| Title: | Imidazolin-5-one Derivative Useful as FASN Inhibitors for the Treatment of Cancer | ||
| Patent Application Number: | WO 2015/095011 A1 | Publication date: | 25 June 2015 |
| Priority Application: | US 61/916,844 | Priority date: | 17 December 2013 |
| Inventors: | Connolly, P. J.; Lu, T. | ||
| Assignee Company: | Janssen Pharmaceutica NV; Turnhoutseweg 30, B-2340 Beerse (BE) | ||
| Disease Area: | Cancer, obesity related disorders, and liver related disorders | Biological Target: | Fatty acid synthase (FASN) |
| Summary: | The invention is in this patent application relates to 1H-imidazol-5(4H)-one derivatives represented generally by formula (I). These compounds are FASN inhibitors and may potentially provide treatments for cancer, obesity related disorders, and liver related disorders. | ||
| Fatty acid synthase (FASN) is a 250 kDa protein that contains seven functional domains; it is a key enzyme in the de novo synthesis of long-chain fatty acids starting with acetyl-coenzyme A (CoA) and malonyl-CoA using NADPH as a reducing cofactor. FASN is highly expressed in lipogenic tissues such as liver, lactating breast, fetal lung, and adipose tissue, but it is minimally expressed in most normal human tissues. The low level of FASN expression in normal cells occurs because these cells obtain their needed fatty acids from diet rather than from lipogenesis. It was discovered that the expression of FASN is highly up-regulated in various cancer tumors such as prostate, breast, colon, and lung cancer. The overexpression of FASN in tumor cells is believed to aid in the growth and survival of the tumors through multiple mechanisms. FASN provides saturated lipids for membrane synthesis; increased saturated lipid content in the membranes increases their resistance to chemotherapy. It also helps in improving the expression of growth factor receptor in lipid rafts and improving cell signaling. In addition, the consumption of NAPDPH due to FASN activities in tumor cells keeps the redox balance in check. | |||
| Studies using siRNA knock down or pharmacological inhibition of FASN in tumor cells have resulted in apoptosis in vitro and delayed tumor growth in vivo. Other studies using transgenic mouse models with FASN overexpression in the prostate established the role of FASN as a potential oncogene. The results of many studies have suggested that FASN exerts its oncogenic effect by inhibiting the intrinsic pathway of apoptosis. FASN expression and activities are up-regulated by androgens and epidermal growth factor. It is also overexpressed in androgen-independent prostate cancers probably through activation of the PI3K/Akt pathway. | |||
| The above data point to the importance of the inhibition of FASN activities as an emerging and promising therapeutic target that may lead to the development of new effective treatment for cancer. FASN inhibitors such as the compounds described in this patent application may potentially provide needed therapeutics for the treatment of several forms of cancer. In addition, FASN has been implicated in diabetes and/or regulation of the general wellness of the liver. Thus, FASN inhibitors may also potentially provide treatments for obesity, type II diabetes mellitus, syndrome X, and disorders of the liver. They may also be useful as treatments for other FASN-mediated disorders including lack of appetite control and inflammatory conditions. | |||
| Important Compound Classes: | 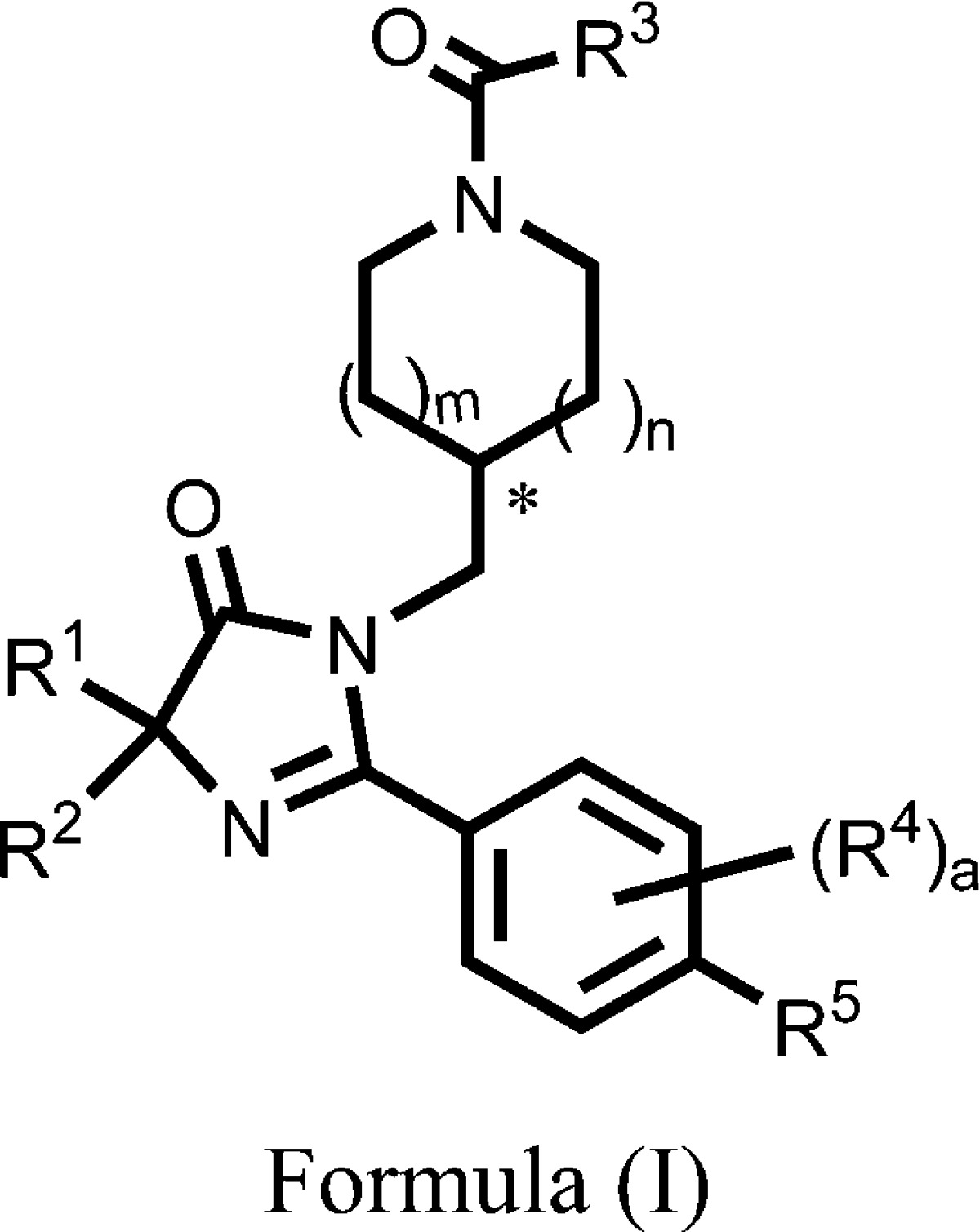 |
||
| Key Structures: | The inventors reported the structures of 99 compounds representing examples of formula (I) including the following compounds: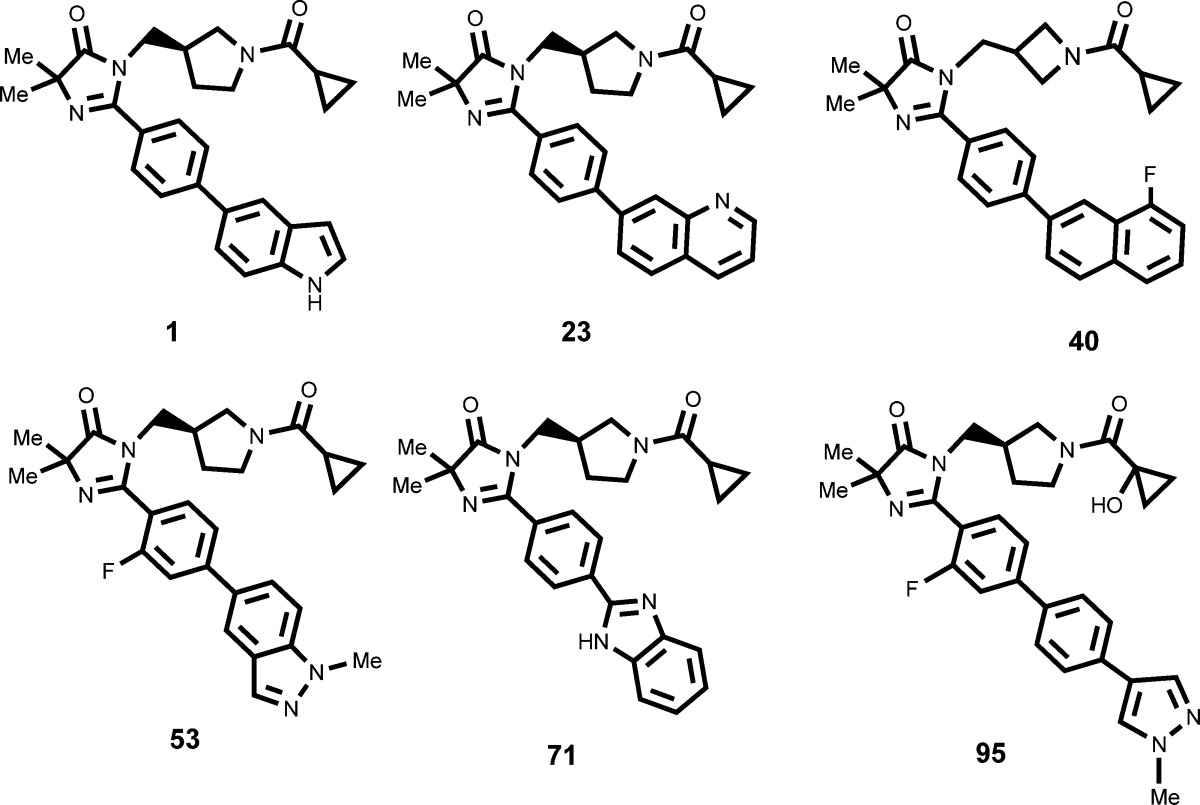
|
||
| Biological Assay: | The inventors described the following biological assays to test formula (I) compounds: | ||
| |||
| Biological Data: | The reported assay results from testing the representative examples (see structures above) using assays 1–4 are listed in the following table: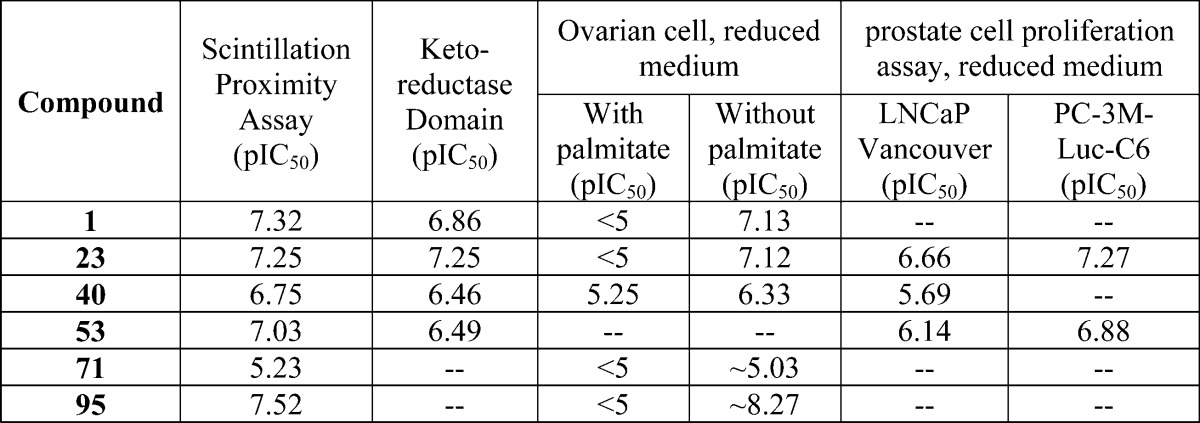
|
||
| Recent Review Articles: | 1. Wu X.; Qin L.; Fako V.; Zhang J.-T.. Adv. Biol. Regul. 2014, 54, 214–221. | ||
| 2. Maruyama T.; Murata S.; Ohkohchi N.. Horiz. Cancer Res. 2012, 49, 153–177. | |||
| 3. Pandey P. R.; Liu W.; Xing F.; Fukuda K.; Watabe K.. Recent Pat. Anticancer Drug Discovery 2012, 7 ( (2), ), 185–197. | |||
| 4. Liu H.; Liu J.-Y.; Wu X.; Zhang J.-T.. Int. J. Biochem. Mol. Biol. 2010, 1 ( (1), ), 69–89. | |||
The authors declare no competing financial interest.


