.
Keywords: Hepatocellular carcinoma, ornithine aminotransferase, GABA aminotransferase, selective inhibitors, antitumor agent, alpha fetoprotein
Hepatocellular carcinoma (HCC), the second most common cause of death from cancer worldwide,1−4 is a highly chemotherapy- and radiotherapy-resistant cancer with only mildly effective systemic therapy.4−6 Several treatment strategies, including surgical resection, liver transplantation, radiofrequency ablation, trans-arterial chemoembolization, systematic therapy using the kinase inhibitor sorafenib, and radiotherapy, are all being used; however, they all have limited efficacy.5,7,8
Three genes involved in glutamine metabolism, encoding glutamine synthetase (GS), ornithine aminotransferase (OAT), and the glutamate transporter GLT-1, are induced by activation of the β-catenin pathway in the liver.9,10 The Wnt/β-catenin cascade has emerged as a critical regulator of cancer cells.11,12 This pathway has been reported to be involved in normal physiological processes and is integrally associated with cancer cell growth and maintenance. Therefore, it has been a target of strategies for anticancer therapy. β-Catenin, a major oncogenic component of the Wnt pathway, is involved in a variety of cancers.13,14 Aberrant Wnt/β-catenin signaling is widely implicated in numerous malignancies, including cancers of the gastrointestinal tract.15 Elevated levels of β-catenin are observed in common forms of human malignancies, indicating that activation of the Wnt pathway plays an important role in tumor development.12
The OAT gene is a β-catenin target gene that is highly expressed in hepatocellular carcinoma (HCC). Overexpression of the OAT gene is associated with activation of β-catenin signaling in the liver.10,16−19 Regulation of OAT gene-associated glutamine metabolism by β-catenin was suggested to be a contributing factor to carcinogenesis,10,20 which links the glutamine pathway to hepatocarcinogenesis.10 The Wnt/β-catenin signaling pathway is activated relatively early during liver regeneration, mostly through post-translational modifications.13,21,22 Once activated, β-catenin signaling drives the expression of target genes that are critical for cell cycle progression and contributes to the initiation of the regeneration process.21 Among human cancers tightly linked to abnormal Wnt/β-catenin signaling, hepatoblastomas, uncommon malignant liver neoplasms occurring in infants and children, occur with the highest rate of β-catenin mutations.23 Among the signaling cascades that are deregulated in HCC, the Wnt/β-catenin signaling pathway plays a role in hepatic oncogenesis.22 Inhibition of β-catenin signaling in HCC cell lines has an antitumor effect; however, no molecules targeting any component of the Wnt/β-catenin pathway are currently being tested in clinical trials for the treatment of HCC.22
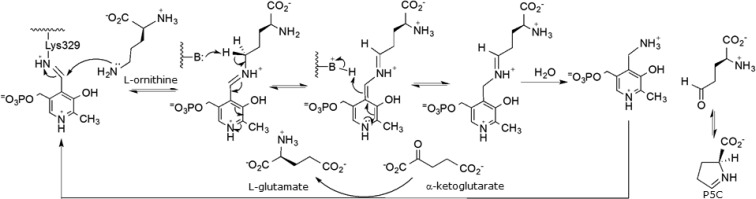
Ornithine aminotransferase7 is a pyridoxal 5′-phosphate (PLP)-dependent mitochondrial matrix enzyme9,24 that catalyzes the interconversion of ornithine and α-ketoglutarate to l-glutamate semialdehyde, which cyclizes to Δ1-pyrroline-5-carboxylate (P5C), and glutamate (Scheme 1).7,9,24 The l-glutamate that is formed from OAT is transported away by GLT-1 so that it does not accumulate and become toxic to the cell, and then it is converted by glutamine synthetase to l-glutamine. Glutamine is essential for growth of both normal and neoplastic cells; however, tumor cells take up glutamine more efficiently than normal cells,25 and tumor growth is enhanced by glutamine.26 Cancer cells distinguish themselves from normal cells in that they have an increased requirement for glutamine to support anabolic processes that stimulate proliferation.27 Glutamine provides a carbon source to maintain pools of tricarboxylic acid (TCA) cycle intermediates and a nitrogen source (for transamination reactions) for nucleotide, nonessential amino acids, and hexosamine biosynthesis.28,28b Glutamine also plays a critical role in suppressing oxidative stress because its catabolism can lead to the biosynthesis of glutathione (GSH), a major intracellular antioxidant.29,29b Cancer cells depend on a continuous supply of glutamine for survival and proliferation. Increased activity of OAT allows tumor cells to grow independently of glutamine supply and may confer a growth advantage to the cell. Reduction of the level of tissue glutamine concentrations by inhibition of OAT was suggested to inhibit cell proliferation and tumor growth.24−26 A suppression subtractive hybridization method applied to hepatitis C virus-associated HCC and adjacent non-HCC liver tissues identified OAT to be one of the genes overexpressed in HCC.30 The OAT content of Morris hepatoma is 15 times higher than that in a normal liver.31 Studies incorporating radioactive leucine into OAT in rats bearing hepatoma showed that the rate of synthesis of this enzyme in tumors was 5-fold higher than in the host liver.31
Scheme 1. OAT Catalytic Mechanism.
Here we show that the OAT gene is one of the most overexpressed genes in the livers of HCC-harboring sand rats. We also have shown that the neurotoxic agent gabaculine (1) inactivates OAT and have identified (1S,3S)-3-amino-4-(hexafluoropropan-2-ylidene)cyclopentane-1-carboxylic acid (2) as a selective OAT inactivator. The effects of 1 and 2 on alpha-fetal protein (AFP), a biomarker for HCC, and on HCC growth were determined both in vitro and in vivo; 2 shows impressive suppression of HCC growth in mice, even at 0.1 mg/kg.
Spontaneous hepatic preneoplastic and hepatoma changes are known to occur in the majority of 24-month old Psammomys obesus (desert sand rat), an animal model for HCC (Figure S1A) as well as for type II diabetes and nonalcoholic steatohepatitis (NASH);32,32b hepatic nodules develop at the age of six months, increasing in multiplicity with advancing age.33 Histologic examination revealed nodules containing hepatocytes characterized by hyperbasophilia, accumulation of glycogen, and eosinophilic cytoplasm; HCC was diagnosed in several animals. Histological malignant changes included excessive pleomorphism, loss of trabecular pattern, and tumor penetration across hepatic vein walls (Figure S1B).
DNA microarray-based gene expression analysis was performed on normal and spontaneous HCC-developing livers from Psammomys obesus. Analysis of the microarray data identified seven genes whose expression levels were increased and 143 genes whose expression levels were decreased in the tumor tissues compared with normal livers (Figure S2A,B). The OAT gene was one of the most prominent genes upregulated in the tumors (Figure S2C).
To determine the importance of OAT for HCC growth, inhibitors were sought. The natural product gabaculine (1) has been reported to be a potent irreversible inhibitor of OAT that forms a stable complex with the active site PLP.34 However, gabaculine is neurotoxic; it also inactivates several other PLP-dependent enzymes,35−37 including l-aspartate aminotransferase, l-alanine aminotransferase, and GABA aminotransferase, an enzyme that degrades the inhibitory neurotransmitter GABA.38,39 We confirmed that gabaculine is a time- and concentration-dependent irreversible inhibitor of OAT (Figure S3A). OAT inactivation constants, KI, and kinact, were 2.1 μM and 0.05 min–1, respectively. Inhibition was irreversible as no enzyme activity was restored by dialysis for 48 h against 0.1 M potassium pyrophosphate buffer, pH 8.0, containing 0.1 mM PLP and 0.1 mM α-ketoglutarate.
As a proof-of-principle test, gabaculine (1) was examined in vitro as an inhibitor of Hep3b, HepA1–6, and HepG2 HCC cell lines. Gabaculine significantly suppressed the proliferation of Hep3B and HepA1–6, by 43–57% (Figure S4A). Although alpha-fetal protein (AFP) secretion, a biomarker for HCC, was not affected by gabaculine in vitro, administration of a single dose of gabaculine in HCC-harboring mice resulted in suppression of AFP levels (Figure S5).
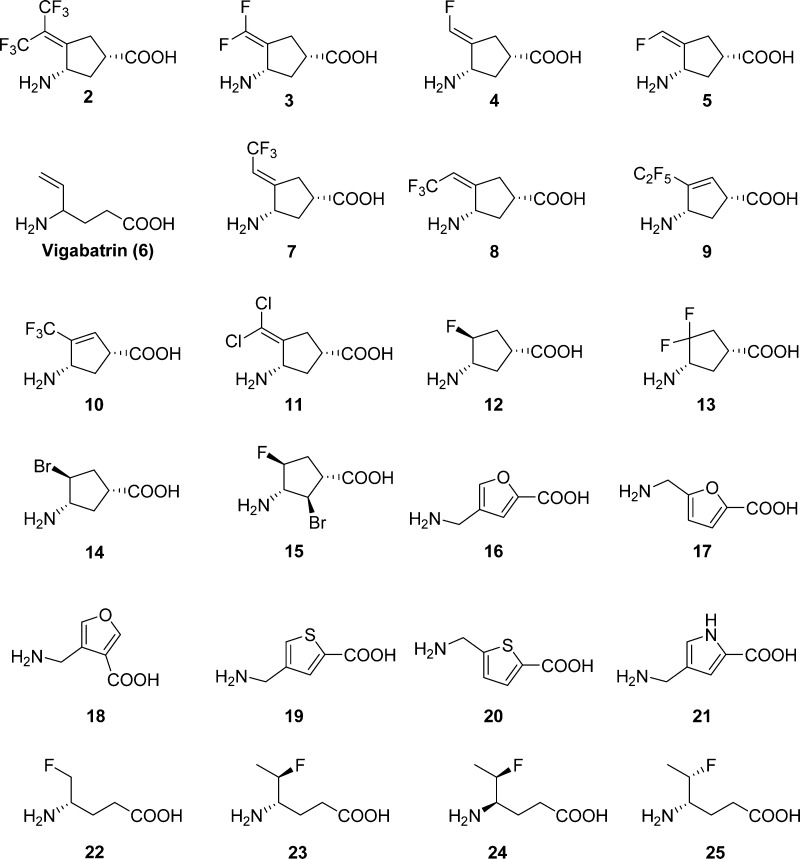
However, because of the lack of selectivity and neurotoxicity of gabaculine, we screened a library of 24 GABA analogues previously studied in the Silverman laboratory (Figure 1) for compounds that might more selectively inactivate OAT. The use of GABA analogues against OAT was based on the known structural similarities of the active sites of OAT and GABA-AT.40 Analogues screened against OAT included cyclic structures, aromatic structures, and flexible structures having a GABA backbone and a variety of functional groups in an attempt to identify appropriate binding site differences between these two enzymes. The kinetic constants for each analogue against OAT are shown in Table S1, and the kinetic constants against GABA-AT are given in Table S2. Gabaculine is the most potent of the compounds tested; however, 2 has one-third the binding potency and one-third the inactivation rate of gabaculine (Figure S3B and Table S1). The third best compound is 3 (now called CPP-115), also a potent GABA-AT inactivator, which is in clinical trials.41 Most importantly, however, we found that 2 does not inactivate GABA-AT and is only a very weak inhibitor (Ki 4.2 mM).42 Furthermore, 2 does not inactivate or inhibit either aspartate aminotransferase or alanine aminotransferase, even at 4 mM concentration (data not shown). Therefore, unlike gabaculine, 2 is highly selective for OAT and is a time- and concentration-dependent irreversible inhibitor; extensive dialysis of 2-inactivated OAT resulted in no return of enzyme activity.
Figure 1.
GABA analogues screened against OAT.
Because of its potency and selectivity, 2 was investigated for its ability to suppress the growth of HCC in vitro and in vivo. A significant decrease (p < 0.01) in AFP secretion in two hepatoma cell lines, Hep3B and HepG2, was observed (Figure S4B). Administration of 2 to HCC-harboring mice at 0.1 mg/kg (2 μg) and 1.0 mg/kg (20 μg) resulted in a significant suppression of AFP secretion in vivo (Figure 2A). Following 14 days of treatment (oral treatment was initiated on day 25), serum AFP levels increased 3.4-fold compared with a 10.9-fold increase in controls (7224 to 24857 vs 2671 to 29155 pg/mL, respectively). Following 21 days of treatment, serum AFP levels increased 49.8-fold in controls but only 8.2-fold and 14.4-fold for treatment with 1 and 0.1 mg/kg of 2, respectively.
Figure 2.
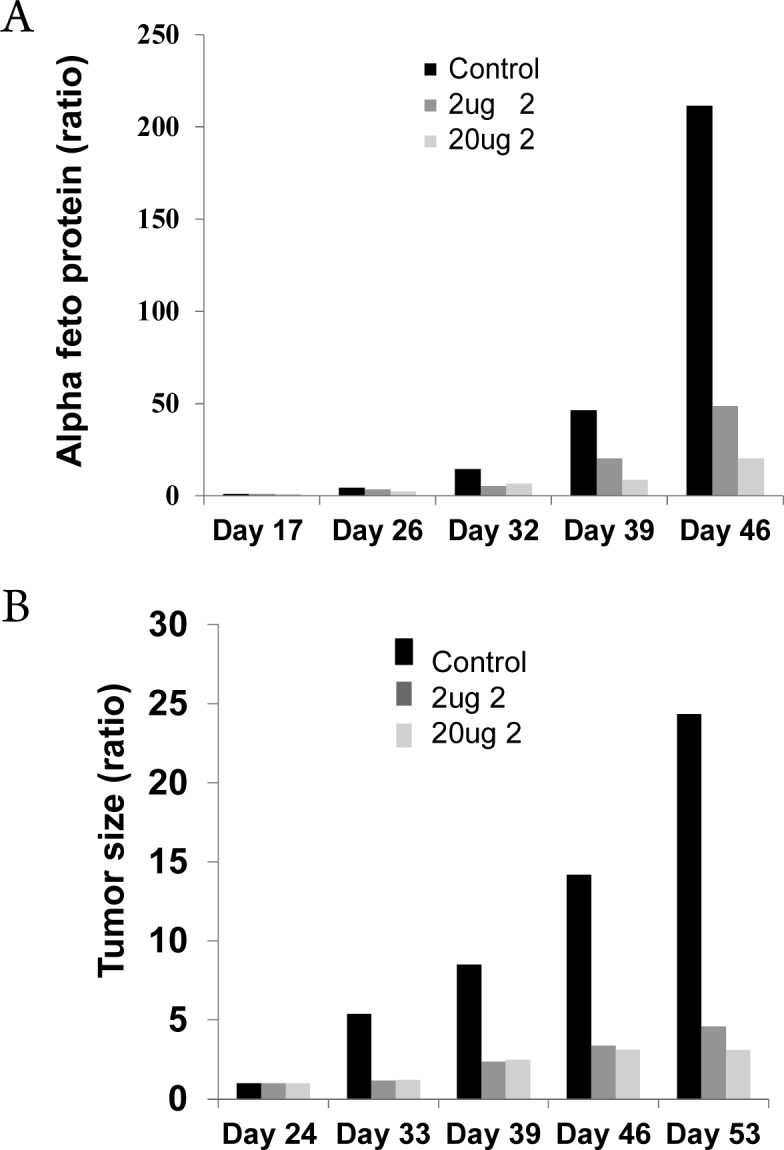
(A) Administration of 2 suppressed serum AFP levels in vivo. Mice were treated for 27 days, 3 times a week, starting 3 weeks following HCC transplantation with two doses of 2 (0.1 mg/kg (2 μg), dark gray bars; 1 mg/kg (20 μg), light gray bars), compared with untreated controls (black bars). Levels are normalized to the starting day of therapy. (B) Compound 2 suppressed tumor volume in both treated groups (0.1 mg/kg [2 μg], dark gray bars; 1.0 mg/kg [20 μg], light gray bars) compared to untreated controls (black bars).
In addition to AFP suppression, there was a highly significant reduction in tumor volume (normalized to the first day of therapy initiation) in both treated groups (0.1 mg/kg [2 μg] and 1.0 mg/kg [20 μg]) compared with controls (Figure 2B). After 28 days of treatment (oral treatment initiated on day 25), tumor sizes increased 24.2-fold in controls but only 3.1-fold and 4.5-fold with 1 and 0.1 mg/kg, respectively, of 2.
In conclusion, we have demonstrated, for the first time, that two potent irreversible inhibitors of OAT (gabaculine and 2) suppress AFP levels in hepatoma cells in vivo, and a selective OAT inhibitor (2), even at 0.1 mg/kg, dramatically reduces the growth of HCC in mice. Overexpression of the OAT gene in HCC and the ability to block the growth of HCC by OAT inhibitors suggest that OAT is an important potential therapeutic target to inhibit the growth of HCC.
Glossary
Abbreviations Used
- AFP
alpha fetoprotein
- GABA-AT
γ-aminobutyric acid aminotransferase
- GS
glutamine synthetase
- HCC
hepatocellular carcinoma
- NASH
nonalcoholic steatohepatitis
- OAT
ornithine aminotransferase
- PLP
pyridoxal 5′-phosphate
Biographies
Richard B. Silverman received his Ph.D. in organic chemistry from Harvard University and then did postdoctoral studies in enzymology at Brandeis University. He joined the chemistry faculty at Northwestern University in 1976; since 2004 he has been the John Evans Professor of Chemistry. Current projects include inactivators of GABA aminotransferase for epilepsy, inhibitors of nitric oxide synthase and modulators of CaV1.3 for Parkinson’s disease, activators of β-glucocerebrosidase for Gaucher’s disease, and inactivators of ornithine aminotransferase for hepatocellular carcinoma. Most recently he was named a Fellow of the National Academy of Inventors and the American Academy of Arts and Sciences.
Yaron Ilan received his MD degree from the Hebrew-University Hadassah Faculty of Medicine. After his residency in internal medicine and gastroenterology, he did his fellowship in liver and gut immunology at Albert Einstein College of Medicine and Mount Sinai Medical Center, both in New York, as well as a research fellowship at the Harvard University Research Institute. Dr. Ilan is a Professor of Medicine and the Chairman of the Department of Medicine at Hadassah-Hebrew University Medical Center in Jerusalem. His current research projects address the development of oral immune therapy compounds and molecules that target liver carcinogenesis.
Supporting Information Available
Figures S1–S5, Scheme S1, Tables S1 and S2, and experimental details. The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.5b00153.
Author Contributions
⊥ These authors contributed equally to this work.
We are grateful to The Roman-Epstein Liver Research Foundation (to Y.I.) and the National Institutes of Health (R01 DA030604 to R.B.S.) for financial support of this research.
The authors declare no competing financial interest.
Supplementary Material
References
- Sherman M.; Bruix J.; Porayko M.; Tran T.; Committee A. P. G. Screening for hepatocellular carcinoma: the rationale for the American Association for the Study of Liver Diseases recommendations. Hepatology 2012, 56, 793. [DOI] [PubMed] [Google Scholar]
- Tan C. H.; Low S. C.; Thng C. H. APASL and AASLD consensus guidelines on imaging diagnosis of hepatocellular carcinoma: a review. Int. J. Hepatol 2011, 2011, 519783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. D.; Roberts L. R. Hepatocellular carcinoma: a global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. K.; Kim J. K.; Kim M. Y.; Rhim H.; Han J. K. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology 2009, 49, 453. [DOI] [PubMed] [Google Scholar]
- Breuhahn K.; Gores G.; Schirmacher P. Strategies for hepatocellular carcinoma therapy and diagnostics: lessons learned from high throughput and profiling approaches. Hepatology 2011, 53, 2112. [DOI] [PubMed] [Google Scholar]
- Llovet J. M.; Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003, 37, 429. [DOI] [PubMed] [Google Scholar]
- de Lope C. R.; Tremosini S.; Forner A.; Reig M.; Bruix J. Management of HCC. J. Hepatol. 2012, 56 (Suppl 1), S75. [DOI] [PubMed] [Google Scholar]
- Xie B.; Wang D. H.; Spechler S. J. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig. Dis. Sci. 2012, 57, 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan M. E.; Brosnan J. T. Hepatic glutamate metabolism: a tale of 2 hepatocytes. Am. J. Clin. Nutr. 2009, 90, 857S. [DOI] [PubMed] [Google Scholar]
- Cadoret A.; Ovejero C.; Terris B.; Souil E.; Levy L.; Lamers W. H.; Kitajewski J.; Kahn A.; Perret C. New targets of beta-catenin signaling in the liver are involved in the glutamine targets of beta-catenin signaling in the liver are involved in the glutamine metabolism. Oncogene 2002, 21, 8293. [DOI] [PubMed] [Google Scholar]
- Lim K. T.; Gupta M. K.; Lee S. H.; Jung Y. H.; Han D. W.; Lee H. T. Possible involvement of Wnt/beta-catenin signaling pathyway in hatching and trophectoderm differentiation of pig blastocysts. Theriogenology 2013, 79, 284. [DOI] [PubMed] [Google Scholar]
- Gupta A.; Verma A.; Mishra A. K.; Wadhwa G.; Sharma S. K.; Jain C. K. The Wnt pathway: emerging anticancer strategies. Recent Pat. Endocr. Metab. Immune Drug Discovery 2013, 7, 138. [DOI] [PubMed] [Google Scholar]
- Voronkov A.; Krauss S. Wnt/beta-catenin signaling and small molecule inhibitors. Curr. Pharm. Des. 2013, 19, 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucero O. M.; Dawson D. W.; Moon R. T.; Chien A. J. A re-evaluation of the ″oncogenic″ nature of Wnt/beta-catenin signaling in melanom and other cancers. Curr. Oncol Rep. 2010, 12, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. D.; Chien A. J.; Dawson D. W. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012, 142, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot S.; Decaens T.; Niwa-Kawakita M.; Godard C.; Hamard G.; Kahn A.; Giovannini M.; Perret C. Liver-targeted disruption of Apc in mice activates beta-catenin signaling and leads to hepatocellulcar carcinomas. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 17216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot S.; Niwa-Kawakita M.; Hamard G.; Godard C.; Le Plenier S.; Houbron C.; Romagnolo B.; Berrebi D.; Giovannini M.; Perret C. Colorectal cancers in a new mouse model of familial adenomatous polyposis: influence of genetic and environmental modifiers. Lab Invest. 2004, 84, 1619. [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J.; Laurent-Puig P. Genetic diversity of hepatocellular carcinomas and its potential impact on targeted therapies. Pharmacogenomics 2007, 8, 997. [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J.; Benhamouche S.; Godard C.; Boyault S.; Grimber G.; Balabaud C.; Cunha A. S.; Bioulac-Sage P.; Perret C. Differential effects of inactivated Axin1 and activated beta-catenin mutations in human hepatocellular carcinomas. Oncogene 2007, 26, 774. [DOI] [PubMed] [Google Scholar]
- Thompson M. D.; Monga S. P. WNT/beta-catenin signaling in liver health and disease. Hepatology 2007, 45, 1298. [DOI] [PubMed] [Google Scholar]
- Nejak-Bowen K. N.; Monga S. P. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin. Cancer Biol. 2011, 21, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahmani R.; Just P. A.; Perret C. The Wnt/beta-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 709. [DOI] [PubMed] [Google Scholar]
- Armengol C.; Cairo S.; Fabre M.; Buendia M. A. Wnt signaling and hepatocarcinogenesis: the hepatoblastoma model. Int. J. Biochem. Cell Biol. 2011, 43, 265. [DOI] [PubMed] [Google Scholar]
- Amadasi A.; Bertoldi M.; Contestabile R.; Bettati S.; Cellini B.; di Salvo M. L.; Borri-Voltattorni C.; Bossa F.; Mozzarelli A. Pyridoxal 5′-phosphate enzymes as targets for therapeutic agents. Curr. Med. Chem. 2007, 14, 1291. [DOI] [PubMed] [Google Scholar]
- Souba W. W. Glutamine and cancer. Ann. Surg. 1993, 218, 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina M. A. Glutamine and cancer. J. Nutr. 2001, 131, 2539S. [DOI] [PubMed] [Google Scholar]
- Wise D. R.; Thompson C. B. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem. Sci. 2010, 35, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dang C. V. Links between metabolism and cancer. Genes Dev. 2012, 26, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- b DeBerardinis R. J.; Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shanware N. P.; Mullen A. R.; DeBerardinis R. J.; Abraham R. T. Glutamine: pleiotropic roles in tumor growth and stress resistance. J. Mol. Med. 2011, 89, 229. [DOI] [PubMed] [Google Scholar]
- b DeBerardinis R. J.; Mancuso A.; Daikhin E.; Nissim I.; Yudkoff M.; Wehrli S.; Thompson C. B. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 19345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka Y.; Enomoto N.; Nagayama K.; et al. Analysis of differentially expressed genes in human hepatocellular carcinoma using suppression subtractive hybridization. Br. J. Cancer 2001, 85, 228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K.; Morris H. P.; Katunuma N. Studies on the turnover rates of ornithine aminotransferase in Morris hepatoma 44 and host liver. J. Biochem 1976, 80, 1085. [DOI] [PubMed] [Google Scholar]
- a Rosenau J.; Hooman N.; Rifai K.; et al. Hepatitis B virus immunization with an adjuvant containing vaccine after liver transplanation for hepatitis B-relatede disease: failure of humoral and cellular immune response. Transpl In. 2006, 19, 828. [DOI] [PubMed] [Google Scholar]
- b Kaiser N.; Cerasi E.; Leibowitz G. Diet-induced diabetes in the sand rat (Psammomys obesus). Methods Mol. Biol. 2012, 933, 89. [DOI] [PubMed] [Google Scholar]
- Ungar H.; Adler J. H. The histogenesis of hepatoma occurring spontaneously in a strain of sand rats (Psammomys obesus). Am. J. Pathol. 1978, 90, 399. [PMC free article] [PubMed] [Google Scholar]
- Shah S. A.; Shen B. W.; Brunger A. T. Human ornithine aminotransferase complexed with L-canaline and gabculine: structural basis for substrate recognition. Structure 1997, 5, 1067. [DOI] [PubMed] [Google Scholar]
- Palfreyman M. G.; Schechter P. J.; Buckett W. R.; Tell G.; Koch-Weser J. The pharmacology of GABA-transaminase inhibitors. Biochem. Pharamacol. 1981, 30, 817. [DOI] [PubMed] [Google Scholar]
- Soper T. S.; Manning J. M. Inactivation of pyridoxal phosphate enzymes by gabaculine. Correlation with enzymic exchange of beta-protons. J. Biol. Chem. 1982, 257, 13930. [PubMed] [Google Scholar]
- Wood J. D.; Kurylo E.; Tsui D. Inhibition of aminotransferase enzyme systems by gabaculine. Neurosci. Lett. 1979, 14, 327. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Mechanism of the irreversible inhibition of gamma-aminobutyric acid-alpha-ketoglutaric acid transaminase by the neutrotoxin gabaculine. Biochemistry 1977, 16, 4604. [DOI] [PubMed] [Google Scholar]
- Fu M.; Silverman R. B. Isolation and characterization of the product of inactivation of gamma-aminobutyric acid aminotransferase by gabaculine. Bioorg. Med. Chem. 1999, 7, 1581. [DOI] [PubMed] [Google Scholar]
- Lee H.; Juncosa J. I.; Silverman R. B. Ornithine aminotransferase versus GABA aminotransferase: implications for the design of new anticancer drugs. Med. Res. Rev. 2015, 35, 286–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman R. B. The 2011 E. B. Hershberg Award for Important Discoveries in Medicinally Active Substances: (1S,3S)-3-Amino-4-difluoromethylenyl-1-cyclopentanoic acid (CPP-115), a GABA aminotransferase inactivator and new treatment for drug addiction and infantile spasms. J. Med. Chem. 2012, 55, 567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.; Silverman R. B. Fluorinated conformationally restricted gamma-aminobutyric acid aminotransferase inhibitors. J. Med. Chem. 2006, 49, 7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.