Abstract
Meropenem dosing in critically ill patients with septic shock and continuous renal replacement therapy (CRRT) is complex, with the recommended maintenance doses being 500 mg to 1,000 mg every 8 h (q8h) to every 12 h. This multicenter study aimed to describe the pharmacokinetics (PKs) of meropenem in this population to identify the sources of PK variability and to evaluate different dosing regimens to develop recommendations based on clinical parameters. Thirty patients with septic shock and CRRT receiving meropenem were enrolled (153 plasma samples were tested). A population PK model was developed with data from 24 patients and subsequently validated with data from 6 patients using NONMEM software (v.7.3). The final model was characterized by CL = 3.68 + 0.22 · (residual diuresis/100) and V = 33.00 · (weight/73)2.07, where CL is total body clearance (in liters per hour), residual diuresis is the volume of residual diuresis (in milliliters per 24 h), and V is the apparent volume of distribution (in liters). CRRT intensity was not identified to be a CL modifier. Monte Carlo simulations showed that to maintain concentrations of the unbound fraction (fu) of drug above the MIC of the bacteria for 40% of dosing interval T (referred to as 40% of the ƒuT>MIC), a meropenem dose of 500 mg q8h as a bolus over 30 min would be sufficient regardless of the residual diuresis. If 100% of the ƒuT>MIC was chosen as the target, oligoanuric patients would require 500 mg q8h as a bolus over 30 min for the treatment of susceptible bacteria (MIC < 2 mg/liter), while patients with preserved diuresis would require the same dose given as an infusion over 3 h. If bacteria with MICs close to the resistance breakpoint (2 to 4 mg/liter) were to be treated with meropenem, a dose of 500 mg every 6 h would be necessary: a bolus over 30 min for oligoanuric patients and an infusion over 3 h for patients with preserved diuresis. Our results suggest that residual diuresis may be an easy and inexpensive tool to help with titration of the meropenem dose and infusion time in this challenging population.
INTRODUCTION
Meropenem is a broad-spectrum carbapenem with high levels of activity against Gram-positive and Gram-negative pathogens, including Pseudomonas aeruginosa, Acinetobacter spp., and anaerobes (1), and is one of the most prescribed antibiotics for the empirical treatment of severe infections (2). It exhibits optimal killing activity when the concentrations of the unbound fraction (fu) of drug in plasma are maintained above the MIC of the bacteria for a certain percentage of dosing interval T (referred to as the percentage of the ƒuT>MIC), which in in vitro and in vivo animal studies has been defined to be about 40% (3). However, some clinical data suggest that critically ill patients may require a higher percentage of the ƒuT>MIC, even 100% (4, 5).
Meropenem is a hydrophilic, small molecule with a low volume of distribution (V; 0.3 liter/kg) and a very low level of protein binding (<2%). These characteristics make meropenem a drug mainly eliminated by the kidneys, as only the unbound fraction is available for glomerular filtration (major elimination pathway) (1). This also makes meropenem a dialyzable drug because the main determinants of drug clearance (CL) while a patient is receiving renal replacement therapy (RRT) are a low molecular size, a high affinity for water, a low V, and a large unbound fraction (6). Thus, there is a potential combined impact of RRT and residual renal function on meropenem total CL, which may be particularly important for critically ill patients with septic shock and a requirement for continuous renal replacement therapy (CRRT). For these patients, available guidelines recommend that 500 to 1,000 mg of meropenem every 8 h (q8h) to every 12 h (q12h) be prescribed (7), which is a considerably broad dose range. However, this population is subject to conditions that may significantly influence meropenem pharmacokinetics (PKs) and, consequently, modify the dosing requirements, such as hypoproteinemia, variable urine output, or diverse CRRT settings (6). It follows that while several studies have described meropenem PKs in critically ill patients with continuous venovenous hemofiltration (CVVHF) and continuous venovenous hemodiafiltration (CVVHDF) (8–19), empirical dosing at the bedside is still challenging in this scenario.
Aims.
The aims of this study were to describe the PKs of meropenem in critically ill patients with septic shock and CRRT, to identify the sources of PK variability in these patients, and to perform different dosing simulations to assess their probability of target attainment by MIC, in order to provide empirical dosing recommendations based on clinical characteristics.
MATERIALS AND METHODS
Patients.
We performed a multicenter, prospective, open-label PK study in the intensive care units of the Hospitals Corporació Sanitària Universitària Parc Taulí of Sabadell (CSUPT), Clínic of Barcelona (HCB), and Joan XXIII (HJ23) of Tarragona, Spain. Patients were enrolled between January 2012 and May 2014. Authorization for the study was granted by the Spanish Regulatory Medicines Agency (code IEM-ANT-2012-1). Ethical approval was obtained from the local ethical committees, and the study was conducted following the Declaration of Helsinki guidelines. Consent to participate was obtained from the patient's legal representative. Inclusion criteria were an age of ≥18 years, a diagnosis of septic shock by the criteria of the Surviving Sepsis Campaign guidelines (20), CRRT, and an indication for treatment with meropenem. The major exclusion criterion was severe chronic kidney disease requiring RRT. The meropenem dose and infusion time were at the discretion of the treating physician. The drug was administered through a separate lumen of a venous catheter using free-fall bolus systems or volumetric infusion pump controllers, as required.
Demographic and clinical data.
The patients' demographic and clinical data were collected. Age, weight, height, sex, site of infection, serum biochemistry, a requirement for vasopressors, CRRT settings, filter downtime, the level of residual diuresis (defined as the volume of urine collected over the 24 h of the natural day of the study), severity scores at admission (acute physiology and chronic health evaluation II [APACHE II] score) (21) and on the day of study (sequential organ failure assessment [SOFA] score) (22), the isolated microorganisms and the meropenem MICs for those microorganisms, the number of days of antibiotic therapy, and hospital survival were recorded (23). These data came from the clinical routine and were registered in a database available only to the researchers.
Continuous renal replacement therapy.
Patients prescribed either CVVHDF or CVVHF were considered for inclusion. Prisma CRRT systems (Hospal, France) were used. A 1.5-m2 surface-treated acrylonitrile and sodium methallyl sulfonate copolymer filter (AN69ST; PrismaFlex ST150; Hospal, France) was used at HJ23, and a 0.9-m2 acrylonitrile and sodium methallyl sulfonate copolymer filter (AN69; PrismaFlex M100; Gambro Hospal, Switzerland) was used at CSUPT and HCB. All CRRT settings were prescribed at the discretion of the treating physician.
Blood sampling.
For each sample, 5 ml of arterial blood was collected after at least 24 h of CRRT and meropenem therapy. For bolus sampling, 6 samples were collected at 10 min predose; at 0 min, 15 min, 60 min, and between 3 and 6 h after the end of the infusion; and just before the next dose. For extended infusion sampling, 5 samples were collected at 10 min predose; at 0 min, 60 min, and 120 min after the end of the infusion; and just before the next dose. Within 1 h of collection, samples were centrifuged at 3,000 rpm at 4°C for 10 min and plasma was frozen at −80°C for posterior analysis.
LC-MS analysis.
The total meropenem concentration in plasma was measured using liquid chromatography (LC) coupled to tandem mass spectrometry (MS/MS) (1200 HPLC binary pump [Agilent Technologies], API 4000 AB Sciex MS) in an external laboratory using a validated method. The method was linear over the range of meropenem concentrations of 0.4 to 300 mg/liter. Within-run and between-run precision and accuracy (coefficients of variation, ≤10%) showed adequate results, according to the guidelines of the European Medicines Agency (24).
Statistical analysis.
Statistical analysis was performed using SPSS software (v20) for Macintosh (IBM SPSS Statistics, USA). Results are expressed as absolute and relative frequencies for categorical variables and as medians (ranges) for continuous variables. A two-tailed Student t test was used to compare normally distributed variables, the Mann-Whitney U test was used to compare nonnormally distributed variables, and the chi-square or Fisher's exact test was used to compare categorical variables, as appropriate. The significance level for all analyses was defined as a P value of ≤0.05.
PK modeling.
Nonlinear effects modeling was performed using NONMEM (v7.3) (25) and XPose (v4.0) (26) software following a three-step strategy: (i) basic population model selection, (ii) covariate selection, and (iii) validation (27, 28). The first-order conditional estimation method with interaction was used for parameter estimation. Interindividual variability (IIV) was modeled as log normal after being tested for log normality. Additive, proportional, and combined error models were tested for residual variance. The goodness of fit for a model was assessed by (i) significant decreases in the −2 log likelihood of the objective function value, (ii) plots of population and individual predicted versus observed concentrations and conditional weighted residuals (CWRES) versus observed concentrations and time (29, 30), and (iii) changes in the standard error of parameter estimates (precision).
In a second step, all reasonable demographic and clinical variables were tested for inclusion as covariates in the basic population PK model. Graphical examination and the generalized additive models procedure (26) were used to investigate their effects on model parameters. Continuous covariates were assessed as a proportional or a power function. Categorical variables were included in the model as Pj = PPOP + θCOV · (1 − Covi), where Pj is the PK parameter for the jth patient, Covi is a numeric index value, PPOP is the typical value of a PK parameter for the reference covariate values, and θCOV is the multiplicative factor for the influence of this covariate on the PK parameter. Each covariate investigated was retained if it led to an improved fit, as evaluated by biological plausibility, graphical displays based on the agreement between the observed and predicted drug concentrations, the uniformity of the distribution of the CWRES, improvement of the precision in parameter estimates, and the log likelihood ratio test. The extent of Bayesian shrinkage, as a measure of model overparameterization, was evaluated for each PK parameter (31).
Model evaluation.
Internal validation of the PK model was performed by graphical and statistical methods, including visual predictive checks (32). The bootstrap resampling technique (200 replicated data sets) was used to build the confidence intervals (CIs) of the PK parameters to assess their stability and evaluate the robustness of the final model (33).
The external predictive performance of the PK model was assessed by analyzing data from new individuals (20 to 30% of the enrolled subjects) (34, 35), following the Food and Drug Administration guidelines (36). Individual predicted meropenem concentrations for all sampling times were obtained by Bayesian estimation. Bias was assessed in terms of individual and population prediction error (IPE and PPE, respectively; in percent). Precision was assessed as absolute individual and population prediction error (IAPE and PAPE, respectively; in percent) (37).
Dosing simulations.
Monte Carlo dosing simulations were performed. Each simulation generated concentration-time profiles for 1,000 subjects per dosing regimen using the final estimated population PK parameters. Three bolus regimens (500 mg q8h, 500 mg every 6 h [q6h], and 1,000 mg q8h over 30 min) and three extended infusion regimens (500 mg q8h, 500 mg q6h, and 1,000 mg q8h over 3 h) were simulated using a mean patient body weight of 70 kg and three categories of residual diuresis (50 ml, 300 ml, and 700 ml), accounting for the definitions of anuria (<100 ml/24 h), oliguria (100 to 500 ml/24 h), and conserved urine output (>500 ml/24 h), respectively (38). From these data, the percentages of patients with 40% of the ƒuT>MIC, 100% of the ƒuT>MIC, and a trough (minimum) concentration (Cmin)/MIC ratio equal to 5, according to meropenem clinical susceptibility breakpoints (39) (probability of target attainment [PTA]), were calculated.
RESULTS
Subjects and samples.
Thirty patients with septic shock and CRRT receiving meropenem were enrolled. Table 1 summarizes the patients' demographic and clinical characteristics. The median age was 66.5 years (range, 34 to 85 years), the median APACHE score on admission was 24 (range, 5 to 44), and the median SOFA score on the day of the study was 12 (range, 4 to 19). Sources of infection were intra-abdominal (n = 13 patients), respiratory (n = 7), bloodstream (n = 4), urinary tract (n = 2), and central nervous system (n = 2). It could not be determined in 2 patients. Twenty-six patients were prescribed CVVHDF, and 4 were prescribed CVVHF. Regarding the CRRT settings, the median intensity on the day of the study was 34.7 ml/kg/h (range, 18.7 to 60.1 ml/kg/h), and the median blood flow was 200 ml/min (range, 130 to 250 ml/min). In four patients, the filters were nonfunctional during a fraction of the sampling interval due to filter clotting and exchange: in one patient during antibiotic administration (30 min), in two patients for 1 h, and in one patient for 2.5 h. Visual inspection did not identify alterations in the meropenem concentration-over-time profiles of these individuals that could be attributed to these incidences. With regard to urine output on the day of the study, 14 patients were anuric (<100 ml/24 h), 11 patients were oliguric (100 to 500 ml/24 h), and 5 patients had preserved diuresis (>500 ml/24 h). The median urine output was 137.5 ml/24 h (range, 0 to 2,050 ml/24 h). For the index and validation data set, subjects were comparable in all characteristics except for vasopressor use at the time of the study: two of the patients in the validation data set were not on vasopressors when samples were collected. Concerning microbiology, positive cultures were obtained from 23 patients (76.7%). The most frequently isolated microorganisms were Escherichia coli (21.4%) and Pseudomonas aeruginosa (14.3%). Table 2 shows the meropenem MIC values for the 28 isolated strains.
TABLE 1.
Demographics and clinical characteristics of subjects included in index data set, validation data set, and overall
| Variable | Model development data set (n = 24) | Validation data set (n = 6) | P value | All data (n = 30) |
|---|---|---|---|---|
| Median (range) age (yr) | 68.5 (50–81) | 56 (34–85) | 0.40 | 66.5 (34–85) |
| No. (%) female | 12 (50) | 2 (33.3) | 0.66 | 14 (46.7) |
| Median (range) wta (kg) | 72.8 (49–95) | 75 (68–126) | 0.24 | 72.8 (49–126) |
| Median (range) APACHE scorea | 26 (5–44) | 20 (15–33) | 0.18 | 24 (5–44) |
| Median (range) SOFA scoreb | 12 (4–19) | 9 (5–19) | 0.67 | 12 (4–19) |
| No. (%) of patients with hepatic impairmentc | 5 (20.8) | 1 (20) | 0.88 | 6 (20) |
| No. (%) of patients receiving: | ||||
| Vasopressorsb | 24 (100) | 4 (66.7) | 0.034d | 28 (93.3) |
| Mechanical ventilationb | 23 (95.8) | 6 (100) | 1 | (29) 96.7 |
| No. of patients receiving CVVHDF/no. of patients receiving CVVHF | 21/3 | 5/1 | 1 | 26/4 |
| Median (range) no. of accumulated days of meropenemb | 4 (2–22) | 2.5 (2–4) | 0.2 | 3 (2–22) |
| Median (range) total CRRT intensityb,e (ml/kg/h) | 34.5 (18.7–60.1) | 39.2 (30.6–49.5) | 0.36 | 34.7 (18.7–60.1) |
| Median (range) dialysate flow rateb (ml/h) | 1,000 (500–1,600) | 900 (800–1,350) | 0.73 | 1,000 (500–1,600) |
| Median (range) ultrafiltrate flow rateb (ml/h) | 1,200 (750–2,000) | 1,800 (1,000–2,500) | 0.06 | 1,550 (750–2,500) |
| Median (range) blood flowb (ml/min) | 200 (130–250) | 200 (200–250) | 0.38 | 200 (130–250) |
| Median (range) albumin concnb (g/liter) | 21.3 (12.4–38) | 24.6 (18.1–32.6) | 0.61 | 23.4 (12.4–38) |
| Median (range) urea concnb (mg/dl) | 64.3 (22–168) | 52 (29–98) | 0.34 | 61.7 (22–168) |
| Median (range) creatinine concnb (mg/dl) | 1.6 (0.7–2.6) | 0.99 (0.4–2.3) | 0.14 | 1.4 (0.4–2.6) |
| Median (range) vol of diuresisb (ml/24 h) | 76.5 (<10–880) | 282.5 (82–2,050) | 0.11 | 137.5 (<10–2,050) |
| % (no.) of patients surviving | 58.3 (14) | 50 (3) | 1 | 56.7 |
On admission.
On the day of the study.
Hepatic impairment was defined as liver function test results with values >2 times the upper limit of normality.
Statistically significant difference (P < 0.05).
CRRT intensity was defined as (filtrate + dialysate flow rate)/(ideal body weight) for CVVHDF and as (filtrate flow rate)/(ideal body weight) for CVVHF, using 24 kg/m2 as the ideal body mass index.
TABLE 2.
Isolated microorganisms and meropenem susceptibility by MIC
| Microorganism | No. of isolates | MIC (mg/liter) |
|---|---|---|
| Burkholderia cepacia | 1 | 1 |
| Clostridium intestinale | 1 | 2 |
| Enterobacter cloacae | 1 | 1 |
| Enterococcus faecalis | 2 | 2 |
| Enterococcus faecalis | 1 | NDa |
| Enterococcus faecium | 1 | 8 |
| Enterococcus faecium | 1 | ND |
| Escherichia coli | 6 | 2 |
| Klebsiella pneumoniae | 1 | 32 |
| Listeria monocytogenes | 1 | ND |
| Moraxella catarrhalis | 1 | 1 |
| Pseudomonas aeruginosa | 1 | 1 |
| Pseudomonas aeruginosa | 1 | 2 |
| Pseudomonas aeruginosa | 1 | 4 |
| Pseudomonas aeruginosa | 1 | 8 |
| Salmonella enterica serovar Enteritidis | 1 | 2 |
| Serratia marcescens | 1 | 2 |
| Staphylococcus aureus | 1 | 2 |
| Staphylococcus epidermidis | 3 | ND |
| Stenotrophomonas maltophilia | 1 | ND |
ND, not determined.
Patients were prescribed meropenem at 500 mg q12h over 30 min (n = 1 subject); 500 mg q8h over 30 min (n = 2) or as a 3-h infusion (n = 3); 500 mg q6h as a 3-h infusion (n = 1); 1,000 mg q12h over 30 min (n = 6), as a 3-h infusion (n = 1), or as a 4-h infusion (n = 1); 1,000 mg q8h over 30 min (n = 8), as a 3-h infusion (n = 5), or as a 4 h-infusion (n = 1); or 2,000 mg q8h over 30 min (n = 1). The median duration of meropenem therapy was 10 days (range, 4 to 28 days).
Population PK analysis.
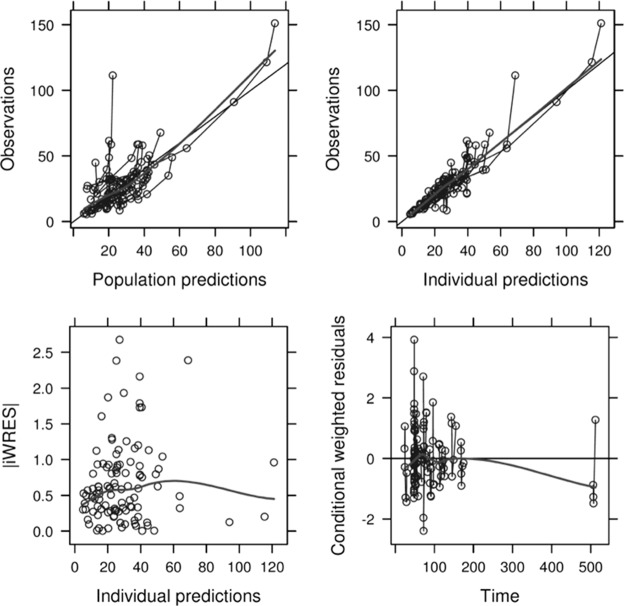
The population PK model was developed using data from 24 subjects (124 samples). Data were better described by a one-compartment linear model characterized by population CL and V at steady state, with interindividual variability being incorporated into both PK parameters. Residual variability consisted of additive and proportional error. Goodness-of-fit plots showed good accordance between observed (OBS), predicted (PRED), and individual predicted (IPRED) concentrations (Fig. 1). The mean ± standard deviation of the CWRES was close to 0, and residual error plots did not show systematic deviations over time. The magnitude of ε shrinkage was 14.5%. The model parameters had moderate levels of η shrinkage for CL (33.3%) and V (20.9%).
FIG 1.
Goodness-of-fit plots for the final population PK model. (Top left) Plot of observed meropenem concentrations versus population predictions. Solid thin line, line of identity; solid thick line, data smoother. (Top right) Plot of observations versus individual predictions. Solid thin line, line of identity; solid thick line, data smoother. (Bottom left) Plot of individual weighted residuals (iWRES) versus individual predictions. Thick line, data smoother. (Bottom right) Plot of conditional weighted residuals versus time. Solid thin line, zero slope line; solid thick line, data smoother. Predicted concentrations are in milligrams per liter; time is in hours.
Concerning the covariate analysis, residual diuresis significantly influenced meropenem CL, whereas CRRT intensity, filter downtime, blood flow, type of membrane, and albumin concentration did not. Concerning V, only total body weight on admission showed a significant impact on the parameter, whereas severity scores, age, and albumin concentration did not. The final model is displayed in Table 3 and summarized as follows: CL = 3.68 + 0.22 · (residual diuresis/100), and V = 33.00 · (weight/73)2.07, where CL is in liters per hour, residual diuresis is in milliliters and is normalized to the defined cutoff for anuria (38), V is in liters, and weight is normalized to the median weight of our patient population.
TABLE 3.
Population pharmacokinetic estimates for the final model and bootstrap resultsa
| Parameter | Estimate (% RSE) | Median bootstrap value (95% CI) |
|---|---|---|
| CL (liters/h) | ||
| θCL | 3.68 (11) | 3.59 (2.90 to 4.46) |
| θDIUR | 0.22 (47) | 0.22 (0.003 to 0.44) |
| V (liters) | ||
| θV | 33.00 (10) | 31.94 (26.65 to 39.35) |
| θWT | 2.07 (24) | 2.27 (0.82 to 3.32) |
| IIV_CL (% CV) | 37 (27) | 37.15 (24.35 to 46.12) |
| IIV_V (% CV) | 45 (61) | 47.89 (12.25 to 65.04) |
| Additive residual error (mg/liter) | 0.0002 (42.76) | 0.0002 (0.0001 to 0.001) |
| Proportional residual error | −0.258 (10) | −0.25 (−0.35 to −0.17) |
RSE, relative standard error; CL, total body clearance; V, apparent volume of distribution; θCL, typical value for CL in the population; θDIUR, multiplicative factor for the influence of residual diuresis on CL; θV, typical value for V in the population; θWT, power factor for the influence of weight on V; IIV_CL, interindividual variability associated with CL; IIV_V, interindividual variability associated with V; CV, coefficient of variation.
Validation.
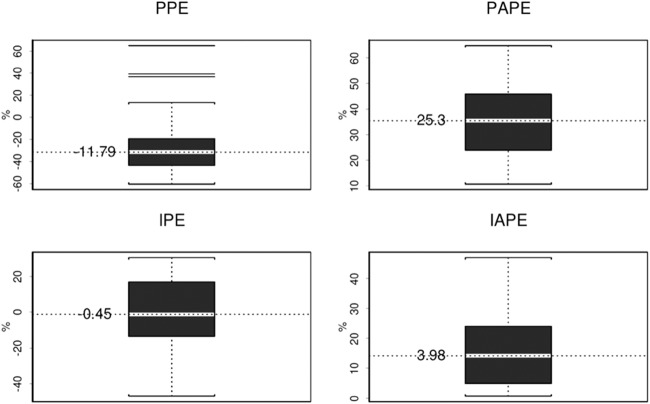
The results from the visual predictive check plot showed that practically all observations dropped into the 95% CI. The statistical distributions of the parameter estimates obtained from the bootstrap analyses are shown in Table 3. The median values of the parameters estimated from the bootstrap analyses were in good agreement with the NONMEM point estimates, and the 95% CIs were reasonably narrow, demonstrating satisfactory precision. With respect to external validation, mean bias and precision for the maximum a posterior Bayesian estimates (IPRED) were −0.45% and 3.98%, respectively, much better than those values obtained from the population PK model-based estimates (PRED), which were −11.79% and 25.3%, respectively (Fig. 2).
FIG 2.
Bias and precision for model estimates for external validation. Box plots of the population prediction error (PPE), population absolute prediction error (PAPE), individual prediction error (IPE), and individual absolute prediction error (IAPE) are shown. The white band in each error box marks the 50th percentile, and the value is presented; the box boundaries are at the 25th and 75th percentiles, and the limits of the whiskers are at the 10th and 90th percentiles. In the top left panel, lines outside the 10th and 90th percentiles represent the outliers from the model estimates for external validation.
Simulations.
PTA versus MIC profiles for simulations of different dosing regimens by residual diuresis and the percentage of the ƒuT>MIC target are presented in Table 4. A PTA of >90% was considered satisfactory. For the attainment of the classical pharmacodynamic (PD) target for carbapenems, i.e., 40% of the ƒuT>MIC, 500 mg q8h as a 30-min bolus would be sufficient for the treatment of bacteria with MICs even close to the susceptibility breakpoint (MICs ≤ 4 mg/liter), regardless of urine output. If 100% of the ƒuT>MIC was chosen as the PD target, oligoanuric patients would require a dose of 500 mg q8h over 30 min for the treatment of susceptible bacteria (MICs < 2 mg/liter), while patients with diuresis of >500 ml/24 h may require the same dose given as a 3-h infusion. If bacteria with MICs close to the resistance breakpoint (MICs, 2 to 4 mg/liter) were to be treated with meropenem, a dose of 500 mg q6h would be necessary and would need to be administered as a 30-min bolus for oligoanuric patients and as a 3-h infusion for patients with preserved diuresis. For the attainment of more aggressive PD targets, such as five times the Cmin/MIC ratio described by Li et al. (5), doses of 1,000 mg q8h as a 3-h infusion or higher would be required regardless of urine output. Table 5 summarizes the recommendations developed from these simulated data.
TABLE 4.
PTA by MIC for simulations of different dosing regimens of meropenem stratified by residual diuresis and pharmacodynamic targeta
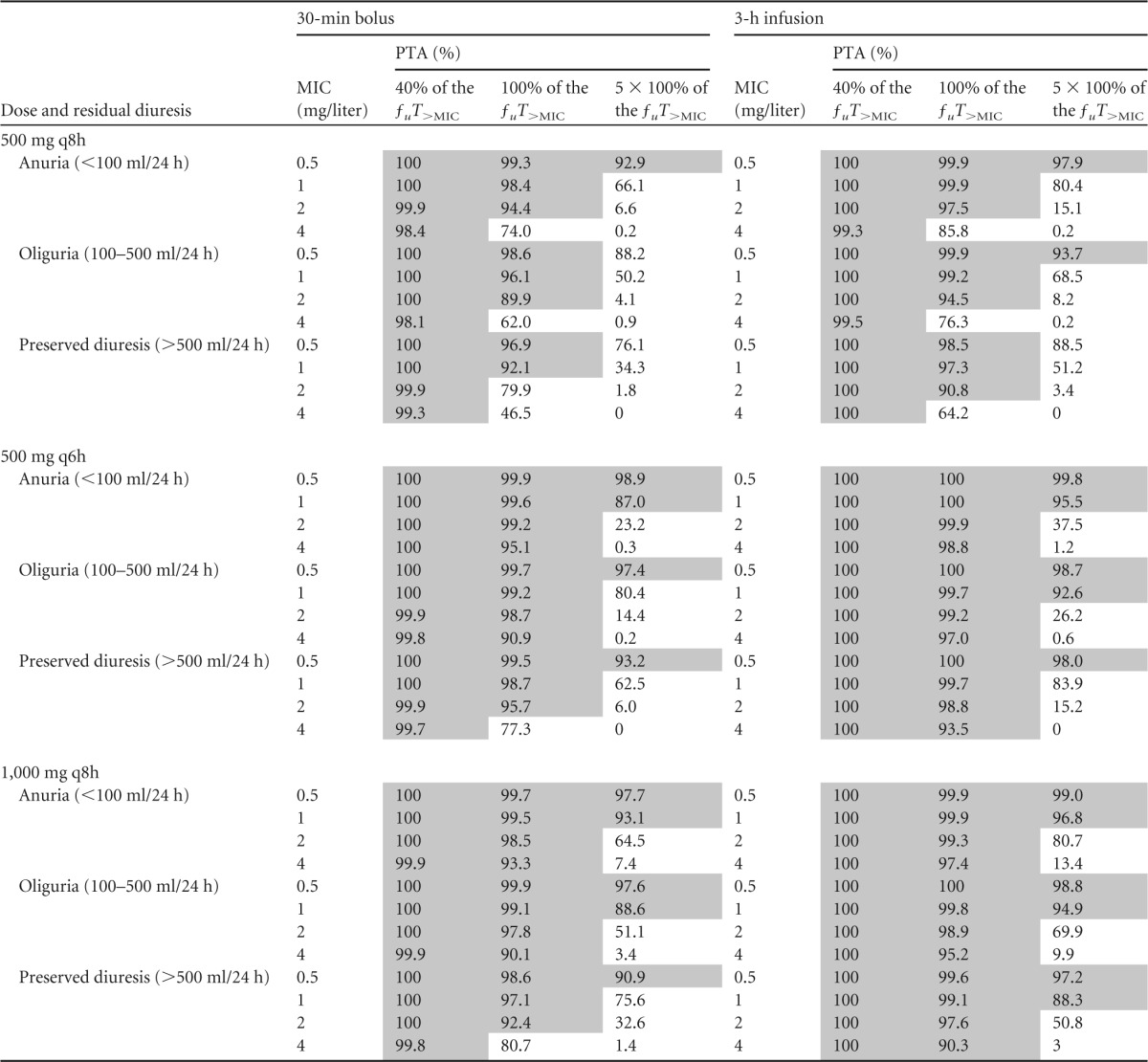
Shaded areas correspond to a PTA of ≥90%.
TABLE 5.
Summary of meropenem maintenance dosing recommendations based on the results of the present study
| PD target | Pathogen MIC (mg/liter) | Dose recommendation |
|---|---|---|
| 40% of the ƒuT>MIC | ≤4 | 500 mg q8h as a 30 min-bolus for all urine outputs |
| 100% of the ƒuT>MIC | ≤2 | 500 mg q8h as a 30-min bolus for oligoanuria, 500 mg q8h as a 3-h infusion for preserved diuresis |
| 2–4 | 500 mg q6h as a 30-min bolus for oligoanuria, 500 mg q6h as a 3-h infusion for preserved diuresis | |
| Cmin/MIC = 5 | ≤1 | 1,000 mg q8h as a 3-h infusion for all urine outputs |
DISCUSSION
To our knowledge, this is the largest multicenter study to have characterized the PKs of meropenem in critically ill patients with septic shock and CRRT. Our PK parameter estimates were in agreement with those from previous studies with a comparable population (15, 18).
Our main finding is the relationship existing among the 24-h urine output, the pathogen MIC, and meropenem dosing requirements for the maintenance phase of therapy, i.e., after 24 h of meropenem therapy and CRRT commencement. In general, antibiotic dose adjustments in critically ill patients are very challenging for the clinician because, unlike other drugs, such as vasopressors or sedatives, among others, the pharmacological effect of antibiotics is not immediately evident but requires a certain period of time, even days, to be visible. For critically ill patients with septic shock and a CRRT requirement, detection of the pharmacological effect of antibiotics is even more challenging due to all the PK changes driven by critical illness and the use of extracorporeal devices (6). In spite of this difficulty, the attainment and maintenance of therapeutic concentrations are crucial, as they have an impact on both clinical outcomes and the development of bacterial resistances. In this context, we have identified that consideration of residual diuresis might be advantageous for meropenem maintenance dose and infusion time adjustment on the basis of the MIC of the pathogen. For the attainment of a PD target of 100% of the ƒuT>MIC, fixed doses would be required, depending on the MIC of the bacteria, but the infusion time would depend on residual diuresis: oligoanuric patients would benefit from a 30-min bolus, while a 3-h extended infusion would be more appropriate for those patients with preserved diuresis. One may hypothesize that residual diuresis may influence meropenem requirements because a given percentage of the administered dose is eliminated with the urine. Conversely, for the attainment of the classical PD target for carbapenems (40% of the ƒuT>MIC), a standard dose of 500 mg q8h as a bolus over 30 min would be sufficient for all cases. Further, for the attainment of a more aggressive target, such as a Cmin/MIC ratio of 5, doses of 1,000 mg q8h as a 3-h infusion or higher would be required. Of note, empirical dosing on the first day would still need to be made on the basis of the predicted V and local antibiogram data, as the use of the 24-h urine output measure can have a meaningful impact only with empirical dosing after 24 h, i.e., during the maintenance phase of therapy.
It is important to highlight that we have principally based our empirical dosing recommendations on targeting of the 100% of the ƒuT>MIC rather than 40% of the ƒuT>MIC described in the classical studies (3). We believe that such a thoughtful pharmacodynamic target is more recommendable for our patient population for several reasons. First, emerging evidence has associated a higher percentage of the ƒuT>MIC with better outcomes (4, 5). For instance, Li et al. reported that trough concentrations higher than 5 times the MIC of the pathogen (Cmin/MIC ratio = 5) were associated with better clinical and microbiological success rates (5). Also, Roberts et al. found that a higher percentage of the time that the concentration is greater than the MIC (T>MIC) had a tendency to better the odds of survival compared to those with a lower percentage of the T>MIC (odds ratios, 1.02 [95% CI, 1.01 to 1.04] for a T>MIC of 50% and 1.56 [95% CI, 1.15 to 2.13] for a T>MIC of 100%), even though these odds data were not statistically compared (4). Further, all this evidence is based on plasma concentrations, but it is well-known that critically ill patients with severe infections exhibit microcirculatory alterations that impair the tissue distribution and lead to a lower percentage of the ƒuT>MIC at the target site. This was shown in a nice study by Varghese et al., who reported that the tissue concentrations of meropenem in critically ill patients with CVVHDF accounted for a median of 60 to 70% of the plasma concentrations (18), which may be even lower in patients with septic shock. Due to the severity of the sickness in patients with septic shock, we believe that more aggressive pharmacodynamic targets should be preferred for ensuring early and adequate antimicrobial therapy. We also report the dosing recommendations for the attainment of a more ambitious target that has been associated with better outcomes in patients treated with meropenem (Cmin/MIC ratio = 5) (5). However, we believe that such an ambitious target is probably too aggressive, and the risks of such high concentrations may outweigh the potential benefits. Also, we arbitrarily accepted a ∼90% PTA to be satisfactory for our dose recommendations, as to our knowledge the optimal PTA breakpoint is still a matter of debate (40).
Interestingly, our model failed to identify CRRT intensity to be a significant modifier of meropenem CL. We initially expected that CRRT intensity would have a significant effect on meropenem CL according to the available literature, which reports differential meropenem CLs when different intensities are used (12, 41). However, exploratory and regression analyses on the effects of covariates on individual CL did not show any visual or statistical trend between intensity and the estimates of individual CL, which may lead to the hypothesis that even the lowest CRRT intensities studied may be enough to maximize meropenem clearance and that higher intensities may add little to total meropenem CL. This explanation is consistent with data from Roberts et al., who also failed in the identification of intensity as a meropenem CL modifier (42). Similarly, we did not observe differences between CRRT techniques, likely because of the underrepresentation of CVVHF (4 out of 30 patients) in our study population. Controversy exists on the impact of CRRT modality on drug CL, as different meropenem CLs between CRRT methods have been reported by some researchers (12), while others have not found any difference (15). Also, we did not find differences in CL between patients according to the different types of membranes used in the various hospitals (1.5-m2 AN69ST in HJ23, 0.9-m2 AN69 in CSUPT and HCB). Importantly, the presence of polyethylenimine and heparin on the membrane surface (AN69ST) did not significantly influence CL, suggesting that meropenem adsorption to the surface-treated filter may not be a major elimination pathway, unlike for other molecules, like colistin (43).
A strong point of our population PK model is that it has been externally validated with new subjects. Before carrying out Monte Carlo simulations to assist with recommending any dosage regimen for a specific patient population, it should be previously established that the population PK model is predictive (34). However, despite the paramount importance of this step, it has been estimated that only 7% of the population PK models are externally validated (44). External validation showed that, by means of bias and precision, our population PK model had mean values within good limits, which supported its utility for undertaking dosing simulations.
Our main limitation was not measuring meropenem urinary and ultrafiltrate concentrations, and so we could not estimate either the sieving coefficient, which has already been well described to be about 1 for meropenem using AN69 membranes (8, 9, 19, 45), or truly quantify the degree of CL during CRRT. Furthermore, we included only patients with septic shock and renal failure requiring CRRT; therefore, our conclusions cannot be extrapolated to other patient populations, like patients without septic shock, without renal failure, with intermittent RRT, or with other extracorporeal blood purification therapies. Also, due to the low level of representation of CVVHF in the patient cohort, our conclusions may be applied only to patients receiving CVVHDF. Finally, the measurement of residual diuresis was performed by the nursing staff as part of their clinical routine, which might not be optimal for obtaining the exact volume of urine but is certainly sufficient for classifying the patients as oligoanuric or as having preserved diuresis. Conversely, the major strengths of this study are its multicenter nature, its large sample size (30 patients), and the fact that the population PK model has been externally validated. Moreover, our recommendations are based on an easy-to-measure and inexpensive clinical parameter such as residual diuresis; hence, our results can easily be implemented in daily care.
Conclusions.
In conclusion, we present the results of the largest multicenter pharmacokinetic study of meropenem prescribed to critically ill patients with septic shock and CRRT. Our population PK model successfully identified residual diuresis to be a modifier of total meropenem CL. CRRT intensity did not significantly modify meropenem CL, for which dose adjustments based on intensity seem to be unnecessary. Given a certain MIC, simulations showed that meropenem dose titration considering residual diuresis was advantageous for the attainment of 100% of the ƒuT>MIC as a PD target. If classical PD targets (40% of the ƒuT>MIC) were targeted, a standard dose of 500 mg q8h as a 30-min bolus would be sufficient, regardless of urine output.
ACKNOWLEDGMENTS
This work was supported by a grant from the Spanish Ministry of Health, Social Policies and Equality (Ministerio de Sanidad, Política Social y Igualdad), project grant number EC11-159. Marta Ulldemolins was supported in part by this project grant.
We have no conflicts of interest to declare.
REFERENCES
- 1.Agencia Española de Medicamentos y Productos Sanitarios. 2015. Meropenem product information. Agencia Española de Medicamentos y Productos Sanitarios, Madrid, Spain: http://www.aemps.gob.es/medicamentosUsoHumano/portada/home.htm Accessed April 2015. [Google Scholar]
- 2.Rello J, Ulldemolins M, Lisboa T, Koulenti D, Manez R, Martin-Loeches I, De Waele JJ, Putensen C, Guven M, Deja M, Diaz E, Group E-VCS. 2011. Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J 37:1332–1339. doi: 10.1183/09031936.00093010. [DOI] [PubMed] [Google Scholar]
- 3.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, Kaukonen KM, Koulenti D, Martin C, Montravers P, Rello J, Rhodes A, Starr T, Wallis SC, Lipman J, DALI Study. 2014. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58:1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Du X, Kuti JL, Nicolau DP. 2007. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother 51:1725–1730. doi: 10.1128/AAC.00294-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulldemolins M, Vaquer S, Llaurado-Serra M, Pontes C, Calvo G, Soy D, Martin-Loeches I. 2014. Beta-lactam dosing in critically ill patients with septic shock and continuous renal replacement therapy. Crit Care 18:227. doi: 10.1186/cc13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heintz BH, Matzke GR, Dager WE. 2009. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy 29:562–577. doi: 10.1592/phco.29.5.562. [DOI] [PubMed] [Google Scholar]
- 8.Krueger WA, Schroeder TH, Hutchison M, Hoffmann E, Dieterich HJ, Heininger A, Erley C, Wehrle A, Unertl K. 1998. Pharmacokinetics of meropenem in critically ill patients with acute renal failure treated by continuous hemodiafiltration. Antimicrob Agents Chemother 42:2421–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krueger WA, Neeser G, Schuster H, Schroeder TH, Hoffmann E, Heininger A, Dieterich HJ, Forst H, Unertl KE. 2003. Correlation of meropenem plasma levels with pharmacodynamic requirements in critically ill patients receiving continuous veno-venous hemofiltration. Chemotherapy 49:280–286. doi: 10.1159/000074527. [DOI] [PubMed] [Google Scholar]
- 10.Thalhammer F, Schenk P, Burgmann H, El Menyawi I, Hollenstein UM, Rosenkranz AR, Sunder-Plassmann G, Breyer S, Ratheiser K. 1998. Single-dose pharmacokinetics of meropenem during continuous venovenous hemofiltration. Antimicrob Agents Chemother 42:2417–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tegeder I, Neumann F, Bremer F, Brune K, Lotsch J, Geisslinger G. 1999. Pharmacokinetics of meropenem in critically ill patients with acute renal failure undergoing continuous venovenous hemofiltration. Clin Pharmacol Ther 65:50–57. doi: 10.1016/S0009-9236(99)70121-9. [DOI] [PubMed] [Google Scholar]
- 12.Valtonen M, Tiula E, Backman JT, Neuvonen PJ. 2000. Elimination of meropenem during continuous veno-venous haemofiltration and haemodiafiltration in patients with acute renal failure. J Antimicrob Chemother 45:701–704. doi: 10.1093/jac/45.5.701. [DOI] [PubMed] [Google Scholar]
- 13.Robatel C, Decosterd LA, Biollaz J, Eckert P, Schaller MD, Buclin T. 2003. Pharmacokinetics and dosage adaptation of meropenem during continuous venovenous hemodiafiltration in critically ill patients. J Clin Pharmacol 43:1329–1340. doi: 10.1177/0091270003260286. [DOI] [PubMed] [Google Scholar]
- 14.Langgartner J, Vasold A, Gluck T, Reng M, Kees F. 2008. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Med 34:1091–1096. doi: 10.1007/s00134-008-1034-7. [DOI] [PubMed] [Google Scholar]
- 15.Seyler L, Cotton F, Taccone FS, De Backer D, Macours P, Vincent JL, Jacobs F. 2011. Recommended beta-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care 15:R137. doi: 10.1186/cc10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isla A, Rodriguez-Gascon A, Troconiz IF, Bueno L, Solinis MA, Maynar J, Sanchez-Izquierdo JA, Pedraz JL. 2008. Population pharmacokinetics of meropenem in critically ill patients undergoing continuous renal replacement therapy. Clin Pharmacokinet 47:173–180. doi: 10.2165/00003088-200847030-00003. [DOI] [PubMed] [Google Scholar]
- 17.Isla A, Maynar J, Sanchez-Izquierdo JA, Gascon AR, Arzuaga A, Corral E, Pedraz JL. 2005. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J Clin Pharmacol 45:1294–1304. doi: 10.1177/0091270005280583. [DOI] [PubMed] [Google Scholar]
- 18.Varghese JM, Jarrett P, Wallis SC, Boots RJ, Kirkpatrick CM, Lipman J, Roberts JA. 2015. Are interstitial fluid concentrations of meropenem equivalent to plasma concentrations in critically ill patients receiving continuous renal replacement therapy? J Antimicrob Chemother 70:528–533. doi: 10.1093/jac/dku413. [DOI] [PubMed] [Google Scholar]
- 19.Giles LJ, Jennings AC, Thomson AH, Creed G, Beale RJ, McLuckie A. 2000. Pharmacokinetics of meropenem in intensive care unit patients receiving continuous veno-venous hemofiltration or hemodiafiltration. Crit Care Med 28:632–637. doi: 10.1097/00003246-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb SA, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 21.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. [DOI] [PubMed] [Google Scholar]
- 23.Li AM, Gomersall CD, Choi G, Tian Q, Joynt GM, Lipman J. 2009. A systematic review of antibiotic dosing regimens for septic patients receiving continuous renal replacement therapy: do current studies supply sufficient data? J Antimicrob Chemother 64:929–937. doi: 10.1093/jac/dkp302. [DOI] [PubMed] [Google Scholar]
- 24.European Medicines Agency. 2014. Guideline on bioanalytical method validation. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu Accessed August 2014. [Google Scholar]
- 25.Beal S, Sheiner LB, Boeckmann A, Bauer RJ. 2009. NONMEM user's guides (1989–2009). Icon Development Solutions, Ellicott City, MD. [Google Scholar]
- 26.Jonsson EN, Karlsson MO. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51–64. [DOI] [PubMed] [Google Scholar]
- 27.Sheiner LB, Steimer JL. 2000. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol 40:67–95. doi: 10.1146/annurev.pharmtox.40.1.67. [DOI] [PubMed] [Google Scholar]
- 28.Sheiner L, Wakefield J. 1999. Population modelling in drug development. Stat Methods Med Res 8:183–193. doi: 10.1191/096228099672920676. [DOI] [PubMed] [Google Scholar]
- 29.Ette EI, Ludden TM. 1995. Population pharmacokinetic modeling: the importance of informative graphics. Pharm Res 12:1845–1855. doi: 10.1023/A:1016215116835. [DOI] [PubMed] [Google Scholar]
- 30.Hooker AC, Staatz CE, Karlsson MO. 2007. Conditional weighted residuals (CWRES): a model diagnostic for the FOCE method. Pharm Res 24:2187–2197. doi: 10.1007/s11095-007-9361-x. [DOI] [PubMed] [Google Scholar]
- 31.Savic RM, Karlsson MO. 2009. Importance of shrinkage in empirical Bayes estimates for diagnostics: problems and solutions. AAPS J 11:558–569. doi: 10.1208/s12248-009-9133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Efron B. 1979. Bootstrap methods: another look at the jackknife. Ann Stat 7:1–26. doi: 10.1214/aos/1176344552. [DOI] [Google Scholar]
- 34.Ette EI, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV. 2003. Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol 43:610–623. doi: 10.1177/0091270003253624. [DOI] [PubMed] [Google Scholar]
- 35.Ette EI. 1997. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol 37:486–495. doi: 10.1002/j.1552-4604.1997.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 36.Food and Drug Administration. 1999. Guidance for industry. Population pharmacokinetics. Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/Guidances/UCM072137.pdf Accessed December 2014. [Google Scholar]
- 37.Sheiner LB, Beal SL. 1981. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 38.Rozman C, Cardellach F (ed). 2012. Farreras-Rozman medicina interna, 17th ed Elsevier, Barcelona, Spain. [Google Scholar]
- 39.European Committee on Antimicrobial Susceptibility Testing. April 2015. Clinical breakpoints. http://www.eucast.org. [Google Scholar]
- 40.Mouton JW, Brown DF, Apfalter P, Canton R, Giske CG, Ivanova M, MacGowan AP, Rodloff A, Soussy CJ, Steinbakk M, Kahlmeter G. 2012. The role of pharmacokinetics/pharmacodynamics in setting clinical MIC breakpoints: the EUCAST approach. Clin Microbiol Infect 18:E37–E45. doi: 10.1111/j.1469-0691.2011.03752.x. [DOI] [PubMed] [Google Scholar]
- 41.Jamal JA, Udy AA, Lipman J, Roberts JA. 2014. The impact of variation in renal replacement therapy settings on piperacillin, meropenem, and vancomycin drug clearance in the critically ill: an analysis of published literature and dosing regimens. Crit Care Med 42:1640–1650. doi: 10.1097/CCM.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 42.Roberts DM, Liu X, Roberts JA, Nair P, Cole L, Roberts MS, Lipman J, Bellomo R, RENAL Replacement Therapy Study Investigators. 2015. A multicenter study on the effect of continuous hemodiafiltration intensity on antibiotic pharmacokinetics. Crit Care 19:818. doi: 10.1186/s13054-015-0818-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Honore PM, Jacobs R, Lochy S, De Waele E, Van Gorp V, De Regt J, Martens G, Joannes-Boyau O, Boer W, Spapen HD. 2013. Acute respiratory muscle weakness and apnea in a critically ill patient induced by colistin neurotoxicity: key potential role of hemoadsorption elimination during continuous venovenous hemofiltration. Int J Nephrol Renovasc Dis 6:107–111. doi: 10.2147/IJNRD.S42791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, Girard P, Laffont CM, Mentre F. 2007. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet 46:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer MM, Munar MY, Kohlhepp SJ, Bryant RE. 1999. Meropenem pharmacokinetics in a patient with multiorgan failure from meningococcemia undergoing continuous venovenous hemodiafiltration. Am J Kidney Dis 33:790–795. doi: 10.1016/S0272-6386(99)70236-2. [DOI] [PubMed] [Google Scholar]