Abstract
The efficacy of cefmetazole and flomoxef (CF) for the treatment of patients with extended-spectrum β-lactamase-producing Escherichia coli (ESBL-EC) bacteremia (ESBL-CF group) was compared with that of carbapenem treatment for ESBL-EC patients (ESBL-carbapenem group) and with that of CF treatment in patients with non-ESBL-EC bacteremia (non-ESBL-CF group). Adult patients treated for E. coli bacteremia in four hospitals were retrospectively evaluated. The 30-day mortality rates in patients belonging to the ESBL-CF, ESBL-carbapenem, and non-ESBL-CF groups were compared as 2 (empirical and definitive therapy) cohorts. The adjusted hazard ratios (aHRs) for mortality were calculated using Cox regression models with weighting according to the inverse probability of propensity scores for receiving CF or carbapenem treatment. The empirical-therapy cohort included 104 patients (ESBL-CF, 26; ESBL-carbapenem, 45; non-ESBL-CF, 33), and the definitive-therapy cohort included 133 patients (ESBL-CF, 59; ESBL-carbapenem, 54; non-ESBL-CF, 20). The crude 30-day mortality rates for patients in the ESBL-CF, ESBL-carbapenem, and non-ESBL-CF groups were, respectively, 7.7%, 8.9%, and 3.0% in the empirical-therapy cohort and 5.1%, 9.3%, and 5.0% in the definitve-therapy cohort. In patients without hematological malignancy and neutropenia, CF treatment for ESBL-EC patients was not associated with mortality compared with carbapenem treatment (empirical-therapy cohort: aHR, 0.87; 95% confidence interval [CI], 0.11 to 6.52; definitive therapy cohort: aHR, 1.04; CI, 0.24 to 4.49). CF therapy may represent an effective alternative to carbapenem treatment for patients with ESBL-EC bacteremia for empirical and definitive therapy in adult patients who do not have hematological malignancy and neutropenia.
Bacteremia caused by Escherichia coli is a common and significant problem in both community and health care settings (1). In recent years, the prevalence of extended-spectrum β-lactamase-producing E. coli (ESBL-EC) has increased dramatically worldwide. Effective treatment of ESBL-EC bacteremia has become challenging because ESBL-EC is usually resistant to cephalosporins, the first-line drug used for E. coli infections (2). At least partially due to the delay in effective treatment regimens, bacteremia caused by ESBL-EC is associated with a higher mortality rate than that caused by non-ESBL-EC (3). Currently, the standard therapy for ESBL-EC bacteremia is virtually limited to carbapenems (4, 5). Alternate therapies involving fluoroquinolones, sulfamethoxazole-trimethoprim, and aminoglycosides offer limited efficacy due to frequent coresistance mechanisms in ESBL-EC (2). To prevent carbapenem overuse and spread of carbapenem-resistant Enterobacteriaceae, alternative drug regimens are needed (6).
β-Lactam/β-lactamase inhibitors and cephamycins (i.e., cefmetazole, cefoxitin, cefotetan, moxalactam, and flomoxef) are usually stable to hydrolysis by ESBLs (2). The MICs of β-lactam/β-lactamase inhibitors can increase as the inoculum increases (7); however, cephamycins (cefotetan and flomoxef) exert in vitro antibacterial activity even in the presence of high inocula (8, 9). Clinical data for β-lactam/β-lactamase inhibitors obtained with a large cohort of patients by using propensity score analysis have been published, but the conclusions are inconsistent (10, 11). In Japan, cefmetazole and flomoxef (CF) are frequently used to treat intra-abdominal infections or as prophylaxis for surgery. Thus, Japanese clinicians have sometimes used CF therapy for sepsis or bacteremia as empirical therapy or as definitive therapy when susceptibility was confirmed. However, clinical data evaluating the effectiveness of cephamycins for ESBL-EC bacteremia have not been reported yet.
In this multicenter retrospective study using propensity score analysis, we evaluated the effectiveness of empirical and definitive CF treatments for ESBL-EC bacteremia in comparison with standard carbapenem therapy. We also evaluated patients with non-ESBL-EC bacteremia who were treated with a CF regimen to investigate potential associations between ESBL production and patient outcomes when E. coli bacteremia was treated with CF therapy.
MATERIALS AND METHODS
Setting and study design.
This retrospective study was conducted at four acute-care hospitals in Japan: the Kyoto University Hospital, the Kyoto Prefectural Medical University Hospital, the Kyoto City Hospital, and the Kyoto-Katsura Hospital. All the bacteremic patients in our hospitals were reported to and followed up by our infectious-disease physicians. Changes in antimicrobial treatment and general management were advised if considered necessary. The Ethics Committee of Kyoto University Graduate School and the Faculty of Medicine (E1835) approved this study and waived the need to obtain informed patient consent.
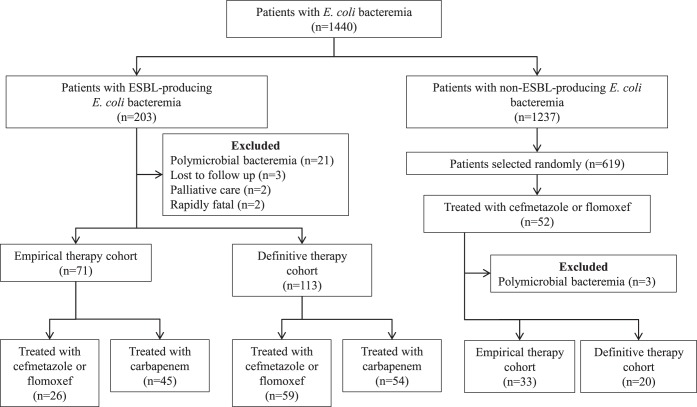
Patients older than 15 years of age with bacteremia due to E. coli were enrolled in this study between January 2005 and April 2014. Each patient was included in the study (limited to one episode per patient) at the time of the initial positive blood culture. Patients who died within 24 h of the onset of bacteremia or due to polymicrobial bacteremia were excluded. Two cohorts were defined and analyzed separately in a manner similar to previous studies (10). The empirical-therapy cohort (ETC) included patients who received empirical therapy with CF or carbapenem monotherapy, whose first dose was administered during the first 24 h after the blood culture had been drawn, and whose isolate was susceptible to the empirical antimicrobial administered. The definitive-therapy cohort (DTC) included patients receiving definitive monotherapy with an active CF or carbapenem administered for ≥50% of the total duration of antimicrobial therapy. Two types of comparisons were performed for both cohorts (Fig. 1): the comparison between patients with ESBL-EC bacteremia who received CF (ESBL-CF) and those who received carbapenem (ESBL-CARBA) and the comparison between the ESBL-CF group and patients with non-ESBL-EC bacteremia who received CF (non-ESBL-CF). To assign the cohorts, we reviewed the medical charts of all of the patients with ESBL-EC bacteremia and reviewed the randomly selected charts of half of the patients with non-ESBL-EC bacteremia to find patients in this demographic who received CF therapy.
FIG 1.
Flow diagram of the patient selection process.
Variables and definitions.
The cases of bacteremia were categorized as nosocomial, health care associated, or community acquired in accordance with criteria by Friedman et al. (12). Neutropenia was defined as an absolute neutrophil count below 500/mm3. Empirical therapy was considered appropriate when an active antimicrobial agent, determined by in vitro susceptibility testing, was administered at the usual recommended dose within the first 24 h after blood sampling.
The clinical information acquired from the medical charts included age, sex, length of hospital stay before bacteremia, history of isolating the ESBL-producing bacterial strain, receipt of immunosuppressive therapies during the previous 30 days, any antimicrobial therapy during the previous 30 days, underlying diseases, Charlson score (13), surgery during the previous 30 days, neutropenia, presence of an intravenous catheter or any other artificial device, SOFA (sepsis-related organ failure assessment) score (14), presence of severe sepsis or septic shock (15) (the previous 4 factors were assessed at the time of blood culture collection), site of infection (all of the above variables were regarded as baseline characteristics), antimicrobial regimen, and patient outcomes.
The primary outcome measure was the 30-day mortality rate. Clinical success within 30 days from the onset of bacteremia was also assessed and was defined as a complete response and no relapse within 30 days or before patient death, and no need to change the antimicrobial regimen due to ineffectiveness or other adverse events. The time of response to treatment was assessed every 24 h after the start of antimicrobial therapy, and a complete response was defined as the resolution of fever, leukocytosis, and all signs of infection. Relapse of infection was defined as the isolation of the same organism from the infection site or blood with the associated clinical symptoms.
Microbiological analysis.
At each hospital, microbiological identification was carried out using the Vitek2 (bioMérieux, Marcy l'Etoile, France) or the MicroScan (Siemens Healthcare Diagnostics, Tokyo, Japan) system. Antibiotic susceptibility was evaluated by microdilution using the Dry Plate Eiken (Eiken, Tokyo, Japan) or the MicroScan system. The results were interpreted based on the 2009 CLSI breakpoints (16). In each hospital, isolates were reported as susceptible to flomoxef at a MIC of ≤8 μg/ml in reference to the CLSI breakpoint for moxalactam (≤8 μg/ml). The same criterion for flomoxef was used in this study. A subset of isolates stored in a freezer was sent to Kyoto University and was re-evaluated by microdilution using the Dry Plate Eiken to obtain MIC distribution data for CF.
ESBL screening was performed according to the CLSI microdilution method (cefpodoxime MIC ≥ 8 μg/ml, cefotaxime MIC ≥ 2 μg/ml, ceftazidime MIC ≥ 2 μg/ml, or aztreonam MIC ≥ 2 μg/ml) (16). The ESBL confirmation test was performed using cefotaxime-clavulanate and ceftazidime-clavulanate disks according to CLSI guidelines (16).
Statistical analysis.
Categorical variables were compared using Fisher's exact test, whereas continuous variables were compared using the Mann-Whitney U test. A Cox proportional hazard regression or logistic regression model was used to determine the association of CF treatment with patient outcomes. To minimize baseline characteristic differences between the ESBL-CF and ESBL-CARBA groups, a propensity score-adjusted analysis was conducted in addition to an unadjusted analysis. The propensity score was calculated using a nonparsimonious multivariate logistic regression model, in which the outcome variable was the use of CF as empirical or definitive therapy. The stabilized inverse probabilities of the treatment weights were used to estimate the average treatment effects of CF compared with carbapenem (17, 18). The balance of each group was determined by standardized biases of <0.25 for all variables representing baseline characteristics (17). If balance was not achieved, other multivariate logistic regression models were attempted by restricting variables using a forward stepwise procedure until balancing was achieved. A P value of <0.05 was considered statistically significant. We conducted our statistical analysis using Stata version 13.1 software (StataCorp, College Station, TX, USA). The standardized biases were calculated using R version 3.1.2 software (R Foundation for Statistical Computing, Vienna, Austria) and the twang R package (R Toolkit for Weighting and Analysis of Nonequivalent Groups).
RESULTS
During the study period, a total of 1,440 E. coli isolates were recovered from patient blood, 203 (14%) of which were positive for ESBL (ESBL-EC) based on results from the confirmation test. The ETC included 104 patients (ESBL-EC, 71; non-ESBL-EC, 33), and the DTC included 133 patients (ESBL-EC, 113; non-ESBL-EC, 20). Of the ETC and DTC patients, 70 (ESBL-EC, 58; non-ESBL-EC, 12) were included in both cohorts (Fig. 1).
Antibiotic susceptibility.
All of the E. coli isolates in this study were susceptible to imipenem and/or meropenem. A total of 121 ESBL-EC and 45 non-ESBL-EC isolates were stored and re-evaluated for the MICs of CF in these strains; 94.2% of ESBL-EC and 100.0% of non-ESBL isolates were susceptible to cefmetazole. The MIC50 and MIC90 (MIC at which 50% and 90% of the isolates were inhibited, respectively) were ≤1 and 2 μg/ml in ESBL-EC and ≤1 and 8 μg/ml in non-ESBL-EC isolates. In total, 98.3% of ESBL-EC and 97.8% of non-ESBL isolates were susceptible to flomoxef. The MIC50 and MIC90 were ≤1 and ≤1 μg/ml in ESBL-EC and non-ESBL-EC isolates. Of the 167 patients in the ETC and DTC, 98.4% and 98.2% of ESBL-EC isolates were susceptible to cefmetazole and flomoxef, respectively, and all of the patients with isolates that were not susceptible to CF received carbapenem. All of the non-ESBL-EC isolates were susceptible to CF.
Empirical therapy cohort.
The ESBL-CF, ESBL-CARBA, and non-ESBL-CF groups were comprised of 26 patients (cefmetazole, 13; flomoxef, 13), 45 patients (meropenem, 36; imipenem, 8; panipenem, 1), and 33 patients (cefmetazole, 21; flomoxef, 12), respectively. Regarding dosage regimens, >90% of the patients in each group received the following intravenous doses (or adjusted equivalent in the case of renal failure): cefmetazole, 1 g/8 h; flomoxef, 1 g/8 h; meropenem, 1 g/8 h or 0.5 g/8 h; imipenem, 1 g/8 h or 0.5 g/8 h; and panipenem, 0.5 g/6 h. The clinical characteristics of each group are shown in Table 1.
TABLE 1.
Characteristics of patients with bacteremia caused by Escherichia coli in the empirical therapy cohort
| Characteristic | Group according to ESBL production and treatmenta |
P valueb |
|||
|---|---|---|---|---|---|
| ESBL-CF (n = 26) | ESBL-CARBA (n = 45) | Non-ESBL-CF (n = 33) | Versus CARBA | Versus non-ESBL | |
| Age (yr)i | 71 (65–83) | 68 (62–78) | 71 (60–79) | 0.22 | 0.41 |
| No. (%) of males | 14 (54) | 24 (53) | 10 (30) | 1 | 0.11 |
| Length of hospital stay before bacteremia (days)i | 6.5 (1–33) | 18 (1–47) | 5 (1–23) | 0.22 | 0.50 |
| No. (%) with: | |||||
| Nosocomial or health care-associated bacteremia | 16 (62) | 31 (69) | 20 (61%) | 0.61 | 1 |
| Prior ESBL producer isolation | 14 (54) | 17 (38) | 1 (3%) | 0.22 | <0.001 |
| Prior use of antimicrobials within 30 days | 15 (58) | 29 (64) | 11 (33%) | 0.62 | 0.07 |
| Charlson comorbidity indexi | 3 (1–6) | 3 (2–3) | 2 (1–4) | 0.51 | 0.31 |
| No. (%) with: | |||||
| Hematological malignancy | 0 (0) | 13 (29) | 1 (3) | 0.001 | 1 |
| Solid malignancy | 11 (42) | 11 (24) | 14 (42) | 0.18 | 1 |
| Diabetes | 7 (27) | 8 (18) | 9 (27) | 0.38 | 1 |
| Transplantation | 2 (8) | 11 (24) | 0 (0) | 0.11 | 0.19 |
| Immunosuppressive therapy | 5 (19) | 13 (29) | 7 (21) | 0.41 | 1 |
| Antitumor chemotherapy | 6 (23) | 12 (27) | 7 (21) | 0.79 | 1 |
| Neutropenia | 0 (0) | 6 (13) | 1 (3) | 0.08 | 1 |
| Hemodialysis | 1 (4) | 2 (4) | 0 (0) | 1 | 0.44 |
| Surgery within 30 days | 3 (12) | 5 (11) | 2 (6) | 1 | 0.65 |
| Intravascular catheterization | 6 (23) | 24 (53) | 10 (30) | 0.02 | 0.57 |
| Urinary catheterization | 4 (15) | 7 (16) | 3 (9) | 1 | 0.69 |
| Artificial devices other than intravascular or urinary catheter | 5 (19) | 13 (29) | 1 (3) | 0.41 | 0.08 |
| Severe sepsis or septic shock | 7 (27) | 21 (47) | 6 (18) | 0.13 | 0.53 |
| SOFA scorei | 1 (0–4) | 3 (2–6) | 1 (0–3) | 0.005 | 0.60 |
| No. (%) with infection at site | |||||
| Urinary tract | 16 (62) | 16 (36) | 23 (70) | 0.048 | 0.59 |
| Intra-abdominal | 6 (23) | 17 (38) | 6 (18) | 0.29 | 0.75 |
| Primary | 3 (12) | 10 (22) | 3 (9) | 0.35 | 1 |
| Other | 1 (4) | 2 (4) | 1 (3) | 1 | 1 |
| No. (%) receiving empirical therapy | |||||
| Cefmetazole | 13 (50) | 0 (0) | 21 (64) | <0.001 | 0.43 |
| Flomoxef | 13 (50) | 0 (0) | 12 (36) | <0.001 | 0.43 |
| Carbapenem | 0 (0) | 45c (100) | 0 (0) | <0.001 | 1 |
| Appropriate therapy | 26 (100) | 45 (100) | 33 (100) | 1 | 1 |
| No. (%) receiving definitive therapy | |||||
| Cefmetazole | 10 (38) | 6 (13) | 7 (21) | 0.02 | 0.16 |
| Flomoxef | 9 (35) | 3 (7) | 6 (18) | 0.006 | 0.23 |
| Carbapenem | 2 (8) | 33 (73) | 0 (0) | <0.001 | 0.19 |
| Cephalosporin | 0 (0) | 0 (0) | 18 (55) | 1 | <0.001 |
| Othersd | 5 (19) | 3 (7) | 2 (6) | 0.13 | 0.22 |
| Total duration of treatment (days)i | 14.5 (11–18) | 14 (10–21) | 13 (10–14) | 0.89 | 0.11 |
| Duration of treatment with the antimicrobial agent used in empirical therapy (days)i | 8 (5–14) | 10 (6–14) | 4 (2–7) | 0.76 | <0.001 |
| No. (%) with: | |||||
| 30-day mortality | 2e (8) | 4 (9) | 1f (3) | 1 | 0.58 |
| Clinical success within 30 days | 26 (100) | 42g (93) | 32h (97) | 0.29 | 1 |
| Duration between onset of the bacteremia and complete response (days)i | 3 (2–4) | 4 (2–7) | 3 (2–4) | 0.30 | 0.80 |
| No. (%) with relapse within 30 days | 0 (0) | 0 (0) | 1h (3) | 1 | 1 |
As empirical therapy, the ESBL-CF group included patients with ESBL-producing E. coli bacteremia who received cefmetazole or flomoxef, the ESBL-CARBA group included patients with ESBL-producing E. coli bacteremia who received carbapenems, and the non-ESBL-CF group included patients with non-ESBL-producing E. coli bacteremia who received cefmetazole or flomoxef.
P values were calculated based on a comparison between the ESBL-CF group and the ESBL-CARBA or non-ESBL-CF group.
Meropenem, imipenem, and panipenem were administered to 36, 8, and 1 patients, respectively.
In the ESBL-EC group receiving cefmetazole or flomoxef, three patients received fluoroquinolones, and two patients received sulfamethoxazole-trimethoprim. In the ESBL-EC group receiving carbapenem, two patients received β-lactam/β-lactamase inhibitors, and one patient received fluoroquinolones. In the non-ESBL-EC cohort, one patient received β-lactam/β-lactamase inhibitor, and one patient received amoxicillin.
These two patients received cefmetazole and died after the completion of treatment due to underlying malignancies. The MICs of cefmetazole were ≤1 and ≤16 μg/ml.
This patient received cefmetazole and died after treatment completion due to underlying liver cirrhosis and malignancy. The MIC of cefmetazole was ≤1 μg/ml.
Three patients died before the achievement of a complete response.
One patient experienced acute pyelonephritis, and recurrent bacteremia was observed 4 days after the completion of the antimicrobial treatment with flomoxef for 3 days followed by cefotaxime for 8 days. The MIC of flomoxef was ≤1 μg/ml. The isolate of the recurrent episode did not develop resistance to flomoxef.
Data are medians (interquartile range).
The crude 30-day mortality rates were 7.7% in the ESBL-CF group, 8.9% in the ESBL-CARBA group, and 3.0% in the non-ESBL-CF group. The relationship between the crude 30-day mortality and the CF MICs is shown in Table 2. Unadjusted Cox regression analyses of the mortality rates indicated that the ESBL-CF group had a hazard ratio (HR) of 0.83 (95% confidence interval [CI], 0.15 to 4.53) compared with the ESBL-CARBA group and an HR of 2.64 (CI, 0.23 to 29.1) compared with the non-ESBL-CF group. The clinical success rates and the times to complete response were similar in the three groups (>90% and 3 to 4 days, respectively). Table 3 presents the propensity score-adjusted analysis, which included only patients without hematological malignancy or neutropenia, because the ESBL-CF group did not include these patients. Compared with carbapenem treatment, empirical treatment with CF for ESBL-EC bacteremia was not associated with mortality (adjusted HR, 0.87; CI, 0.11 to 6.52) and demonstrated similar clinical success rates (100% and 96.5%, respectively).
TABLE 2.
Thirty-day mortality in patients with Escherichia coli bacteremia, according to the MIC of the antimicrobial useda
| Cohort, ESBL production, and treatment | No. of deceased patients/no. treated with a MIC (μg/ml) of: |
|||
|---|---|---|---|---|
| ≤1 | 2 | 4 | 8 | |
| Empirical therapy cohort | ||||
| ESBL-EC | ||||
| Cefmetazole | 1/5 | 0/2 | ||
| Flomoxef | 0/11 | |||
| Non-ESBL-EC | ||||
| Cefmetazole | 1/18 | 0/1 | ||
| Flomoxef | 0/13 | |||
| Definitive therapy cohort | ||||
| ESBL-EC | ||||
| Cefmetazole | 1/13 | 0/2 | 0/2 | |
| Flomoxef | 0/22 | 0/1 | ||
| Non-ESBL-EC | ||||
| Cefmetazole | 1/10 | |||
| Flomoxef | 0/8 | |||
Only patients with isolates that were re-evaluated for the detailed cefmetazole and flomoxef MIC data were included.
TABLE 3.
Crude and propensity score-adjusted Cox proportional hazards regression analysis of 30-day mortality and logistic regression analysis of 30-day clinical success in patients with bacteremia caused by ESBL-producing Escherichia colia
| Cohort and adjustment | Maximum/mean standardized biasb | % with 30-day mortality |
HR (95% CI) | % with 30-day clinical success |
OR (95% CI) | ||
|---|---|---|---|---|---|---|---|
| ESBL-CF | ESBL-CARBA | ESBL-CF | ESBL-CARBA | ||||
| Empirical therapy cohort | |||||||
| Unadjusted | 0.74/0.28 | 7.7 | 8.9 | 0.83 (0.15–4.53) | 100.0 | 93.3 | Not donec |
| Propensity score adjustedd,e | 0.24/0.09 | 5.2 | 5.8 | 0.87 (0.11–6.52)f | 100.0 | 96.5 | Not donec |
| Definitive therapy cohort | |||||||
| Unadjusted | 1.05/0.30 | 5.1 | 9.3 | 0.53 (0.12–2.20) | 98.3 | 92.6 | 4.64 (0.50–42.9) |
| Propensity score adjustedd,g | 0.22/0.08 | 7.4 | 7.0 | 1.04 (0.24–4.49)h | 98.4 | 96.3 | 2.46 (0.20–29.6) |
ESBL-CF, treatment with cefmetazole or flomoxef; ESBL-CARBA, treatment with carbapenem; HR, hazard ratio; CI, confidence interval; OR, odds ratio.
The standardized bias was calculated by dividing the difference in the means of the covariate between the two groups by the standard deviation for all of the baseline characteristic variables.
Odds ratios for clinical success were not calculated because all of the patients in the ESBL-CF group achieved clinical success.
Patients with hematological malignancy and neutropenia were included only in the carbapenem treatment group. Only patients without hematological malignancy and neutropenia were used to make an appropriate multivariate logistic regression model for the propensity score calculations and to achieve balancing of the ESBL-CF and ESBL-CARBA groups. Propensity score adjustments were performed using the stabilized inverse probabilities of treatment weights.
The propensity score was calculated using a multivariate logistic regression model including age, prior ESBL colonization, Charlson score, artificial devices other than intravascular or urinary catheter, and SOFA score for 26 patients in the ESBL-CF group and 31 patients in the ESBL-CARBA group.
The HR (CI) with adjustment for severe sepsis or septic shock or a source other than the urinary tract was 0.86 (0.11 to 6.51) or 0.94 (0.12 to 7.02).
The propensity score was calculated using a nonparsimonious multivariate logistic regression model including all of the baseline characteristic variables (age, sex, length of hospital stay, nosocomial or health care-associated bacteremia, prior ESBL producer isolation, prior use of antimicrobials, Charlson score, solid malignancy, diabetes, transplantation, immunosuppressive therapy, antitumor chemotherapy, neutropenia, hemodialysis, surgery, intravenous catheterization, urinary catheterization, artificial devices other than intravascular or urinary catheter, severe sepsis or septic shock, SOFA score, and site of infection) for 59 patients in the ESBL-CF group and 30 patients in the ESBL-CARBA group.
The HR (CI) with adjustment for severe sepsis or septic shock or source other than the urinary tract was 1.04 (0.24 to 4.49) or 1.04 (0.24 to 4.49).
Definitive therapy cohort.
The ESBL-CF, ESBL-CARBA, and non-ESBL-CF groups were comprised of 59 patients (cefmetazole, 33; flomoxef, 26), 54 patients (meropenem, 47; imipenem, 6; panipenem, 1), and 20 patients (cefmetazole, 11; flomoxef, 9), respectively. The dosage regimens were similar to those described for the ETC. The clinical characteristics of each group are presented in Table 4.
TABLE 4.
Characteristics of patients with bacteremia caused by Escherichia coli in the definitive therapy cohort
| Characteristic | Cohort according to ESBL production and treatmenta |
P valueb |
|||
|---|---|---|---|---|---|
| ESBL-CF (n = 59) | ESBL-CARBA (n = 54) | Non-ESBL-CF (n = 20) | Versus carbapenem | Versus non-ESBL | |
| Age (yr)i | 71 (64–81) | 68.5 (60–78) | 68 (60.5–76) | 0.09 | 0.26 |
| No. (%) of males | 40 (68) | 28 (52) | 10 (50) | 0.12 | 0.19 |
| Length of hospital stay before bacteremia (days)i | 7 (1–34) | 20 (2–67) | 11.5 (0–35.5) | 0.02 | 0.92 |
| No. (%) with: | |||||
| Nosocomial or health care-associated bacteremia | 37 (63) | 41 (76) | 15 (75) | 0.16 | 0.42 |
| Prior ESBL-producer isolation | 13 (22) | 17 (31) | 2 (10) | 0.29 | 0.33 |
| Prior use of antimicrobials within 30 days | 33 (56) | 38 (70) | 9 (45) | 0.12 | 0.45 |
| Charlson comorbidity indexi | 2 (1–4) | 2 (2–4) | 2 (1–5.5) | 0.10 | 0.43 |
| No. (%) with: | |||||
| Hematological malignancy | 0 (0) | 23 (43) | 1 (5) | <0.001 | 0.25 |
| Solid malignancy | 16 (27) | 14 (26) | 11 (55) | 1 | 0.03 |
| Diabetes | 15 (25) | 11 (20) | 6 (30) | 0.66 | 0.77 |
| Transplantation | 5 (8) | 10 (19) | 0 (0) | 0.17 | 0.32 |
| Immunosuppressive therapy | 9 (15) | 13 (24) | 6 (30) | 0.34 | 0.19 |
| Antitumor chemotherapy | 5 (8) | 20 (37) | 4 (20) | <0.001 | 0.22 |
| Neutropenia | 0 (0) | 12 (22) | 1 (5) | <0.001 | 0.25 |
| Hemodialysis | 2 (3) | 3 (6) | 0 (0) | 0.67 | 1 |
| Surgery within 30 days | 4 (7) | 5 (9) | 2 (10) | 0.74 | 0.64 |
| Intravascular catheterization | 18 (31) | 29 (54) | 9 (45) | 0.01 | 0.28 |
| Urinary catheterization | 13 (22) | 8 (15) | 2 (10) | 0.35 | 0.33 |
| Artificial devices other than intravascular or urinary catheter | 12 (20) | 10 (19) | 2 (10) | 1 | 0.50 |
| Severe sepsis or septic shock | 25 (42) | 21 (39) | 4 (20) | 0.85 | 0.11 |
| SOFA scorei | 2 (1–5) | 3.5 (2–5) | 2 (0–4.5) | 0.13 | 0.39 |
| No. (%) with infection at site | |||||
| Urinary tract | 34 (58) | 23 (43) | 9 (45) | 0.13 | 0.44 |
| Intra-abdominal | 16 (27) | 16 (30) | 8 (40) | 0.84 | 0.40 |
| Primary | 5 (8) | 14 (26) | 2 (10) | 0.02 | 1 |
| Others | 4 (7) | 1 (2) | 1 (5) | 0.37 | 1 |
| No. (%) receiving empirical therapy | |||||
| Cefmetazole | 7 (12) | 0 (0) | 6 (30) | 0.01 | 0.08 |
| Flomoxef | 10 (17) | 2 (4) | 6 (30) | 0.03 | 0.22 |
| Carbapenem | 10 (17) | 31 (57) | 2 (10) | <0.001 | 0.72 |
| Cephalosporin | 20 (34) | 15 (28) | 3 (15) | 0.54 | 0.16 |
| β-Lactam/β-lactamase inhibitor | 11 (19) | 6 (11) | 3 (15) | 0.30 | 1 |
| None | 1c (2) | 0 (0) | 0 (0) | 1 | 1 |
| Appropriate therapy | 47 (80) | 46 (85) | 20 (100) | 0.47 | <0.001 |
| No. (%) receiving definitive therapy | |||||
| Cefmetazole | 33 (56) | 0 (0) | 11 (55) | <0.001 | 1 |
| Flomoxef | 26 (44) | 0 (0) | 9 (45) | <0.001 | 1 |
| Carbapenem | 0 (0) | 54d (100) | 0 (0) | <0.001 | 1 |
| Total duration of treatment (days)i | 14 (11–16) | 14 (11–19) | 11.5 (9–14) | 0.65 | 0.11 |
| Duration of treatment with the antimicrobial agent used in definitive therapy (days)i | 11 (8–14) | 11 (9–15) | 9 (7–11.5) | 0.22 | 0.24 |
| No. (%) with: | |||||
| 30-day mortality | 3e (5) | 5 (9) | 1f (5) | 0.48 | 1 |
| Clinical success within 30 days | 58g (98) | 50h (93) | 20 (100) | 0.19 | 1 |
| Duration between onset of the bacteremia and complete response (days)i | 3 (2–5) | 4 (3–7) | 3.5 (2–5) | 0.12 | 0.77 |
| No. (%) with relapse within 30 days | 1g (2) | 1h (2) | 0 (0) | 1 | 1 |
As a definitive therapy, the ESBL-CF group included patients with ESBL-producing E. coli bacteremia who received cefmetazole or flomoxef, the ESBL-CARBA group included patients with ESBL-producing E. coli bacteremia who received carbapenem, and the non-ESBL-CF group included patients with non-ESBL-producing E. coli bacteremia who received cefmetazole or flomoxef.
P values were calculated based on comparisons between the ESBL-CF group and the ESBL-CARBA or non-ESBL-CF group.
One patient who received definitive treatment with cefmetazole did not receive any empirical antimicrobial therapy.
Meropenem, imipenem, and panipenem were administered to 47, 6, and 1 patients, respectively.
All of the patients received cefmetazole and died after the completion of treatments due to underlying malignancies or choking. The MICs of cefmetazole were ≤1, ≤4, and ≤16 μg/ml.
This patient received cefmetazole and died after completion of the treatment due to underlying liver cirrhosis and malignancy. The MIC of cefmetazole was ≤1 μg/ml.
One patient experienced acute prostatitis, which recurred 12 days after the completion of the antimicrobial treatment, including cefepime for 2 days followed by cefmetazole for 6 days and faropenem for 3 days. The MIC of cefmetazole was ≤1 μg/ml.
Three patients died before achieving a complete response. Another patient experienced acute pyelonephritis, and recurrent bacteremia was observed 4 days after the completion of an antimicrobial regimen consisting of cefotiam for 3 days followed by meropenem for 18 days. The MIC of meropenem was ≤0.25 μg/ml. Neither of the isolates of the recurrent episodes developed resistance to cefmetazole or meropenem.
Data are medians (interquartile range).
The crude 30-day mortality rates were 5.1% in the ESBL-CF group, 9.3% in the ESBL-CARBA group, and 5.0% in the non-ESBL-CF group. Unadjusted Cox regression analyzes of the mortality rates indicated that the ESBL-CF group had an HR of 0.53 (CI, 0.12 to 2.20) compared with the ESBL-CARBA group and an HR of 1.04 (CI, 0.10 to 10.0) compared with the non-ESBL-CF group. The clinical success rates were similar among the three groups (>90%). Table 3 presents the propensity score-adjusted analysis, which included only patients without hematological malignancy or neutropenia, because the ESBL-CF group did not include these patients. Compared with carbapenem treatment, definitive CF treatment for ESBL-EC bacteremia was not associated with mortality (adjusted HR, 1.04; CI, 0.24 to 4.49) and demonstrated similar clinical success rates (98.4% and 96.3%, respectively).
DISCUSSION
In this multicenter retrospective study using a propensity score-adjusted analysis, the superiority of carbapenems to CF for empirical and definitive treatment of ESBL-EC bacteremia could not be shown regarding the 30-day mortality rates and clinical success. To date, 5 reports have evaluated the efficacy of cephamycins for the treatment of infections ESBL-producing members of the Enterobacteriaceae (8, 19–22). All of these single-center retrospective studies included a small number of patients and a control group comprised of patients receiving carbapenem. Lee et al. evaluated 7 patients who were diagnosed with Klebsiella pneumoniae bacteremia and received definitive flomoxef treatment; the 14-day mortality was similar to that of the controls (8). Doi et al. evaluated 10 patients who were diagnosed with E. coli or K. pneumoniae pyelonephritis and received definitive cefmetazole treatment. The 4-week clinical cure rate was similar to that of the controls (19). Pilmis et al. evaluated 9 patients who were diagnosed with urinary tract infections or bacteremia due to K. pneumoniae or Enterobacter cloacae and received definitive cefoxitin treatment; the clinical and microbiological outcomes of these patients did not differ from those of the controls (20). Yang et al. evaluated 19 patients who were diagnosed with K. pneumoniae hemodialysis catheter-related bacteremia and received definitive flomoxef treatment (21, 22). Flomoxef treatment was associated with higher mortality than that of the controls. The results from these studies are inconsistent; however, this could be explained at least in part by the heterogeneity in the bacterial species, the sources of infection studied, differences in the population demographics, and the definitions of patient outcomes.
In nonrandomized controlled trials, antimicrobial regimens are selected by the clinicians, which entails a degree of bias and makes direct comparison the outcomes difficult (17). Clinicians often treat severely ill patients with carbapenem, which affects the mortality rate of these patients (10). In fact, the crude mortality rates and severity of illness of the ESBL-CARBA group were higher than those of the ESBL-CF group, which is consistent with our expectations. Propensity score analysis is the current gold standard among strategies to analyze observational data (23) and is considered superior to multivariate regression models in reducing biases between groups, because it uses many covariates, entails a degree of adjustment (balancing), and can be used even for a small number of events (24). Therefore, we believe that our propensity score-adjusted analysis may provide more reliable data than previous reports.
In the ETC, the ESBL-CF group had a higher mortality rate than the non-ESBL-CF group, but clinical success was observed in all of the ESBL-CF patients. These results suggest that the clinical efficacy of CF is not associated with ESBL production. Of the ESBL-CF and non-ESBL-CF groups in the ETC and DTC, only patients who received cefmetazole died or did not achieve clinical success within 30 days. However, the deceased patients achieved a complete response before death, and a single patient who relapsed did not receive adequate duration of treatment. These results do not indicate inferiority of cefmetazole to flomoxef.
Some limitations of this study should be noted. First, due to the retrospective nature of our study, all measured or unmeasured confounders may not have been properly controlled. Second, we analyzed patients who received CF as a combined group, not separately. Clinicians usually do not discriminate between these agents. Moreover, the ESBL-EC isolates analyzed demonstrated high rates of susceptibility to both antibiotics. Third, the number of patients included in this study is small with respect to statistical power; however, to our knowledge, this is the largest study to date evaluating cephamycins for the treatment of ESBL-producing Enterobacteriaceae and the first study evaluating cephamycins for the treatment of ESBL-EC. Fourth, we could not characterize the ESBL genes or investigate the MIC distribution for all of the study isolates.
In conclusion, cefmetazole and flomoxef represent viable alternative antimicrobial agents for the treatment of ESBL-EC bacteremia as empirical and definitive therapies in adult patients who do not have hematological malignancy, neutropenia, or polymicrobial bacteremia. Larger prospective studies or, ideally, randomized controlled trials are required to define the exact efficacies of cephamycins.
ACKNOWLEDGMENTS
We thank Sayo Shitashiro for technical assistance.
This study was supported by internal funding.
We have no conflicts of interest to declare.
REFERENCES
- 1.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 2.Pitout JD, Laupland KB. 2008. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis 8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 3.Rottier WC, Ammerlaan HS, Bonten MJ. 2012. Effects of confounders and intermediates on the association of bacteraemia caused by extended-spectrum β-lactamase-producing Enterobacteriaceae and patient outcome: a meta-analysis. J Antimicrob Chemother 67:1311–1320. doi: 10.1093/jac/dks065. [DOI] [PubMed] [Google Scholar]
- 4.Ramphal R, Ambrose PG. 2006. Extended-spectrum beta-lactamases and clinical outcomes: current data. Clin Infect Dis 42(Suppl 4):S164–S172. doi: 10.1086/500663. [DOI] [PubMed] [Google Scholar]
- 5.Vardakas KZ, Tansarli GS, Rafailidis PI, Falagas ME. 2012. Carbapenems versus alternative antibiotics for the treatment of bacteraemia due to Enterobacteriaceae producing extended-spectrum β-lactamases: a systematic review and meta-analysis. J Antimicrob Chemother 67:2793–2803. doi: 10.1093/jac/dks301. [DOI] [PubMed] [Google Scholar]
- 6.Hussein K, Sprecher H, Mashiach T, Oren I, Kassis I, Finkelstein R. 2009. Carbapenem resistance among Klebsiella pneumoniae isolates: risk factors, molecular characteristics, and susceptibility patterns. Infect Control Hosp Epidemiol 30:666–671. doi: 10.1086/598244. [DOI] [PubMed] [Google Scholar]
- 7.Thomson KS, Moland ES. 2001. Cefepime, piperacillin-tazobactam, and the inoculum effect in tests with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother 45:3548–3554. doi: 10.1128/AAC.45.12.3548-3554.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CH, Su LH, Tang YF, Liu JW. 2006. Treatment of ESBL-producing Klebsiella pneumoniae bacteraemia with carbapenems or flomoxef: a retrospective study and laboratory analysis of the isolates. J Antimicrob Chemother 58:1074–1077. doi: 10.1093/jac/dkl381. [DOI] [PubMed] [Google Scholar]
- 9.Jacoby GA, Carreras I. 1990. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 34:858–862. doi: 10.1128/AAC.34.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodríguez-Baño J, Navarro MD, Retamar P, Picón E, Pascual Á. 2012. β-Lactam/β-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum β-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin Infect Dis 54:167–174. doi: 10.1093/cid/cir790. [DOI] [PubMed] [Google Scholar]
- 11.Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, Cosgrove SE. 2015. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum β-lactamase bacteremia. Clin Infect Dis 60:1319–1325. doi: 10.1093/cid/civ003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman N, Kaye K, Stout J, McGarry S, Trivette S, Briggs J, Lamm W, Clark C, MacFarquhar J, Walton A, Reller L, Sexton D. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. 1996. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710. [DOI] [PubMed] [Google Scholar]
- 15.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. 1992. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874. doi: 10.1097/00003246-199206000-00025.1597042 [DOI] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Harder VS, Stuart EA, Anthony JC. 2010. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychol Methods 15:234–249. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robins JM, Hernán MA, Brumback B. 2000. Marginal structural models and causal inference in epidemiology. Epidemiology 11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Doi A, Shimada T, Harada S, Iwata K, Kamiya T. 2013. The efficacy of cefmetazole against pyelonephritis caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae. Int J Infect Dis 17:e159-163. doi: 10.1016/j.ijid.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Pilmis B, Parize P, Zahar JR, Lortholary O. 2014. Alternatives to carbapenems for infections caused by ESBL-producing Enterobacteriaceae. Eur J Clin Microbiol Infect Dis 33:1263–1265. doi: 10.1007/s10096-014-2094-y. [DOI] [PubMed] [Google Scholar]
- 21.Yang CC, Li SH, Chuang FR, Chen CH, Lee CH, Chen JB, Wu CH, Lee CT. 2012. Discrepancy between effects of carbapenems and flomoxef in treating nosocomial hemodialysis access-related bacteremia secondary to extended spectrum beta-lactamase producing Klebsiella pneumoniae in patients on maintenance hemodialysis. BMC Infect Dis 12:206. doi: 10.1186/1471-2334-12-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CC, Wu CH, Lee CT, Liu HT, Chen JB, Chiu CH, Chen CH, Chuang FR. 2014. Nosocomial extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteremia in hemodialysis patients and the implications for antibiotic therapy. Int J Infect Dis 28:3–7. doi: 10.1016/j.ijid.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Perez F, Bonomo RA. 2015. Bloodstream infection caused by extended-spectrum β-lactamase-producing gram-negative bacteria: how to define the best treatment regimen? Clin Infect Dis 60:1326–1329. doi: 10.1093/cid/civ007. [DOI] [PubMed] [Google Scholar]
- 24.Austin PC. 2011. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]