Abstract
The drug target profile proposed by the Medicines for Malaria Venture for a malaria elimination/eradication policy focuses on molecules active on both asexual and sexual stages of Plasmodium, thus with both curative and transmission-blocking activities. The aim of the present work was to investigate whether the class of monovalent ionophores, which includes drugs used in veterinary medicine and that were recently proposed as human anticancer agents, meets these requirements. The activity of salinomycin, monensin, and nigericin on Plasmodium falciparum asexual and sexual erythrocytic stages and on the development of the Plasmodium berghei and P. falciparum mosquito stages is reported here. Gametocytogenesis of the P. falciparum strain 3D7 was induced in vitro, and gametocytes at stage II and III or stage IV and V of development were treated for different lengths of time with the ionophores and their viability measured with the parasite lactate dehydrogenase (pLDH) assay. The monovalent ionophores efficiently killed both asexual parasites and gametocytes with a nanomolar 50% inhibitory concentration (IC50). Salinomycin showed a fast speed of kill compared to that of standard drugs, and the potency was higher on stage IV and V than on stage II and III gametocytes. The ionophores inhibited ookinete development and subsequent oocyst formation in the mosquito midgut, confirming their transmission-blocking activity. Potential toxicity due to hemolysis was excluded, since only infected and not normal erythrocytes were damaged by ionophores. Our data strongly support the downstream exploration of monovalent ionophores for repositioning as new antimalarial and transmission-blocking leads.
INTRODUCTION
In 2012, malaria caused 627,000 deaths, and there were 207 million reported cases (1). Among the five species that infect humans, Plasmodium falciparum is responsible for the majority of deaths and severe cases. The recommended malaria control measures include drug treatment, in particular with artemisinin-based combination therapy (ACT), and protection from the vectors with insecticide-treated bed nets and indoor residual spraying (1). However, the effectiveness of control tools is seriously threatened by the emergence and spread of drug and insecticide resistance (2). Even for artemisinins, which until now were safe and effective, resistance is a growing issue in Asia, particularly on the Cambodia-Thailand border, which is the cradle of antimalarial resistance (3, 4). New drugs and new lead compounds for antimalarial drug development are greatly needed (5).
In the past few years, the international strategy against malaria has changed toward malaria elimination and, ultimately, eradication. The Medicines for Malaria Venture (MMV) has defined four target candidate profiles (TCPs) that describe the requirements for novel tools for the control and elimination of malaria (6). In particular, new multistage antimalarial drugs able to kill the liver or sexual stages of the parasite and/or that are capable of preventing the parasite development in the mosquito are needed (5, 6). Gametocytes are the sexual stage of Plasmodium, which appear concomitantly in the circulation or after the asexual intraerythrocytic stage. They undergo five stages of maturation, from I to V. Stage V gametocytes, when taken up by Anopheles mosquitoes during a blood meal, become gametes and fuse to form a zygote (7). Subsequently, the zygote transforms into a motile ookinete and becomes an oocyst, which divides to produce sporozoites that are ready to restart the cycle. As the stage responsible for transmission, gametocytes are an essential target for malaria elimination/eradication, and the identification of gametocytocidal compounds has become an absolute priority.
The strategy of drug repositioning (the usage of existing drugs for new therapeutic indications) allows a significant reduction in development costs, time to market, and risks of failure. This is particularly important for diseases in developing countries, for which research funds are limited (8). Here, we provide evidence in support of the repurposing of salinomycin and/or other ionophores as antimalarial and transmission-blocking agents. Salinomycin is a polyether antibiotic isolated from Streptomyces spp. and a monovalent ionophore for alkali ions with relative K+ selectivity, able thus to interfere with mitochondrial functions (9). Salinomycin was patented in 1974 as an anticoccidial agent and has been used since then in poultry and other livestock. More recently, salinomycin and other ionophores, such as monensin and nigericin (sodium and potassium antiporters, respectively), were found to inhibit cancer stem cell growth by modulating the Wnt pathway (10–12). This finding has prompted the usage of salinomycin for compassionate use in a few cancer patients, with promising results (13).
Salinomycin and other ionophores (gramicidin, lasalocid, and monensin) have already been reported to be active against P. falciparum parasites (14–16), but the potency of this class of compounds as transmission-blocking agents has not yet been fully investigated. The aim of the present work was to evaluate the antimalarial activity of salinomycin, monensin, and nigericin on both the asexual and transmission stages of P. falciparum.
MATERIALS AND METHODS
P. falciparum cultures.
P. falciparum cultures were carried out according to the method of Trager and Jensen (17), with minor modifications. The P. falciparum strains used in this study are either CQ sensitive (D10, 3D7, and the Ghana isolate) or CQ resistant (W2 and the Burkina Faso isolate). The resistance profile of W2 is well documented in the literature (18), whereas the P. falciparum Burkina Faso isolate is considered resistant, since its 50% inhibitory concentration (IC50) of chloroquine is >100 nM, which is commonly accepted as the threshold for resistance (19). All the strains were maintained at 5% hematocrit (human type A-positive red blood cells for D10, W2, and the Burkina Faso and Ghana isolates and O-positive red blood cells for 3D7) in RPMI 1640 medium containing 24 mM sodium bicarbonate (EuroClone; Celbio), with the addition of 0.01% hypoxanthine, 20 mM HEPES, and 2 mM glutamine. All the parasites were cultured in the presence of 1% AlbuMAX II (lipid-rich bovine serum albumin), except the 3D7 strain, which was cultured in the presence of 10% (vol/vol) naturally clotted heat-inactivated O+ human serum (Interstate Blood Bank, Inc.), which ensures constant and high gametocyte production. All the cultures were maintained at 37°C in a standard gas mixture consisting of 1% O2, 5% CO2, and 94% N2. The P. falciparum Ghana and Burkina Faso isolates derived from two different patients with malaria admitted to the Ospedale Sacco (Milan, Italy) returning to Milan from Ghana or Burkina Faso, respectively. The parasites were adapted to grow in culture and are not clonal strains.
Gametocytogenesis was triggered as previously described (20). Briefly, P. falciparum 3D7 asexual parasite cultures were diluted to 0.5% parasitemia, and the medium was changed daily without the addition of fresh red blood cells (RBC). When a parasitemia of >5% was obtained and the parasites were stressed by nutrient deprivation, the cultures were treated for 48 to 72 h with N-acetylglucosamine (NAG) (Sigma-Aldrich) to clear residual asexual parasites. Stage II and III gametocytes were obtained and used for the experiments after 4 days after the addition of NAG to the culture, while stage IV and V gametocytes were used after 7 to 8 days. Gametocyte stages were routinely checked in the Giemsa-stained smears.
In vitro P. falciparum drug susceptibility assay.
Salinomycin, monensin, and nigericin sodium salts (Sigma-Aldrich) (Fig. 1) were dissolved in either dimethyl sulfoxide (DMSO) (salinomycin and nigericin) or ethanol (monensin) and then diluted with medium to achieve the required concentrations (final DMSO or ethanol concentration of <1%, which is nontoxic to the parasites). The compounds were placed in 96-well plates (EuroClone) and serial dilutions were made in a final volume of 100 μl/well. Asexual parasite stages derived from asynchronous cultures with parasitemia of 1 to 1.5% or gametocyte cultures with parasitemia of 0.5 to 1% were distributed into the plates (100 μl/well; final hematocrit, 1%) and incubated for 72 h at 37°C. Chloroquine (CQ) and dihydroartemisinin (DHA) were used as a reference control for the asexual stage, and DHA and epoxomicin were used for gametocytes. Three experiments in duplicate were performed with asexual parasites, with at least two experiments in duplicate or triplicate with gametocytes in each stage (II and III or IV and V). Parasite growth was determined spectrophotometrically by measuring the activity of the parasite lactate dehydrogenase (pLDH), according to a modified version of Makler's method in control and treated cultures (21). Briefly, the drug-treated culture was resuspended, and 20 μl/well was transferred to a plate containing 100 μl of Malstat reagent (0.11% [vol/vol] Triton-100, 115.7 mM lithium l-lactate, 30.27 mM Tris, 0.62 mM 3-acetylpyridine adenine dinucleotide [APAD] [Sigma-Aldrich], adjusted to pH 9 with 1 M HCl) and 25 μl of PES/NBT (1.96 mM nitroblue tetrazolium chloride-0.24 mM phenazine ethosulfate) to perform the pLDH assay. The plate was read at a wavelength of 650 nm using a microplate reader, Synergy4 (BioTek), and the results were expressed as the 50% inhibitory concentration (IC50).
FIG 1.
Chemical structures of the ionophores used in this study.
In the gametocyte assay, the pLDH assay was performed at both 72 and 144 h, as described previously (20). After 72 h of incubation, 150 μl/well supernatant was collected and checked for hemolysis, and 150 μl fresh medium was added. Twenty microliters per well of resuspended culture was used to perform the pLDH assay, the plate was incubated for a further 72 h, and the pLDH assay was performed again under the same conditions.
In vitro mammalian cell toxicity assay.
A long-term cell line of human dermal microvascular endothelial cells (HMEC-1) immortalized by simian virus 40 (SV40) large T antigen was kindly provided by the Centers for Disease Control and Prevention, Atlanta, GA, USA (22). The cells were maintained under standard conditions at 37°C in a 5% CO2 incubator in MCDB 131 medium (Gibco-BRL, Paisley, Scotland) supplemented with 10% fetal calf serum (HyClone, Logan, UT, USA), 10 ng/ml epidermal growth factor (PeproTech, Rocky Hill, NY, USA), 1 μg/ml hydrocortisone (Sigma Italia, Milan, Italy), 2 mM glutamine (EuroClone, Pero, Italy), 100 U/ml penicillin, 100 mg/ml streptomycin (EuroClone), and 20 mM HEPES buffer (pH 7.3) (EuroClone). For the toxicity experiments, HMEC-1 cells at 1.0 × 104 cells/100 μl/well were plated in 96-well plates and incubated at 37°C and 5% CO2 overnight. The cells were then treated for 72 h with different doses of salinomycin, monensin, or nigericin diluted as described above (final volume, 200 μl/well). Three independent experiments were performed in duplicate. The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H tetrazolium bromide (MTT) (Sigma) cytotoxicity assay was used to measure cell viability, as described elsewhere (23). Cytotoxicity was expressed as the 50% inhibitory concentration (IC50).
IC50 and selectivity index.
The results of the chemosensitivity assays were expressed as the percent viability compared to the untreated controls, calculated with the following formula: 100 × ([OD of treated sample − blank]/[OD of untreated sample − blank]) (OD, optical density). As a blank, uninfected RBCs were used. The percent viability was plotted as a function of drug concentrations, and the curve fitting was obtained by nonlinear regression analysis using a four-parameter logistic method (software Gen5 1.10 provided with the Synergy4 plate reader [Biotek]). The IC50 was extrapolated as the dose that induced a 50% inhibition of gametocyte viability.
The selectivity index (SI) was calculated to evaluate the toxicity impact of salinomycin, monensin, or nigericin against normal human cells compared to the toxicity against the parasite, and this allows an assessment of the selectivity of these drugs for the parasite. The SI was calculated as the ratio between the cytotoxic IC50s against HMEC-1, calculated as previously described, and the parasitic IC50s against 3D7 gametocytes at stages IV and V for both the time points 72 h and 72 plus 72 h.
Time course experiments.
Two different time course protocols were employed. In the first case, stage IV and V gametocytes were incubated with salinomycin, monensin, or nigericin at 1, 10, or 100 nM for different lengths of time (2, 6, 24, 48, or 72 h), and the pLDH assay was performed at the end of each treatment.
In the second series of experiments, stage IV and V gametocytes were incubated with salinomycin at 1, 10, or 100 nM for different lengths of time (2, 6, 24, 48, or 72 h), the drugs were removed, fresh medium was added, the plate was centrifuged, and the medium was changed again. The pLDH assay was performed 72 h after the onset of the experiment, i.e., 72 h after the addition of the drugs to the cultures.
For both experimental schemes, two independent experiments were performed in triplicate, and the results were expressed as the percent viability compared to that of the untreated controls.
Hemolysis.
Gametocyte cultures (about 0.5 and 1% parasitemia), fresh RBCs, or RBCs kept for 10 days under the same culture conditions as the gametocytes were diluted to 1% hematocrit and treated for 72 h with different doses (1, 10, and 100 nM) of salinomycin, monensin, or nigericin. Hemolysis was evaluated by measuring spectrophotometrically the release of hemoglobin in the supernatants (absorbance at 405 nm, Soret band) and calculating the ratio of untreated to ionophore-treated samples. Similar experiments were performed on mouse RBCs. Blood from uninfected or Plasmodium berghei-infected mice, diluted to 7.5% hematocrit, was incubated for 24 h at 19°C with monensin and salinomycin at doses ranging from 1 to 50 nM. Hemoglobin release in the well supernatants was measured as described above.
The percentages of hemolysis for both human and mouse RBCs were estimated by referring to a standard curve prepared with serially diluted RBCs, which were lysed with saponin (1%).
Early sporogonic development P. berghei assay.
P. berghei CTRPp.GFP, a strain expressing the green fluorescent protein (GFP) exclusively at early sporogonic stages (zygotes, ookinetes, and early oocysts), was used (kindly provided by R. Sinden, Imperial College, London, United Kingdom) to infect BALB/c mice and recover P. berghei gametocytes for the early sporogonic development assay. Experimental animal rearing and handling were in compliance with the Italian legislative decree on the protection of animals used for experimental and other scientific purposes (24) and in full adherence with the European Directive 2010/63/UE (25).
The early sporogonic development assay was performed according to the protocol developed by Delves and colleagues (26), with slight modifications. Salinomycin and monensin were dissolved in DMSO and ethanol, respectively, and then diluted further to obtain the desired concentrations with ookinete medium (RPMI 1640 containing 25 mM HEPES, 25 mM sodium bicarbonate, 50 mg/liter hypoxanthine, 100 μM xanthurenic acid [pH 7.6 to 8], supplemented with 20% heat-inactivated fetal bovine serum, 50 U/ml penicillin, and 50 μg/ml streptomycin). All chemicals were purchased from Sigma-Aldrich. Aliquots (20 μl) of compounds at a concentration 10 times higher than the desired test concentrations were then added to the microplate wells (96-well plates; Nunc, Denmark) containing 165 μl of ookinete medium. Subsequently, 15 μl of gametocytemic blood obtained by cardiac puncture from P. berghei CTRPp.GFP-infected mice were added and the plates incubated at 19°C. DMSO and ethanol at 0.3% were used as solvent controls. After 24 h of incubation, the well contents were diluted (1:550) in another plate, and the zygotes and ookinetes expressing green fluorescent protein were enumerated with the help of an ocular grid with a fluorescence microscope (×400 magnification; Zeiss). Compounds were tested in triplicate in two independent experiments using different gametocyte donor mice. The data are expressed as the percent inhibition of zygote and ookinete formation in drug-treated samples compared to that of the controls.
Luminescent standard membrane feeding assays.
Compounds were serially diluted in DMSO and subsequently in parasite culture medium to reach a final DMSO concentration of 0.1%. Luminescent standard membrane feeding assays were performed essentially as described by Stone et al. (27), with the exception that we used an hsp70 promoter instead of the elongation factor 1 alpha (EF-1α) promoter to drive the expression of the luciferase reporter gene (M. W. Vos, W. J. R. Stone, K. M. J. Koolen, G. van Gemert, B. van Schaijk, R. W. Sauerwein, T. Bousema, and K. J. Dechering., unpublished data). Luminescence intensities were analyzed for 24 mosquitoes per sample. To determine the background luminescence levels, 24 uninfected mosquitoes were analyzed in parallel. For the determination of oocyst prevalence, mosquitoes were considered infected when the luminescence activity was 5 standard deviations above the average luminescence level observed in the uninfected mosquitoes.
Statistical analysis.
The data were expressed as the mean ± standard deviation (SD) and analyzed using a two-tailed Student t test with a level of significance of a P value of <0.05 or <0.01.
RESULTS
Ionophores inhibit asexual P. falciparum growth.
Salinomycin, monensin, and nigericin were tested in vitro for antiplasmodial activity against three CQ-sensitive (D10, 3D7, and the Ghana isolate) and two CQ-resistant (W2 and the Burkina Faso isolate) strains of P. falciparum using CQ and DHA as reference drugs. All the ionophores displayed a strong inhibition of asexual parasite growth against all the tested strains (Table 1). The activity of monensin (IC50 range, 0.5 to 1.0 nM) and nigericin (IC50 range, 1.8 to 1.9 nM) against P. falciparum laboratory strains was superior to that of DHA (IC50 range, 2.1 to 5.6 nM). Against the CQ-sensitive parasites, the potency of salinomycin was slightly lower than that of CQ (IC50 range, 22 to 40 versus 11 to 19 nM CQ), while monensin and nigericin were approximately 10-fold more active than CQ. Differently from CQ, all the ionophores showed low-nanomolar activity against the W2 and Burkina Faso strains, which indicates the absence of cross-resistance.
TABLE 1.
In vitro antimalarial activities of salinomycin, monensin, and nigericin against asexual P. falciparum parasites
| Drug | IC50 (nM) for strain (mean ± SD)a: |
||||
|---|---|---|---|---|---|
| D10 | W2 | 3D7 | Ghana | Burkina Faso | |
| Salinomycin | 39.6 ± 1.1 | 26.3 ± 11.4 | 21.9 ± 12.3 | 30.7 ± 4.0 | 50.0 ± 2.1 |
| Monensin | 0.5 ± 0.1 | 0.5 ± 0.2 | 1.0 ± 0.2 | NTb | NT |
| Nigericin | 1.9 ± 0.6 | 1.8 ± 0.1 | 1.8 ± 1.0 | NT | NT |
| CQc | 18.6 ± 6.6 | 255.7 ± 77.8 | 11.0 ± 5.4 | 15.8 ± 2.0 | 146.4 ± 31.0 |
| DHAc | 5.6 ± 1.1 | 2.1 ± 0.3 | 2.8 ± 0.7 | 0.6 ± 0.5 | 1.7 ± 0.3 |
Data are from three different experiments performed in duplicate. P. falciparum strains D10 and 3D7 (CQ sensitive), strain W2 (CQ resistant), and two isolates (not clonal) from different patients coming from Ghana and Burkina Faso were used in this study.
NT, not tested.
Chloroquine (CQ) and dihydroartemisinin (DHA) were used as positive controls.
Ionophores inhibit P. falciparum gametocyte viability.
Using the pLDH assay, we were able to calculate the IC50 for all the ionophores, even after the first 72 h of incubation. The ionophores are far more potent than the reference compound epoxomicin or DHA. Neither compound induced a 50% reduction in gametocyte viability at the 72-h time point, even at the highest concentration tested, but required an additional 72 h without drugs to obtain a >50% inhibition of viability and thus to calculate the IC50 (Table 2) (20). Therefore, the ionophores appear to be the most potent compounds tested so far in our assay, in that they reduce gametocyte viability over a very short period, with an IC50 in the nanomolar range.
TABLE 2.
In vitro antimalarial activities of salinomycin, monensin, and nigericin against different stages of 3D7 P. falciparum gametocytes
| Drug | Gametocyte stages | IC50 (nM) for incubation for (h)a: |
SI for incubation for (h)b: |
HMEC-1 IC50 (nM)a | ||
|---|---|---|---|---|---|---|
| 72 | 72 + 72 | 72 | 72 + 72 | |||
| Salinomycin | II–III | 29.7 ± 9.2c | 14.5 ± 7.4d | 157.5 ± 45.0 | ||
| IV–V | 13.8 ± 1.4 | 6.3 ± 1.7 | 11.4 | 25.0 | ||
| Monensin | II–III | 4.6 ± 1.0 | 1.9 ± 1.3 | 169.6 ± 48.0 | ||
| IV–V | 6.1 ± 1.1 | 5.7 ± 1.1 | 27.8 | 29.8 | ||
| Nigericin | II–III | 10.6 ± 4.0 | 2.7 ± 1.2 | 86.4 ± 31.0 | ||
| IV–V | 9.3 ± 4.9 | 0.9 ± 0.4 | 9.3 | 96.0 | ||
| DHA | II–III | >500 | 14.3 ± 8.7 | 3,094.8 ± 316.5 | ||
| IV–V | >500 | 156.3 ± 78.3 | NAe | 19.8 | ||
| Epoxomicin | II–III | >100 | 12.1 ± 4.5 | 11.7 ± 3.1 | ||
| IV–V | >100 | 6.7 ± 1.9 | NA | 1.7 | ||
Data are the means ± SD from at least two independent experiments in duplicate or triplicate.
SI, selectivity index, calculated as the IC50 HMEC-1/IC50 gametocytes at stage IV/V at the indicated incubation time.
P < 0.005, effect of salinomycin on stage II and III versus stage IV and V gametocytes at 72 h.
P < 0.005, effect of salinomycin on stage II and III versus stage IV and V gametocytes at 72 plus 72 h.
NA, not applicable.
Selective chemosensitivity of gametocytes in different phases of their development was previously described (28). When the IC50 of salinomycin was determined against stage II and III young gametocytes or stage IV and V mature gametocytes, the results were significantly different (Table 2). After 72 h of treatment, salinomycin appeared to be more effective (lower IC50) on mature than on young gametocyte stages. After 72 plus 72 h of incubation, the differences between stages II and III and IV and V remained significant. Monensin and nigericin did not show a significant difference in activity against young versus mature gametocytes.
The IC50 of the ionophores on human endothelial cells (HMEC-1) was lower than that of DHA, suggesting potential toxicity. However, due to the higher activity on mature gametocytes, the selectivity indices of salinomycin and monensin (25 and 29.8, respectively) are comparable to that of DHA (19.8), and that of nigericin was even higher (96.0).
To investigate the time to kill, time course experiments were performed by treating gametocytes with different doses of compounds for various pulse-inhibitory periods (2, 6, 24, 48, and 72 h) (Fig. 2a and b). When the pLDH activity was evaluated immediately after salinomycin treatment (Fig. 2a), the gametocyte viability was reduced by 41% with the 100 nM dose only and with a minimum incubation time of 24 h. Lower doses were ineffective. Complete inhibition was observed after 48 h. The time-dependent inhibition of gametocyte viability by monensin or nigericin was similar to that of salinomycin at 100 nM, at a higher rate than with the 10 nM dose (at 72 h, inhibition of gametocyte viability was 27% for salinomycin, 68% for monensin, and 88% for nigericin).
FIG 2.
Time course of the activity of salinomycin, monensin, or nigericin on mature gametocytes. Gametocytes were incubated with the ionophores at 1 nM (circles), 10 nM (triangles), or 100 nM (squares) for different lengths of time (2, 6, 24, 48, and 72 h). (a) The pLDH viability assay was performed at the end of each incubation time. (b) The compounds were removed after each incubation time, fresh medium was added, and the pLDH assay was performed at 72 h. The results are the means ± SD from two independent experiments in triplicate.
In a subsequent series of experiments, the drugs were removed after the different pulse-inhibitory periods, fresh medium was added, and the incubation was continued up to 72 h, when the pLDH activity was evaluated (Fig. 2b). The results showed that salinomycin at 100 nM already inhibited pLDH activity by 65% after 2 h, whereas the same percentage of inhibition was obtained at the 10 nM dose only after 24 h. In this set of experiments, for each dose, the maximal inhibition was observed at 24 h, without any further increase up to 72 h. The activity of monensin and nigericin was similar to that of salinomycin, with 61% and 82% inhibition, respectively, already after 2 h at the 100 nM dose.
Hemolytic effect of ionophores is limited to infected RBCs at doses higher than the IC50.
To study the possible toxic effects of ionophores on RBCs, hemolysis was measured spectrophotometrically by determining the hemoglobin release in the supernatants of infected RBCs and, for comparison, of fresh uninfected RBCs or cultured uninfected RBCs. The cells were treated with different doses of ionophores for 72 h. The percentage of hemolysis induced on fresh RBCs was <5% in the control untreated RBCs and in RBCs treated with different doses of all the ionophores. Both infected and uninfected RBCs kept at 37°C for 10 days showed a spontaneous hemolysis of about 15%. However, only in the case of infected RBCs did the percentages of hemolysis increase in a dose-dependent manner after ionophore treatment, reaching maximum values of 32, 22, and 34% after treatment with 100 nM salinomycin, monensin, and nigericin, respectively. To better compare the hemolytic effect of the ionophores in the different experimental groups, the results were expressed as the ratio of the optical density at 405 nm (OD405) of ionophore-treated RBCs to that of untreated RBCs. As shown in Fig. 3, the 100 nM dose of salinomycin, monensin, or nigericin caused a 3.1-fold, 2.3-fold, or 2.9-fold increase, respectively, in the release of hemoglobin in the supernatants of treated compared to those of the untreated gametocyte cultures. The increase in hemoglobin release was much lower (salinomycin, 1.4-fold; monensin, 1.2-fold; nigericin, 1.3-fold) for cultured uninfected RBCs. Moreover, a dose close to the IC50 on gametocytes (10 nM) was hemolytic on gametocyte-infected RBCs but not on normal uninfected RBCs.
FIG 3.

Hemolytic effects of salinomycin (a), monensin (b), and nigericin (c) evaluated by measuring the release of hemoglobin (OD405) in test supernatants. The histograms represent the ratio of the OD of the treated cells to that of the untreated cells. Black bars, gametocyte-infected RBCs; striped bars, fresh RBCs; white bars, normal RBCs kept under the same culture conditions as infected RBCs at 37°C for ≥7 days. The data are the means ± SD from at least three independent experiments in triplicate.
Ionophores inhibit early sporogonic P. berghei development in vitro.
Using an early sporogonic stage-specific GFP reporter strain of the rodent parasite P. berghei (CTRPp.GFP), the capacity of gametocytes to undergo in vitro early sporogonic development (gamete formation, zygote formation, and ookinete maturation) was examined in the presence of the ionophores.
As illustrated in Fig. 4, early sporogonic development was inhibited by both ionophores in a dose-dependent manner. Inhibition of ≥90% was recorded for salinomycin at 80 nM and for monensin at 40 nM. From a comparison of the IC50s, it appeared that monensin (IC50, 16.8 ± 2.5) was about twice as active as salinomycin (IC50, 34.9 ± 5.1).
FIG 4.
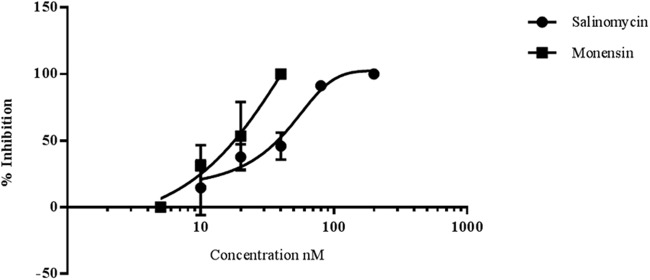
Salinomycin and monensin block early sporogonic-stage development of P. berghei in vitro. The figure illustrates the mean percentages of inhibition and standard deviations for early sporogonic stages (zygotes and ookinetes) by salinomycin and monensin as a function of the compound concentration indicated on the x axis. The data are from 2 or 3 replicate experiments in triplicate wells, except for the 5 nM and 200 nM doses, which are based on a single experiment.
To assess the toxicity of ionophores on infected and uninfected mouse RBCs, the release of hemoglobin was measured in wells incubated for 24 h with monensin or salinomycin at concentrations ranging from 1 to 50 nM. No appreciable hemolytic activity (<2%) on uninfected RBCs was observed with monensin or salinomycin at doses between 1 and 10 nM or 1 and 25 nM, respectively. However, a 6- or 8-fold increase in hemolysis was recorded in wells containing infected RBCs after treatment with monensin at 10 nM or salinomycin at 25 nM. Given that early sporogonic development is an extracellular process, it is unlikely that the effects of ionophores observed on early sporogonic stages and red blood cells lysis are related.
Ionophores block P. falciparum oocyst development.
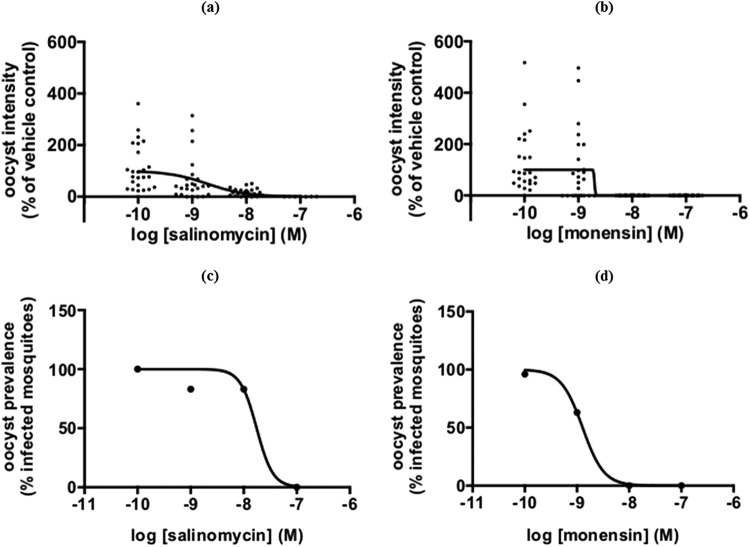
P. falciparum stage V gametocytes of an hsp70-GFP:luc reporter strain were incubated with compounds for 24 h and subsequently fed to Anopheles stephensi mosquitoes in the standard membrane feeding assays (27). Figure 5 shows that salinomycin and monensin dose-dependently reduced oocyst intensity in the mosquito midgut, as indicated by a reduction in luciferase activity. Both compounds showed IC50s in the low-nanomolar range (IC50, 1.9 nM for both drugs). The reduction in oocyst intensity translated into a reduction in oocyst prevalence or the number of infected mosquitoes (Fig. 5c and d). The IC50 of monensin was 1.3 nM and that of salinomycin was 18 nM. These data indicate that monensin and salinomycin effectively block P. falciparum transmission in the nanomolar range.
FIG 5.
Salinomycin and monensin block oocyst development in the mosquito. P. falciparum stage V gametocytes of a transgenic hsp70-GFP::luc reporter strain were incubated for 24 h with different doses of compounds and fed to A. stephensi mosquitoes. (a and b) Luminescence intensities in individual mosquitoes as a function of the compound concentration indicated on the x axis. (c and d) Oocyst prevalence (percentage of infected mosquitoes) as a function of the compound concentration indicated on the x axis.
DISCUSSION
Repositioning or repurposing of existing drugs for another application has already been proposed in the malaria field and applied to existing libraries against the asexual stages of P. falciparum. Successful repositioning of drugs significantly benefits the clinical development pipeline in term of costs and time (8). Here, we demonstrated the antimalarial and transmission-blocking activities of a specific class of compounds, the monovalent ionophores (i.e., salinomycin, monensin, and nigericin), polyether antibiotics that are used in several countries as anticoccidial agents in poultry. Despite being registered and FDA approved for veterinary use since 1974, only recently have the ionophores, and salinomycin in particular, experienced renewed interest as potential human anticancer agents (10, 13).
Our data show that salinomycin, monensin, and nigericin are very active in vitro against a number of P. falciparum isolates, including drug-resistant strains, in accordance with other reports (16, 29–33). Importantly, we also show that these molecules possess in vitro nanomolar activity against mature P. falciparum stage IV and V gametocytes and against P. berghei ookinetes, the earliest development stage in the mosquito vector. These data corroborate previous findings with salinomycin and monensin on P. falciparum gametocytes after a large library screening (15). The cytocidal activity against gametocytes and ookinetes apparently leads to a functional block of oocyst development in the standard membrane feeding assays at concentrations in the nanomolar range.
Monovalent ionophores are known for their activity as antifungal, antiparasitic, and antiviral agents, especially against multidrug-resistant pathogens. Recent reports highlighted their activity against Trypanosoma brucei, Toxoplasma gondii, and cytomegalovirus (33–36). Considerable attention has been devoted recently to salinomycin and derivatives due to their selective activity against tumor stem cells (13, 37, 38). A pilot study has been conducted in a few cancer patients, under compassionate use, and salinomycin was shown to induce tumor regression with minor side effects, which were lower than those of conventional chemotherapeutic drugs (13). In vitro, salinomycin has been shown to be active against multidrug-resistant cancer cells of different origins by inducing cell cycle arrest in G1 phase (39), inhibiting cancer cell motility (40), modulating the autophagy process (41), or inducing leukemia cell apoptosis via the inhibition of Wnt signaling (42). The inhibition of Wnt signaling also seems to be involved in the inhibition of cytomegalovirus replication by salinomycin (35). In P. falciparum, the autophagy pathway is not yet completely characterized, but autophagy-related (ATG) proteins are abundantly expressed in P. falciparum gametocytes, suggesting that they may play a role in gametocytogenesis (43). Since monensin has been shown to induce autophagy and death of T. gondii (44), we cannot exclude a similar effect of ionophores on P. falciparum.
Our data and reports in the literature confirm that the spectrum of action of monovalent ionophores against P. falciparum is quite broad and not stage specific. Here, we demonstrate that ionophores inhibit the viability of stage IV and V gametocytes, as already described for all stages of asexual parasites (14). In addition, there is evidence that salinomycin and monensin are effective against liver stages, both in vitro (HepG2 cells infected by P. berghei sporozoites) and orally in P. berghei-infected C57BL/6 mice in vivo (32, 33). Unlike many reference drugs, like DHA (28, 45), salinomycin was more active on mature (stage IV and V) than young (stage II and III) gametocytes, which reinforces the transmission-blocking potential of this molecule. Mature gametocytes (stages IV and V) are metabolically quiescent (no protein synthesis or hemoglobin digestion) and thus relatively more resistant than early stage gametocytes or asexuals (46, 47). Only primaquine is effective in reducing late-stage gametocytemia in vivo (48). In that respect, the ionophores, and salinomycin in particular, compare quite well with the most advanced new antimalarial drug candidates of the MMV portfolio, including the spiroindolone KAE609 (49), the imidazolopiperazine KAF 156, and the quinolone-3-diarylether ELQ-300 (50, 51). All these compounds have been shown to inhibit early and late P. falciparum gametocytes at concentrations between 50 and 500 nM.
Ionophores have a relatively fast speed of action. More than 50% inhibition of gametocyte viability was seen at the first 72 h of the pLDH assay, whereas a longer incubation (72 plus 72 h) was necessary to observe the same effect with the reference drugs DHA, epoxomicin (Table 2), and methylene blue (20). This is clearly related to the mode of action of the ionophores, but it also emphasizes the advantages of the dual-time pLDH assay, which discriminates between fast- and slow-acting compounds. The minimal contact time required to achieve >50% inhibition of gametocyte viability was 24 h for salinomycin and monensin at 10 nM and only 2 h for all three compounds at 100 nM. Indeed, salinomycin showed a similar time course activity on the intraerythrocytic parasite Babesia gibsoni, whereas 4 h was reported as the time for monensin to achieve 50% inhibition of asexual P. falciparum parasites (14, 52). As far as we know, an activity so fast has not yet been described for any other molecule (53).
Due to the importance of ion balance for RBCs, one of the potential side effects of Na+ and K+ monovalent ionophores is the risk of hemolysis. However, few and controversial data are reported in the literature (54). Here, we show that a dose of ionophores close to the IC50 for gametocytes (10 nM) was hemolytic only for gametocyte-infected but not uninfected normal human RBCs. Similarly, little hemolysis of uninfected mouse erythrocytes was observed after ionophore treatment in the concentration range of 10 to 25 nM, whereas on P. berghei-infected RBCs, a 6- to 8-fold increase in hemoglobin release in ionophore-treated compared to untreated samples was observed.
These data highlight the specificity of ionophores for infected versus normal RBCs. Similar findings were reported by Gumila et al. (55) using asexual parasites and were attributed to the modification of the membrane properties of infected RBCs and the different phospholipid and lipid composition of parasite membranes. All ionophores strongly interact with phospholipid monolayers and facilitate the incorporation and transport of ions in low-cholesterol-containing membranes typical of asexual P. falciparum parasites (55, 56). Whether this may also apply to gametocyte-infected RBCs is not known yet. Selectivity and antiparasitic activity might also depend on the ability of monovalent ionophores to induce K+ efflux from the cytosol with a consequent increase in the influx of Na+ and water. The parasite compartment is characterized by high K+ and Ca2+ levels and low Na+ levels with respect to the infected RBC cytosol, which is poor in K+ and rich in Na+ (57, 58). This is different from normal RBCs, which are maintained at very high K+ and low Na+ concentrations by the Na+-K+ ATPase pump. Perturbation of the cation gradient and/or content in infected versus normal RBCs or in the parasite cytosol may be responsible for the selective antimalarial effects of ionophores. A similar mechanism has been proposed to explain the activity of salinomycin against T. brucei and B. gibsoni (34, 52) and the egress of T. gondii from host cells (59).
To consider the potential use of ionophores as antimalarial drugs, it is of paramount importance to address clinical safety. In animals, monovalent ionophores can induce considerable neurotoxicity and cardiotoxicity after accidental exposure in nontarget species, such as calves, cats, and horses (60–62). No appreciable risk for humans was demonstrated following the consumption of products from nontarget animals (63). However, preclinical pharmacological and safety data are scarce. Toxicity in humans has been reported in cases of accidental high-dose ingestion in people working with livestock (64, 65). In our assays, salinomycin appeared to be the most toxic for HMEC-1 cells among the ionophores tested, but the selectivity index was similar to that of the antimalarial drug DHA. Salinomycin exhibits an IC50 similar to that of mefloquine when tested against HepG2 cells, whereas monensin showed no significant toxicity at the highest tested concentration (46 μM) (15). The narrow therapeutic index of salinomycin as a potential anticancer drug has already boosted the development of chemically modified derivatives or innovative drug delivery systems (66–68), which should be considered in their future development as new antimalarials.
The pharmacokinetic properties of salinomycin as an anticoccidial agent have been fully investigated in several animal species. As a highly lipophilic drug, salinomycin is well absorbed and distributed throughout the plasma and tissues (especially fat in chickens) (66). In mice, its oral and intravenous bioavailability was shown to be limited by the presence of the P-glycoprotein; at a dose of 1 mg/kg of body weight, the peak concentration was reached after 2 h, and the half-life was approximately 6 h (69). So far, no detailed information is available on salinomycin pharmacokinetic properties or plasma levels in humans. In a pilot study of metastatic cancer patients, the dose of 200 μg/kg of intravenous salinomycin was given every second day for 12 to 14 days; this protocol was well tolerated and led to partial clinical regression of heavily pretreated and therapy-resistant cancers (13). The suitability of long-term treatment might be an advantage for a transmission-blocking agent, since mature gametocytes persist in the blood for up to 2 weeks. However, the limited absorption, distribution, metabolism, and excretion (ADME)/Tox data presently available in animal models suggest that new derivatives with longer half-lives and improved safety profiles need to be developed.
In conclusion, our data suggest a strong and fast activity of monovalent ionophores against asexual stages and against mature gametocytes and ookinetes, resulting in a block of sporogonic development in the mosquito. These compounds, being active against several different P. falciparum life stages, thus appear to be particularly attractive as potential curative and transmission-blocking agents, as advocated by the International Malaria Control Program and by the MMV (6).
ACKNOWLEDGMENTS
We thank Pietro Alano and his group for sharing gametocyte culture technology and for helpful suggestions, Daniela Jabes (NAICONS, Milan, Italy) for providing the molecules that initiated this work, and Laura Galastri, Paola Verducci, and Tiziana Bianchi from AVIS Comunale Milano for providing blood samples for parasite culture.
This work was supported by the Global Health Program of the Bill & Melinda Gates Foundation (grant OPP1040394).
REFERENCES
- 1.WHO. 2013. World malaria report. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2013/wmr2013_no_profiles.pdf?ua=1. [Google Scholar]
- 2.White NJ, Pukrittayakamee S, Hien TT, Faiz MA, Mokuolu OA, Dondorp AM. 2014. Malaria. Lancet 383:723–735. doi: 10.1016/S0140-6736(13)60024-0. [DOI] [PubMed] [Google Scholar]
- 3.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NJ. 2014. Malaria: a molecular marker of artemisinin resistance. Lancet 383:1439–1440. doi: 10.1016/S0140-6736(14)60656-5. [DOI] [PubMed] [Google Scholar]
- 5.Burrows JN, Burlot E, Campo B, Cherbuin S, Jeanneret S, Leroy D, Spangenberg T, Waterson D, Wells TN, Willis P. 2014. Antimalarial drug discovery—the path towards eradication. Parasitology 141:128–139. doi: 10.1017/S0031182013000826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrows JN, van Huijsduijnen RH, Möhrle JJ, Oeuvray C, Wells TN. 2013. Designing the next generation of medicines for malaria control and eradication. Malar J 12:187. doi: 10.1186/1475-2875-12-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alano P. 2007. Plasmodium falciparum gametocytes: still many secrets of a hidden life. Mol Microbiol 66:291–302. doi: 10.1111/j.1365-2958.2007.05904.x. [DOI] [PubMed] [Google Scholar]
- 8.Lotharius J, Gamo-Benito FJ, Angulo-Barturen I, Clark J, Connelly M, Ferrer-Bazaga S, Parkinson T, Viswanath P, Bandodkar B, Rautela N, Bharath S, Duffy S, Avery VM, Möhrle JJ, Guy RK, Wells T. 2014. Repositioning: the fast track to new anti-malarial medicines? Malar J 13:143. doi: 10.1186/1475-2875-13-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitani M, Yamanishi T, Miyazaki Y, Ōtake N. 1976. Salinomycin effects on mitochondrial ion translocation and respiration. Antimicrob Agents Chemother 9:655–660. doi: 10.1128/AAC.9.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang QL, Zhao ZQ, Li JC, Liang Y, Yin JQ, Zou CY, Xie XB, Zeng YX, Shen JN, Kang T, Wang J. 2011. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett 311:113–121. doi: 10.1016/j.canlet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Zhou HM, Dong TT, Wang LL, Feng B, Zhao HC, Fan XK, Zheng MH. 2012. Suppression of colorectal cancer metastasis by nigericin through inhibition of epithelial-mesenchymal transition. World J Gastroenterol 18:2640–2648. doi: 10.3748/wjg.v18.i21.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumova L, Pombinho AR, Vojtechova M, Stancikova J, Gradl D, Krausova M, Sloncova E, Horazna M, Kriz V, Machonova O, Jindrich J, Zdrahal Z, Bartunek P, Korinek V. 2014. Monensin inhibits canonical Wnt signaling in human colorectal cancer cells and suppresses tumor growth in multiple intestinal neoplasia mice. Mol Cancer Ther 13:812–822. doi: 10.1158/1535-7163.MCT-13-0625. [DOI] [PubMed] [Google Scholar]
- 13.Naujokat C, Steinhart R. 2012. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol 2012:950658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gumila C, Ancelin ML, Delort AM, Jeminet G, Vial HJ. 1997. Characterization of the potent in vitro and in vivo antimalarial activities of ionophore compounds. Antimicrob Agents Chemother 41:523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun W, Tanaka TQ, Magle CT, Huang W, Southall N, Huang R, Dehdashti SJ, McKew JC, Williamson KC, Zheng W. 2014. Chemical signatures and new drug targets for gametocytocidal drug development. Sci Rep 4:3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adovelande J, Schrével J. 1996. Carboxylic ionophores in malaria chemotherapy: the effects of monensin and nigericin on Plasmodium falciparum in vitro and Plasmodium vinckei petteri in vivo. Life Sci 59:PL309-PL315. [DOI] [PubMed] [Google Scholar]
- 17.Trager W, Jensen JB. 2005. Human malaria parasites in continuous culture. 1976. J Parasitol 91:484–486. doi: 10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Rathod PK, McErlean T, Lee PC. 1997. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A 94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekland EH, Fidock DA. 2008. In vitro evaluations of antimalarial drugs and their relevance to clinical outcomes. Int J Parasitol 38:743–747. doi: 10.1016/j.ijpara.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D'Alessandro S, Silvestrini F, Dechering K, Corbett Y, Parapini S, Timmerman M, Galastri L, Basilico N, Sauerwein R, Alano P, Taramelli D. 2013. A Plasmodium falciparum screening assay for anti-gametocyte drugs based on parasite lactate dehydrogenase detection. J Antimicrob Chemother 68:2048–2058. doi: 10.1093/jac/dkt165. [DOI] [PubMed] [Google Scholar]
- 21.Makler MT, Ries JM, Williams JA, Bancroft JE, Piper RC, Gibbins BL, Hinrichs DJ. 1993. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am J Trop Med Hyg 48:739–741. [DOI] [PubMed] [Google Scholar]
- 22.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. 1992. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Investig Dermatol 99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 23.D'Alessandro S, Gelati M, Basilico N, Parati EA, Haynes RK, Taramelli D. 2007. Differential effects on angiogenesis of two antimalarial compounds, dihydroartemisinin and artemisone: implications for embryotoxicity. Toxicology 241:66–74. doi: 10.1016/j.tox.2007.08.084. [DOI] [PubMed] [Google Scholar]
- 24.Gazzetta Ufficiale. 2014. Protection of animals used for experimental and other scientific purposes. Decreto legislativo 4 marzo 2014, no. 26. Gazzetta Ufficiale, Rome, Italy: http://www.gazzettaufficiale.it/eli/id/2014/03/14/14G00036/sg (In Italian.) [Google Scholar]
- 25.European Parliament and Council. 2010. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. European Parliament and Council, Brussels, Belgium: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32010L0063. [Google Scholar]
- 26.Delves MJ, Ramakrishnan C, Blagborough AM, Leroy D, Wells TN, Sinden RE. 2012. A high-throughput assay for the identification of malarial transmission-blocking drugs and vaccines. Int J Parasitol 42:999–1006. doi: 10.1016/j.ijpara.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Stone WJ, Churcher TS, Graumans W, van Gemert GJ, Vos MW, Lanke KH, van de Vegte-Bolmer MG, Siebelink-Stoter R, Dechering KJ, Vaughan AM, Camargo N, Kappe SH, Sauerwein RW, Bousema T. 2014. A scalable assessment of Plasmodium falciparum transmission in the standard membrane-feeding assay, using transgenic parasites expressing green fluorescent protein-luciferase. J Infect Dis 210:1456–1463. doi: 10.1093/infdis/jiu271. [DOI] [PubMed] [Google Scholar]
- 28.Lucantoni L, Avery V. 2012. Whole-cell in vitro screening for gametocytocidal compounds. Future Med Chem 4:2337–2360. doi: 10.4155/fmc.12.188. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoudi N, de Julián-Ortiz JV, Ciceron L, Gálvez J, Mazier D, Danis M, Derouin F, García-Domenech R. 2006. Identification of new antimalarial drugs by linear discriminant analysis and topological virtual screening. J Antimicrob Chemother 57:489–497. doi: 10.1093/jac/dki470. [DOI] [PubMed] [Google Scholar]
- 30.Yuan J, Cheng KC, Johnson RL, Huang R, Pattaradilokrat S, Liu A, Guha R, Fidock DA, Inglese J, Wellems TE, Austin CP, Su XZ. 2011. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science 333:724–729. doi: 10.1126/science.1205216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gumila C, Ancelin ML, Jeminet G, Delort AM, Miquel G, Vial HJ. 1996. Differential in vitro activities of ionophore compounds against Plasmodium falciparum and mammalian cells. Antimicrob Agents Chemother 40:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derbyshire ER, Prudêncio M, Mota MM, Clardy J. 2012. Liver-stage malaria parasites vulnerable to diverse chemical scaffolds. Proc Natl Acad Sci U S A 109:8511–8516. doi: 10.1073/pnas.1118370109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leitao R, Rodriguez A. 2010. Inhibition of Plasmodium sporozoites infection by targeting the host cell. Exp Parasitol 126:273–277. doi: 10.1016/j.exppara.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steverding D, Sexton DW. 2013. Trypanocidal activity of salinomycin is due to sodium influx followed by cell swelling. Parasit Vectors 6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapoor A, He R, Venkatadri R, Forman M, Arav-Boger R. 2013. Wnt modulating agents inhibit human cytomegalovirus replication. Antimicrob Agents Chemother 57:2761–2767. doi: 10.1128/AAC.00029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lavine MD, Arrizabalaga G. 2011. The antibiotic monensin causes cell cycle disruption of Toxoplasma gondii mediated through the DNA repair enzyme TgMSH-1. Antimicrob Agents Chemother 55:745–755. doi: 10.1128/AAC.01092-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. 2009. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antoszczak M, Huczyński A. 2015. Anticancer activity of polyether ionophone-salinomycin. Anticancer Agents Med Chem 15:575–591. [DOI] [PubMed] [Google Scholar]
- 39.Koo KH, Kim H, Bae YK, Kim K, Park BK, Lee CH, Kim YN. 2013. Salinomycin induces cell death via inactivation of Stat3 and downregulation of Skp2. Cell Death Dis 4:e693. doi: 10.1038/cddis.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopp F, Hermawan A, Oak PS, Herrmann A, Wagner E, Roidl A. 2014. Salinomycin treatment reduces metastatic tumor burden by hampering cancer cell migration. Mol Cancer 13:16. doi: 10.1186/1476-4598-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verdoodt B, Vogt M, Schmitz I, Liffers ST, Tannapfel A, Mirmohammadsadegh A. 2012. Salinomycin induces autophagy in colon and breast cancer cells with concomitant generation of reactive oxygen species. PLoS One 7:e44132. doi: 10.1371/journal.pone.0044132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. 2011. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A 108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cervantes S, Bunnik EM, Saraf A, Conner CM, Escalante A, Sardiu ME, Ponts N, Prudhomme J, Florens L, Le Roch KG. 2014. The multifunctional autophagy pathway in the human malaria parasite, Plasmodium falciparum. Autophagy 10:80–92. doi: 10.4161/auto.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavine MD, Arrizabalaga G. 2012. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS One 7:e42107. doi: 10.1371/journal.pone.0042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. 2011. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A 108:E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker DA. 2010. Malaria gametocytogenesis. Mol Biochem Parasitol 172:57–65. doi: 10.1016/j.molbiopara.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peatey CL, Leroy D, Gardiner DL, Trenholme KR. 2012. Anti-malarial drugs: how effective are they against Plasmodium falciparum gametocytes? Malar J 11:34. doi: 10.1186/1475-2875-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chotivanich K, Sattabongkot J, Udomsangpetch R, Looareesuwan S, Day NP, Coleman RE, White NJ. 2006. Transmission-blocking activities of quinine, primaquine, and artesunate. Antimicrob Agents Chemother 50:1927–1930. doi: 10.1128/AAC.01472-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Pelt-Koops JC, Pett HE, Graumans W, van der Vegte-Bolmer M, van Gemert GJ, Rottmann M, Yeung BK, Diagana TT, Sauerwein RW. 2012. The spiroindolone drug candidate NITD609 potently inhibits gametocytogenesis and blocks Plasmodium falciparum transmission to anopheles mosquito vector. Antimicrob Agents Chemother 56:3544–3548. doi: 10.1128/AAC.06377-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nilsen A, LaCrue AN, White KL, Forquer IP, Cross RM, Marfurt J, Mather MW, Delves MJ, Shackleford DM, Saenz FE, Morrisey JM, Steuten J, Mutka T, Li Y, Wirjanata G, Ryan E, Duffy S, Kelly JX, Sebayang BF, Zeeman AM, Noviyanti R, Sinden RE, Kocken CH, Price RN, Avery VM, Angulo-Barturen I, Jiménez-Díaz MB, Ferrer S, Herreros E, Sanz LM, Gamo FJ, Bathurst I, Burrows JN, Siegl P, Guy RK, Winter RW, Vaidya AB, Charman SA, Kyle DE, Manetsch R, Riscoe MK. 2013. Quinolone-3-diarylethers: a new class of antimalarial drug. Sci Transl Med 5:177ra137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhen KL, Chatterjee AK, Rottmann M, Gagaring K, Borboa R, Buenviaje J, Chen Z, Francek C, Wu T, Nagle A, Barnes SW, Plouffe D, Lee MC, Fidock DA, Graumans W, van de Vegte-Bolmer M, van Gemert GJ, Wirjanata G, Sebayang B, Marfurt J, Russell B, Suwanarusk R, Price RN, Nosten F, Tungtaeng A, Gettayacamin M, Sattabongkot J, Taylor J, Walker JR, Tully D, Patra KP, Flannery EL, Vinetz JM, Renia L, Sauerwein RW, Winzeler EA, Glynne RJ, Diagana TT. 2014. KAF156 is an antimalarial clinical candidate with potential for use in prophylaxis, treatment and prevention of disease transmission. Antimicrob Agents Chemother 58:5060–5067. doi: 10.1128/AAC.02727-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamasaki M, Nakamura K, Tamura N, Hwang SJ, Yoshikawa M, Sasaki N, Ohta H, Yamato O, Maede Y, Takiguchi M. 2009. Effects and mechanisms of action of ionophorous antibiotics valinomycin and salinomycin-Na on Babesia gibsoni in vitro. J Parasitol 95:1532–1538. doi: 10.1645/GE-2036.1. [DOI] [PubMed] [Google Scholar]
- 53.Bolscher JM, Koolen KM, van Gemert GJ, van de Vegte-Bolmer MG, Bousema T, Leroy D, Sauerwein RW, Dechering KJ. 2015. A combination of new screening assays for prioritization of transmission-blocking antimalarials reveals distinct dynamics of marketed and experimental drugs. J Antimicrob Chemother 70:1357–1366. doi: 10.1093/jac/dkv003. [DOI] [PubMed] [Google Scholar]
- 54.Bissinger R, Malik A, Jilani K, Lang F. 2014. Triggering of erythrocyte cell membrane scrambling by salinomycin. Basic Clin Pharmacol Toxicol 115:396–402. doi: 10.1111/bcpt.12250. [DOI] [PubMed] [Google Scholar]
- 55.Gumila C, Miquel G, Seta P, Ancelin ML, Delort AM, Jeminet G, Vial HJ. 1999. Ionophore-phospholipid interactions in langmuir films in relation to ionophore selectivity toward Plasmodium-infected erythrocytes. J Colloid Interface Sci 218:377–387. doi: 10.1006/jcis.1999.6432. [DOI] [PubMed] [Google Scholar]
- 56.Omodeo-Salè F, Motti A, Basilico N, Parapini S, Olliaro P, Taramelli D. 2003. Accelerated senescence of human erythrocytes cultured with Plasmodium falciparum. Blood 102:705–711. doi: 10.1182/blood-2002-08-2437. [DOI] [PubMed] [Google Scholar]
- 57.Ginsburg H, Handeli S, Friedman S, Gorodetsky R, Krugliak M. 1986. Effects of red blood cell potassium and hypertonicity on the growth of Plasmodium falciparum in culture. Z Parasitenkd 72:185–199. doi: 10.1007/BF00931146. [DOI] [PubMed] [Google Scholar]
- 58.Kirk K, Lehane AM. 2014. Membrane transport in the malaria parasite and its host erythrocyte. Biochem J 457:1–18. doi: 10.1042/BJ20131007. [DOI] [PubMed] [Google Scholar]
- 59.Fruth IA, Arrizabalaga G. 2007. Toxoplasma gondii: induction of egress by the potassium ionophore nigericin. Int J Parasitol 37:1559–1567. doi: 10.1016/j.ijpara.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pakozdy A, Challande-Kathman I, Doherr M, Cizinauskas S, Wheeler SJ, Oevermann A, Jaggy A. 2010. Retrospective study of salinomycin toxicosis in 66 cats. Vet Med Int 2010:147142. doi: 10.4061/2010/147142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holliman A, Howie F, Payne J, Scholes S. 2011. Salinomycin toxicity in dairy calves. Vet Rec 169:561. doi: 10.1136/vr.d7423. [DOI] [PubMed] [Google Scholar]
- 62.Aleman M, Magdesian KG, Peterson TS, Galey FD. 2007. Salinomycin toxicosis in horses. J Am Vet Med Assoc 230:1822–1826. doi: 10.2460/javma.230.12.1822. [DOI] [PubMed] [Google Scholar]
- 63.Dorne JL, Fernández-Cruz ML, Bertelsen U, Renshaw DW, Peltonen K, Anadon A, Feil A, Sanders P, Wester P, Fink-Gremmels J. 2013. Risk assessment of coccidostatics during feed cross-contamination: animal and human health aspects. Toxicol Appl Pharmacol 270:196–208. doi: 10.1016/j.taap.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 64.Story P, Doube A. 2004. A case of human poisoning by salinomycin, an agricultural antibiotic. N Z Med J 117:U799. [PubMed] [Google Scholar]
- 65.Kouyoumdjian JA, Morita MP, Sato AK, Pissolatti AF. 2001. Fatal rhabdomyolysis after acute sodium monensin (Rumensin) toxicity: case report. Arq Neuropsiquiatr 59:596–598. doi: 10.1590/S0004-282X2001000400022. [DOI] [PubMed] [Google Scholar]
- 66.Zhou S, Wang F, Wong ET, Fonkem E, Hsieh TC, Wu JM, Wu E. 2013. Salinomycin: a novel anti-cancer agent with known anti-coccidial activities. Curr Med Chem 20:4095–4101. doi: 10.2174/15672050113109990199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Antoszczak M, Popiel K, Stefańska J, Wietrzyk J, Maj E, Janczak J, Michalska G, Brzezinski B, Huczyński A. 2014. Synthesis, cytotoxicity and antibacterial activity of new esters of polyether antibiotic–salinomycin. Eur J Med Chem 76:435–444. doi: 10.1016/j.ejmech.2014.02.031. [DOI] [PubMed] [Google Scholar]
- 68.Surolia R, Pachauri M, Ghosh PC. 2012. Preparation and characterization of monensin loaded PLGA nanoparticles: in vitro anti-malarial activity against Plasmodium falciparum. J Biomed Nanotechnol 8:172–181. [DOI] [PubMed] [Google Scholar]
- 69.Lagas JS, Sparidans RW, van Waterschoot RA, Wagenaar E, Beijnen JH, Schinkel AH. 2008. P-glycoprotein limits oral availability, brain penetration, and toxicity of an anionic drug, the antibiotic salinomycin. Antimicrob Agents Chemother 52:1034–1039. doi: 10.1128/AAC.01041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]